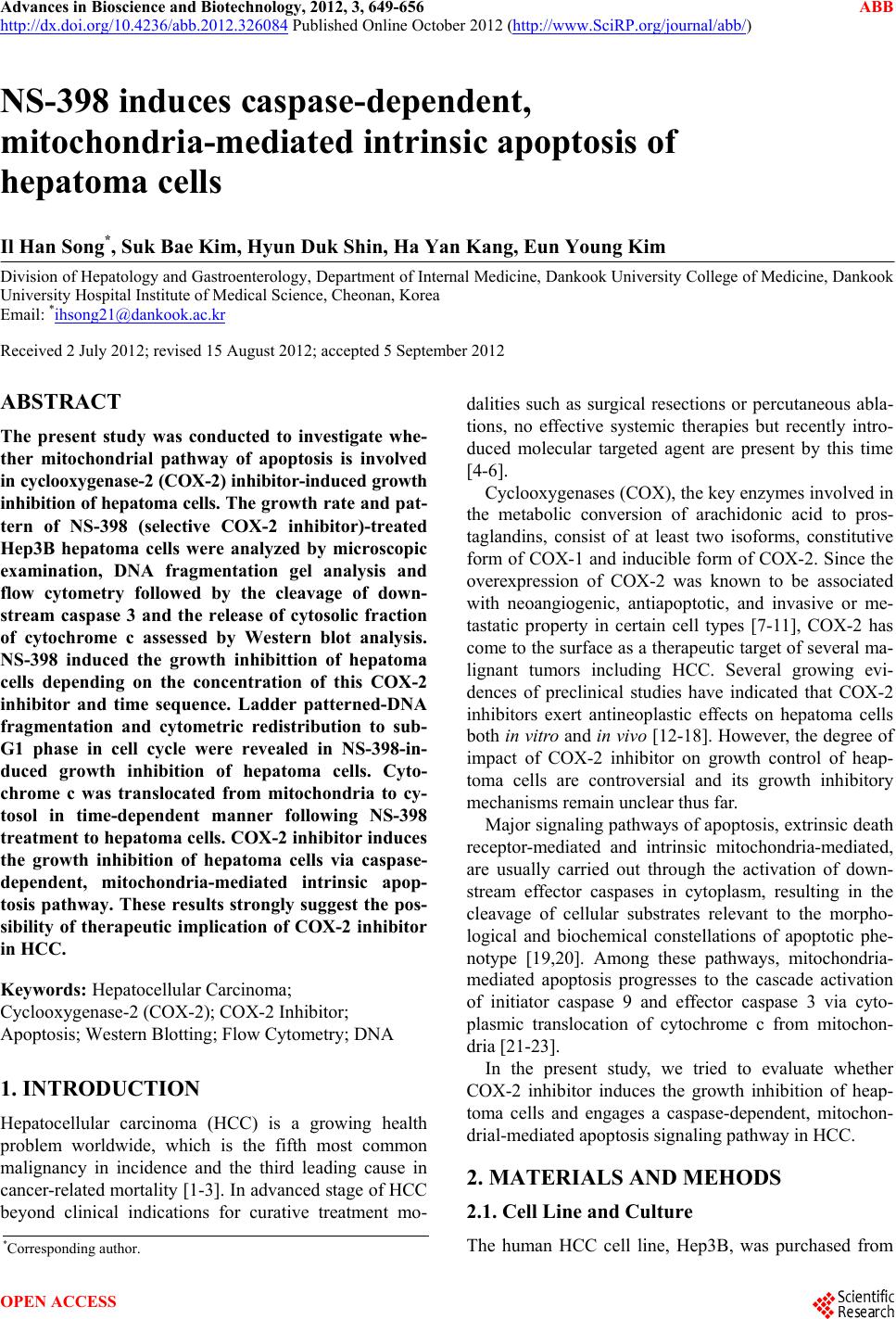
 Advances in Bioscience and Biotechnology, 2012, 3, 649-656 ABB http://dx.doi.org/10.4236/abb.2012.326084 Published Online October 2012 (http://www.SciRP.org/journal/abb/) NS-398 induces caspase-dependent, mitochondria-mediated intrinsic apoptosis of hepatoma cells Il Han Song*, Suk Bae Kim, Hyun Duk Shin, Ha Yan Kang, Eun Young Kim Division of Hepatology and Gastroenterology, Department of Internal Medicine, Dankook University College of Medicine, Dankook University Hospital Institute of Medical Science, Cheonan, Korea Email: *ihsong21@dankook.ac.kr Received 2 July 2012; revised 15 August 2012; accepted 5 September 2012 ABSTRACT The present study was conducted to investigate whe- ther mitochondrial pathway of apoptosis is involved in cyclooxygenase-2 (COX-2) inhibitor-induced growth inhibition of hepatoma cells. The g ro wt h ra t e a nd pa t- tern of NS-398 (selective COX-2 inhibitor)-treated Hep3B hepatoma cells were analyzed by microscopic examination, DNA fragmentation gel analysis and flow cytometry followed by the cleavage of down- stream caspase 3 and the release of cytosolic fraction of cytochrome c assessed by Western blot analysis. NS-398 induced the growth inhibittion of hepatoma cells depending on the concentration of this COX-2 inhibitor and time sequence. Ladder patterned-DNA fragmentation and cytometric redistribution to sub- G1 phase in cell cycle were revealed in NS-398-in- duced growth inhibition of hepatoma cells. Cyto- chrome c was translocated from mitochondria to cy- tosol in time-dependent manner following NS-398 treatment to hepatoma cells. COX-2 inhibitor induces the growth inhibition of hepatoma cells via caspase- dependent, mitochondria-mediated intrinsic apop- tosis pathway. These results strongly suggest the pos- sibility of therapeutic implication of COX-2 inhibitor in HCC. Keywords: Hepatocellular Carcinoma; Cyclooxygenase-2 (COX-2); COX-2 Inhibitor; Apoptosis; Western Blotting; Flow Cytometry; DNA 1. INTRODUCTION Hepatocellular carcinoma (HCC) is a growing health problem worldwide, which is the fifth most common malignancy in incidence and the third leading cause in cancer-related mortality [1-3]. In advanced stage of HCC beyond clinical indications for curative treatment mo- dalities such as surgical resections or percutaneous abla- tions, no effective systemic therapies but recently intro- duced molecular targeted agent are present by this time [4-6]. Cyclooxygenases (COX), the key enzymes involved in the metabolic conversion of arachidonic acid to pros- taglandins, consist of at least two isoforms, constitutive form of COX-1 and inducible form of COX-2. Since the overexpression of COX-2 was known to be associated with neoangiogenic, antiapoptotic, and invasive or me- tastatic property in certain cell types [7-11], COX-2 has come to the surface as a therapeutic target of several ma- lignant tumors including HCC. Several growing evi- dences of preclinical studies have indicated that COX-2 inhibitors exert antineoplastic effects on hepatoma cells both in vitro and in vivo [12-18]. However, the degree of impact of COX-2 inhibitor on growth control of heap- toma cells are controversial and its growth inhibitory mechanisms remain unclear thus far. Major signaling pathways of apoptosis, extrinsic death receptor-mediated and intrinsic mitochondria-mediated, are usually carried out through the activation of down- stream effector caspases in cytoplasm, resulting in the cleavage of cellular substrates relevant to the morpho- logical and biochemical constellations of apoptotic phe- notype [19,20]. Among these pathways, mitochondria- mediated apoptosis progresses to the cascade activation of initiator caspase 9 and effector caspase 3 via cyto- plasmic translocation of cytochrome c from mitochon- dria [21-23]. In the present study, we tried to evaluate whether COX-2 inhibitor induces the growth inhibition of heap- toma cells and engages a caspase-dependent, mitochon- drial-mediated apoptosis signaling pathway in HCC. 2. MATERIALS AND MEHODS 2.1. Cell Line and Culture The human HCC cell line, Hep3B, was purchased from *Corresponding author. OPEN ACCESS  I. H. Song et al. / Advances in Bioscience and Biotechnology 3 (2012) 649-656 650 Korean Cell Line Bank (Seoul, Korea). Hep3B cells were cultured in Dulbecco’s Modified Eagle Medium (Gibco BRL, Hyclone laboratories, Lagan, Utah, USA), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in a humidified incubator supplied with 5% CO2 at 37˚C atmosphere. 2.2. Treatment of Selective COX-2 Inhibitor to Hepatoma Cells NS-398 (N-[2-(cyclohexyloxy)-4-nitrophenyl]methane- sulfonamide), dissolved in demethyl sulfoxide (DMSO), was used as a selective COX-2 inhibitor. We prepared the culture media in concentrations of 0, 10, 100, and 200 μM NS-398 for concentration-oriented experiments. Hepa- toma cells were plated at a density of 1 × 105 cells/well in six-well plastic dishes with 2 mL of 10% FBS-sup- plemented medium. After 24 h exposure of NS-398, the media were changed with other new media containing same concentration of NS-398, and then the cells were incubated for 72 h. 2.3. Microscopic Examination After discarding the media with floating cells, we mi- croscopically observed the cells continuously for three days for time-oriented experiments under ×20 magnifica- tion and compared the growth pattern of cell prolifera- tion between controls (DMSO-treated cells) and NS-398- treated cells according to sequential time course of 24, 48, and 72 h. 2.4. DNA Fragmentation Gel Analysis Hepatoma cells were harvested at 24, 48, and 72 h after treatment of various concentrations of NS-398. The cells dissolved with lysis buffer were centrifuged at 10,000 g for 30 min. For DNA extraction, the supernatant was digested with 50 ng/mL proteinase K at 37˚C for 24 h, and precipitated with a equal volume of absolute ethanol. For RNA elimination, the pellet was incubated with a Tris-EDTA buffer containing 10 μg/mL RNase A at 37˚C for 1 h. The amount of extracted DNA was measured by spectrophotometric analysis. Each DNA sample was electrophoresed on 1.8% agarose gel containing 0.5 mg/L ethidium bromide, and photographed under ultra- violet (UV) light. 2.5. Flow Cytometric Analysis Cell cycle distribution was determined by flow cytomet- ric analysis. After treatment of NS-398, the cells were collected by trypsinization, washed twice with phos- phate-buffered saline (PBS), and fixed overnight in 70% ethanol at 4˚C. The cells were stained with 50 μg/mL propidium iodide at room temperature for 30 min in the dark, following to be incubated with 50 μg/mL RNase A at 37˚C for 1 h. Then cell cycle components were ana- lyzed by a flow cytometer and CellQuest software. 2.6. Western Blot Analysis NS-398-treated hepatoma cells were prepared by wash- ing in PBS and dissolving in lysis buffer (50 mmol/L Tris pH 7.5, 250 mmol/L NaCl2, 0.5% Triton X-100, 1 mmol/L EDYA, 1 mmol/L PMSF, 1 mmol/L Na3VO4, 1 mmol/L dithiothreitol, 10 μg of leupeptin/mL and 10 μg of aprotinin/mL). After centrifugation of cell lysates for 10 min at 14,000 g, the protein concentrations of super- natant in the homogenate were determined by bicin- choninic acid assay (Pierce Co, Rockford, IL, USA) ac- cording to the manufacturer’s instructions. 40 μg of pro- tein in each extract was separated by 15% SDS-poly- acrylamide gel, and electronically transferred to nitro- cellulose membrane. The membrane was blocked with 5% fat free dry milk in TBS-T (25 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, 0.5 Tween-20), incubated with primary antibody for overnight at 4˚C, washed three times in TBS-T for 10 min, and then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The immunoblotting signals were developed with an ECL system (Amersham Life Sciences, Buckinghamshire, UK). Anti-β-actin (1:1000, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) was used as a protein loading control. 2.7. Preparation of Mitochondrial and Cytosolic Extracts for Localization of Cytochrome c The cell pellets were obtained from NS-398-treated hepatoma cells that were washed, centrifugated, and re- suspended in a buffer containing 25 mM Tris (pH 7.4), 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT. The resuspened cells were homogenized ten times with Dounce homoge- nizer (Wheaton Scientific Products, Millville, New Jer- sey, USA) after adding protease inhibitor (10 μg of leu- peptin/mL, 10 μg of aprotinin/mL, and 1 mM PMSF) and phosphatase inhibitor (10 μM Na3VO4). Unlysed cells and nuclei were discarded by centrifugation at 750 g for 10 min. The supernatant was centrifugated at 10,000 g for 30 min, and the resulting pellet, which indicates the mitochondrial-enriched fraction, was washed once with the same buffer. The remnant supernatant was further centrifugated at 10,000 g for 1 h, representing the cytosol fraction of final supernatant. Each 40 μg of cytosolic and mitochondrial fractions were used for cytochome c im- munoblotting described above. 2.8. Statistical Analysis Statistical analysis was carried out using SPSS software Copyright © 2012 SciRes. OPEN ACCESS 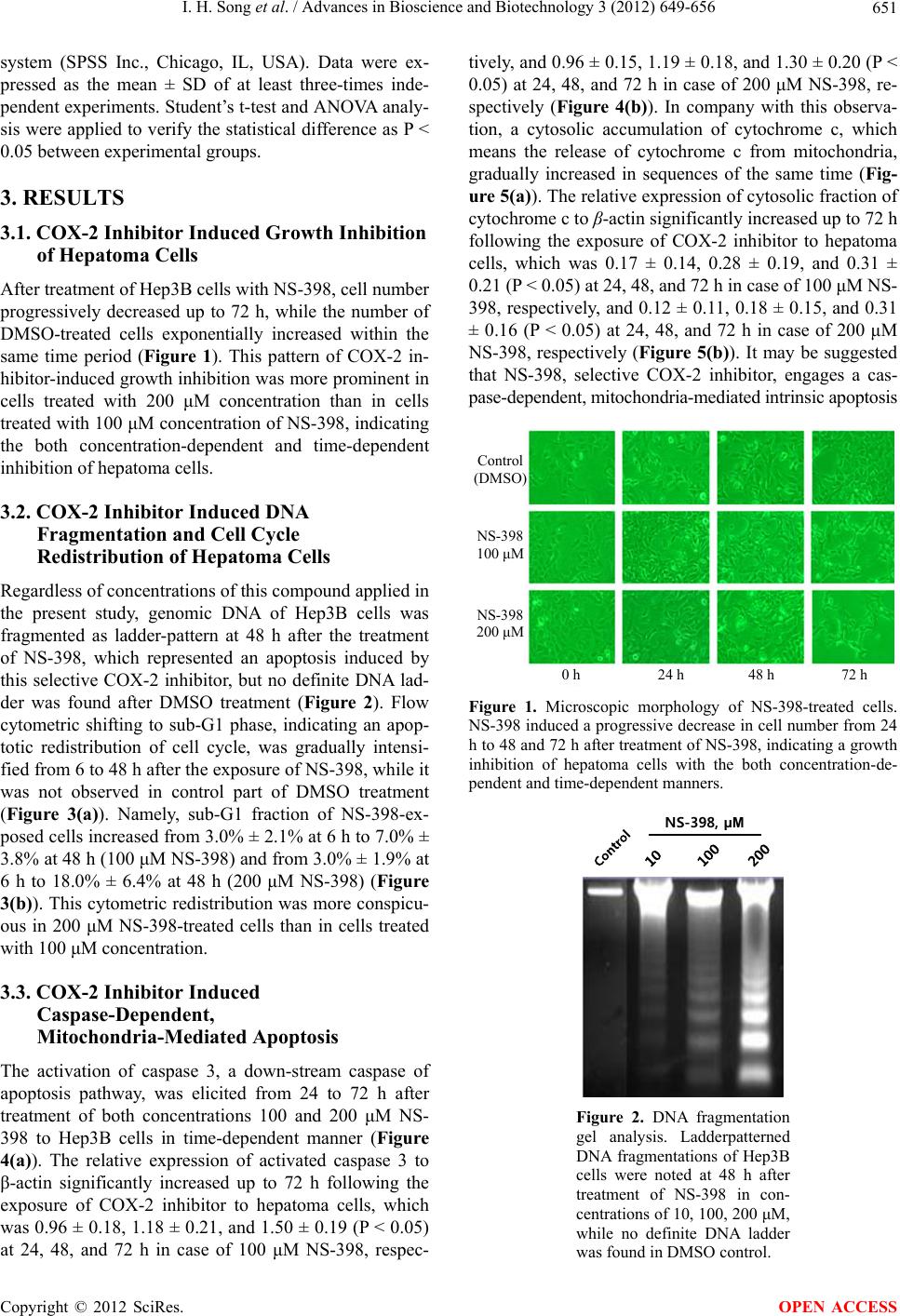 I. H. Song et al. / Advances in Bioscience and Biotechnology 3 (2012) 649-656 651 system (SPSS Inc., Chicago, IL, USA). Data were ex- pressed as the mean ± SD of at least three-times inde- pendent experiments. Student’s t-test and ANOVA analy- sis were applied to verify the statistical difference as P < 0.05 between experimental groups. 3. RESULTS 3.1. COX-2 Inhibitor Induced Growth Inhibition of Hepatoma Cells After treatment of Hep3B cells with NS-398, cell number progressively decreased up to 72 h, while the number of DMSO-treated cells exponentially increased within the same time period (Figure 1). This pattern of COX-2 in- hibitor-induced growth inhibition was more prominent in cells treated with 200 μM concentration than in cells treated with 100 μM concentration of NS-398, indicating the both concentration-dependent and time-dependent inhibition of hepatoma cells. 3.2. COX-2 Inhibitor Induced DNA Fragmentation and Cell Cycle Redistribution of Hepatoma Cells Regardless of concentrations of this compound applied in the present study, genomic DNA of Hep3B cells was fragmented as ladder-pattern at 48 h after the treatment of NS-398, which represented an apoptosis induced by this selective COX-2 inhibitor, but no definite DNA lad- der was found after DMSO treatment (Figure 2). Flow cytometric shifting to sub-G1 phase, indicating an apop- totic redistribution of cell cycle, was gradually intensi- fied from 6 to 48 h after the exposure of NS-398, while it was not observed in control part of DMSO treatment (Figure 3(a)). Namely, sub-G1 fraction of NS-398-ex- posed cells increased from 3.0% ± 2.1% at 6 h to 7.0% ± 3.8% at 48 h (100 μM NS-398) and from 3.0% ± 1.9% at 6 h to 18.0% ± 6.4% at 48 h (200 μM NS-398) (Figure 3(b)). This cytometric redistribution was more conspicu- ous in 200 μM NS-398-treated cells than in cells treated with 100 μM concentration. 3.3. COX-2 Inhibitor Induced Caspase-Dependent, Mitochondria-Mediated Apoptosis The activation of caspase 3, a down-stream caspase of apoptosis pathway, was elicited from 24 to 72 h after treatment of both concentrations 100 and 200 μM NS- 398 to Hep3B cells in time-dependent manner (Figure 4(a)). The relative expression of activated caspase 3 to β-actin significantly increased up to 72 h following the exposure of COX-2 inhibitor to hepatoma cells, which was 0.96 ± 0.18, 1.18 ± 0.21, and 1.50 ± 0.19 (P < 0.05) at 24, 48, and 72 h in case of 100 μM NS-398, respec- tively, and 0.96 ± 0.15, 1.19 ± 0.18, and 1.30 ± 0.20 (P < 0.05) at 24, 48, and 72 h in case of 200 μM NS-398, re- spectively (Figure 4(b)). In company with this observa- tion, a cytosolic accumulation of cytochrome c, which means the release of cytochrome c from mitochondria, gradually increased in sequences of the same time (Fig- ure 5(a)). The relative expression of cytosolic fraction of cytochrome c to β-actin significantly increased up to 72 h following the exposure of COX-2 inhibitor to hepatoma cells, which was 0.17 ± 0.14, 0.28 ± 0.19, and 0.31 ± 0.21 (P < 0.05) at 24, 48, and 72 h in case of 100 μM NS- 398, respectively, and 0.12 ± 0.11, 0.18 ± 0.15, and 0.31 ± 0.16 (P < 0.05) at 24, 48, and 72 h in case of 200 μM NS-398, respectively (Figure 5(b)). It may be suggested that NS-398, selective COX-2 inhibitor, engages a cas- pase-dependent, mitochondria-mediated intrinsic apoptosis Control (DMSO) NS-398 100 μM NS-398 200 μM 0 h24 h 48 h 72 h Figure 1. Microscopic morphology of NS-398-treated cells. NS-398 induced a progressive decrease in cell number from 24 h to 48 and 72 h after treatment of NS-398, indicating a growth inhibition of hepatoma cells with the both concentration-de- pendent and time-dependent manners. NS-398, μM Figure 2. DNA fragmentation gel analysis. Ladderpatterned DNA fragmentations of Hep3B cells were noted at 48 h after treatment of NS-398 in con- centrations of 10, 100, 200 μM, while no definite DNA ladder as found in DMSO control. w Copyright © 2012 SciRes. OPEN ACCESS 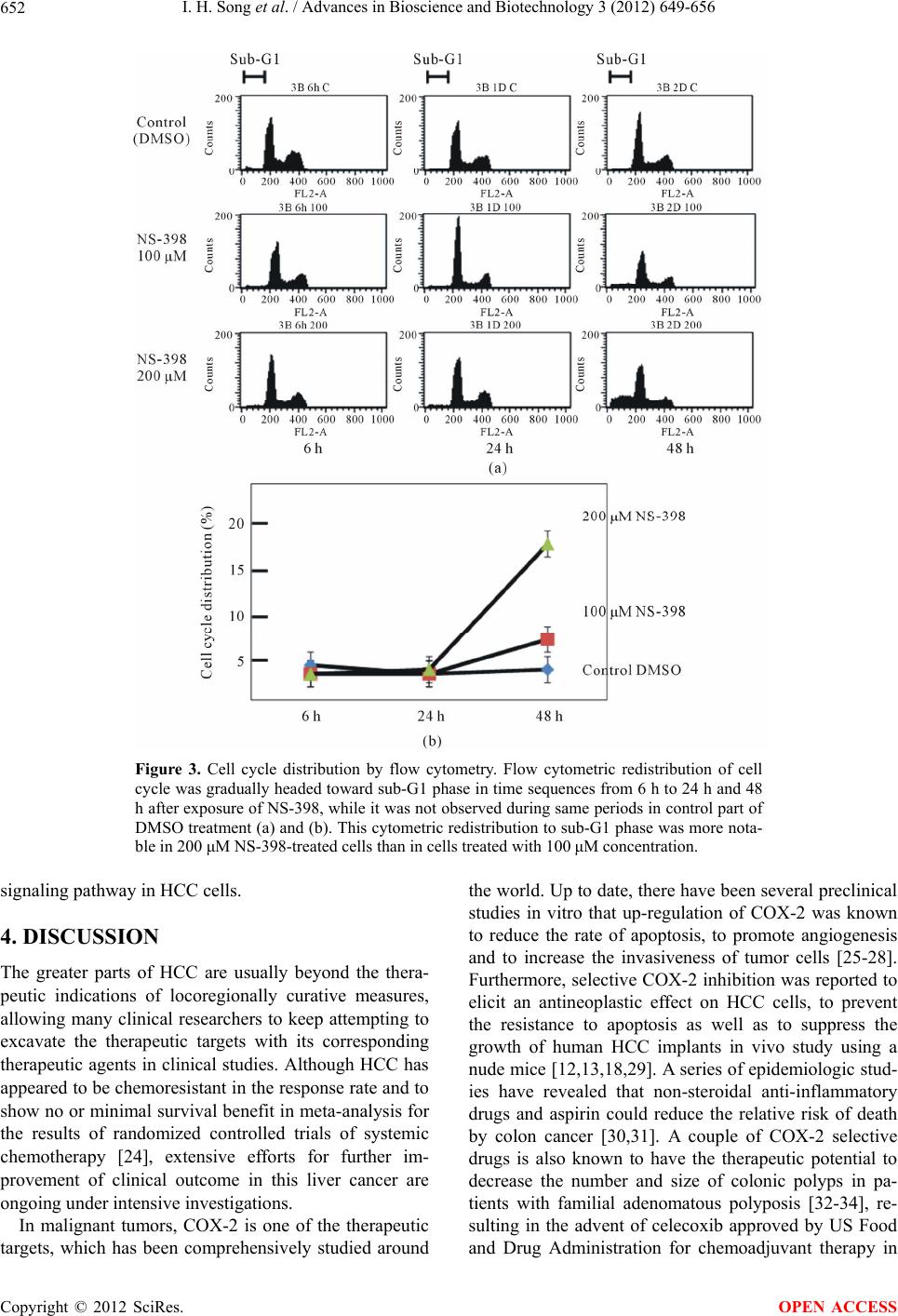 I. H. Song et al. / Advances in Bioscience and Biotechnology 3 (2012) 649-656 Copyright © 2012 SciRes. 652 Figure 3. Cell cycle distribution by flow cytometry. Flow cytometric redistribution of cell cycle was gradually headed toward sub-G1 phase in time sequences from 6 h to 24 h and 48 h after exposure of NS-398, while it was not observed during same periods in control part of DMSO treatment (a) and (b). This cytometric redistribution to sub-G1 phase was more nota- ble in 200 μM NS-398-treated cells than in cells treated with 100 μM concentration. signaling pathway in HCC cells. 4. DISCUSSION The greater parts of HCC are usually beyond the thera- peutic indications of locoregionally curative measures, allowing many clinical researchers to keep attempting to excavate the therapeutic targets with its corresponding therapeutic agents in clinical studies. Although HCC has appeared to be chemoresistant in the response rate and to show no or minimal survival benefit in meta-analysis for the results of randomized controlled trials of systemic chemotherapy [24], extensive efforts for further im- provement of clinical outcome in this liver cancer are ongoing under intensive investigations. In malignant tumors, COX-2 is one of the therapeutic targets, which has been comprehensively studied around the world. Up to date, there have been several preclinical studies in vitro that up-regulation of COX-2 was known to reduce the rate of apoptosis, to promote angiogenesis and to increase the invasiveness of tumor cells [25-28]. Furthermore, selective COX-2 inhibition was reported to elicit an antineoplastic effect on HCC cells, to prevent the resistance to apoptosis as well as to suppress the growth of human HCC implants in vivo study using a nude mice [12,13,18,29]. A series of epidemiologic stud- ies have revealed that non-steroidal anti-inflammatory drugs and aspirin could reduce the relative risk of death by colon cancer [30,31]. A couple of COX-2 selective drugs is also known to have the therapeutic potential to decrease the number and size of colonic polyps in pa- tients with familial adenomatous polyposis [32-34], re- sulting in the advent of celecoxib approved by US Food and Drug Administration for chemoadjuvant therapy in OPEN ACCESS  I. H. Song et al. / Advances in Bioscience and Biotechnology 3 (2012) 649-656 653 β-actin Caspase 3 Activat ed caspase 3 24 h 48 h 72 h 100 μM 200 μM NS-398 24 h 48 h 72 h Control (a) (a) 24 h 48 h 72 h 100 μM 200 μM 24 h 48 h 72 h 0 0.4 0.8 1.2 1.6 NS-398 Expresionratio (Caspase 3/β-actin) Caspase 3Activated caspase 3 ** (b) Figure 4. Caspase activity after treatment of NS-398. (a) The activity of caspase 3, a down-stream caspase of apoptosis, was evaluated by Western blot analysis; (b) The expression of cleaved form (19 kDa) of caspase 3 gradually increased on time sequence from 24 h to 72 h after both concentrations 100 and 200 μM of NS-398 treatment to Hep3B cells in time-dependent manner. It may be indicated that NS-398 involves a caspase- dependent apoptosis signaling pathway in HCC cells. *P < 0.05, compared with the relative expression of activated caspase 3 at 24 h after treatment of NS-398. these patients. However, the exact mechanisms responsi- ble for explaining these growth-inhibitory effects of se- lective COX-2 inhibitor are not clear even to this time. Based on our knowledges, together with experimental and clinical evidences mentioned above, that COX-2 might play a pivotal role in tumorigenesis and overex- pression of COX-2 has been observed in a number of tumor tissues, including colorectal cancer [9], pancreatic cancer [35], gastric cancer [27], esophageal cancer [36] and hepatocellular carcinoma [37], we conducted the present study that was designed for the clarification of inhibitory mechanisms and chemotherapeutic impact of NS-398, a selective COX-2 inhibitor, on the growth of hepatoma cells. Our results showed that NS-398 definitely suppressed the growth of Hep3B HCC cells in both concentration- dependent and time-dependent manner with a resultant consequence of decreased tumor cell number. Besides the decrease of cell number, cell morphology was changed to be microscopically elongated with its significance being not defined. This growth-inhibitory effect of NS-398 to hepatoma cells was verified as a result of the induction of apoptosis by indicating a distinct ladder patterned- fragmentation of genomic DNA and significant redistri- Cytochrome c (mitochondria) 24 h 48 h 72 h 100 μM 200 μM NS-398 24 h 48 h 72 h Contol (a) Cytochrome c (cytosol) β-actin (a) 0 0.2 0.4 Expresion ratio (Cytochrome c/β-actin) (b) 0.6 100 μM 200 μM NS-398 Cytochromec (mitochondria)Cytochromec (cytosol) 24 h 48 h 72 h24 h 48 h 72 h ** (b) Figure 5. Localization of cytochrome c after treatment of NS- 398. (a) The activity of cytochrome c was evaluated by Western blot analysis; (b) The activity of cytosolic fraction of cyto- chrome c (14 kDa) gradually increased on time sequence from 24 h to 72 h, in opposition to that of mitochondrial fraction, following the treatment of both concentrations 100 and 200 μM of NS-398, which means the cytoplasmic release of cytochrome c from mitochondria. It may be indicated that NS-398 engages a mitochondria-mediated intrinsic apoptosis signaling pathway in HCC cells. *P < 0.05, compared with the relative expression of cytosolic fraction of cytochrome c at 24 h after treatment of NS-398. bution of cell cycle shifted to sub-G1 phase following NS-398 treatment. The strength of apoptosis-mediated growth inhibition of hepatoma cells was proportionally intensified according to the concentration of COX-2 in- hibitor, which is allowed to be able to predict the dose- dependent growth suppression of tumor cell. Not only NS-398 but other COX-2 inhibitors such as nimesulide [13], CAY 10404 [13,17,38], celecoxib [16, 18], 2,5-dimethyl-celecoxib [39], meloxicam [12], JTE- 522 [40], sulindac [12] and indomethacin [13], have been introduced to the preclinical and clinical investigations designed for the identification of its growth-inhibitory mechanisms in tumorous conditions. Until now, the regu- latory mechanisms of COX-2 inhibitors on the growth of tumor cells have appeared to depend on the various con- ditions, namely, a kind of COX-2 inhibitor compounds, a selectivity of COX-2 inhibition, a type of tumor cells, whether or not COX-2 expression in tumor cells, and an inhibitory dominancy of COX-2 inhibitor to COX-2 ac- tivity. Especially, The pattern of NS-398-induced growth suppression is thought to be variable according to the type of tumor cells: COX-2 expressing tumor cell lines such as GKCI-4 as well as Hep3B and COX-2 nonex- pressing cell line such as HepG2 and PLC/PRE/5 [41]. Copyright © 2012 SciRes. OPEN ACCESS  I. H. Song et al. / Advances in Bioscience and Biotechnology 3 (2012) 649-656 654 The antitumor effects of NS-398 COX-2 inhibitor in HCC cells have been reported to be performed through the inhibitory signals via apoptosis, necrosis, or cell cy- cle arrest [19,40-43], however, most COX-2 inhibitors are generally accepted to be involved in the activation of apoptosis pathways which are advanced through the death receptor-mediated, mitochondria-mediated, or both signaling. There are several death receptors such as CD 95 (Fas receptor), tumor necrosis factor (TNF)-R, TNF- related apoptosis-inducing ligand (TRAIL)-R1, and TRAIL-R2, that are known to be triggered by COX-2 inhibition [17,18]. Besides apoptotic activity via death receptors, several different mechanisms of COX-2 inhibitor relevant to apoptosis signal were investigated as follows: 1) down- regulation of myeloid cell leukemia-1 (Mcl-1); 2) de- creased expression of Bcl-2 antiapoptotic family; 3) down-regulation of surviving; 4) inhibition of mitogen- antivated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase (MEK)/ERK (MEK/ERK) signaling pathway; 5) reduction of serine/threonine protein kinase B (Akt) phosphorylation; 6) up-regulation of peroxisome proliferator-activated receptor (PPAR)-γ protein; 7) in- creased expression of Fas ligand [18,26,38,40-42,44]. Caspase 3, one of down-stream proteases for the exe- cution of apoptotic programmed death in any type of insulted cells, is a final common pathway for the propa- gation of both extrinsic/death receptor-mediated or in- trinsic/mitochondria-mediated apoptosis signals [19]. Our study revealed that the enzymatic activation of cas- pase 3 increased up to 72 h following treatment of Hep3B cells with NS-398. This finding was accompanied by a gradual accumulation of cytoplasmic fraction of cytochrome c up to 72 h after NS-398 treatment in both concentrations of 100 and 200 μM, suggesting a gradual release of cytochrome c from mitochondria. Both active- tion of cleaved form of down-stream caspase and cyto- plasmic translocation of cytochrome c are a hallmark of intrinsic aspoptosis signaling pathway. 5. CONCLUSION NS-398, a selective COX-2 inhibitor, induced the growth inhibition of hepatoma cells through the caspase-de- pendent, mitochondria-mediated intrinsic apoptosis, pro- viding a strong insight into the anti-neoplastic effects of selective COX-2 inhibitors as novel one of therapeutic agents for hepatocellular carcinoma. In the near future selective COX-2 inhibitors would be expected to try for the application to managements of HCC in clinical fields. 6. ACKNOWLEDGEMENTS The financial support provided by the Institute of Medical Science Research of Dankook University Medical Center in 2008 for the pre- sent work is thankfully acknowledged. REFERENCES [1] El-Serag, H.B. and Rudolph, K.L. (2007) Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology, 132, 2557-2576. doi:10.1053/j.gastro.2007.04.061 [2] Sherman, M. (2005) Hepatocellular carcinoma: Epidemi- ology, risk factors, and screening. Seminar in Liver Dis- ease, 25, 143-154. doi:10.1055/s-2005-871194 [3] Song, I.H. and Kim, K.S. (2009) Current status of liver diseases in Korea: Hepatocellular carcinoma. Korean Journal of Hepatology, 15, S50-S59. doi:10.3350/kjhep.2009.15.S6.S50 [4] Lopez, P.M., Villanueva, A. and Llovet, J.M. (2006) Systematic review: Evidence-based management of heap- tocellular carcinoma—An updated analysis of random- ized controlled trials. Alimentary Pharmacology and The- rapeutics, 23, 1535-1547. doi:10.1111/j.1365-2036.2006.02932.x [5] Llovet, J.M., Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J.F., de Oliveira, A.C., Santoro, A., Raoul, J.L., Forner, A., Schwartz, M., Porta, C., Zeuzem, S., Bolondi, L., Greten, T.F., Galle, P.R., Seitz, J.F., Borbath, I., Häussinger, D., Giannaris, T., Shan, M., Moscovici, M., Voliotis, D. and Bruix, J.; SHARP Investigators Study Group. (2008) Sorafenib in advanced hepatocellular car- cinoma. New England Journal Medicine, 359, 378-390. doi:10.1056/NEJMoa0708857 [6] Cheng, A.L., Kang, Y.K., Chen, Z., Tsao, C.J., Qin, S., Kim, J.S., Luo, R., Feng, J., Ye, S., Yang, T.S., Xu, J., Sun, Y., Liang, H., Liu, J., Wang, J., Tak, W.Y., Pan, H., Burock, K., Zou, J., Voliotis, D. and Guan, Z. (2009) Ef- ficacy and safety of sorafenib in patients in the Asia-Pa- cific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncology, 10, 25-34. doi:10.1016/S1470-2045(08)70285-7 [7] Koga, H. (2003) Hepatocellular carcinoma: Is there a potential for chemoprevention using cyclooxygenase-2 inhibitors? Cancer, 98, 661-667. doi:10.1002/cncr.11576 [8] Chang, W.S., Yang, M.D., Tsai, C.W., Cheng, L.H., Jeng, L.B., Lo, W.C., Lin, C.H., Huang C.Y. and Bau, D.T. (2012) Association of cyclooxygenase 2 single-nucleotide polymorphisms and hepatocellular carcinoma in Taiwan. Chinese Journal of Physiology, 55, 1-7. [9] Ogunwobi, O.O., Wang, T., Zhang, L. and Liu, C. (2012) Cyclooxygenase-2 and Akt mediate multiple growth- factor-induced epithelial-mesenchymal transition in hu- man hepatocellular carcinoma. Journal of Gastroen- terology and Hepatology, 27, 566-578. doi:10.1111/j.1440-1746.2011.06980.x [10] Nzeako, U.C., Guicciardi, M.E., Yoon, J.H., Bronk, S.F. and Gores, G.J. (2002) COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology, 35, 552-559. doi:10.1053/jhep.2002.31774 [11] Abiru, S., Nakao, K., Ichikawa, T., Migita, K., Shigeno, Copyright © 2012 SciRes. OPEN ACCESS  I. H. Song et al. / Advances in Bioscience and Biotechnology 3 (2012) 649-656 655 M., Sakamoto, M., Ishikawa, H., Hamasaki, K., Nakata, K. and Eguchi, K. (2002) Aspirin and NS-398 inhibit hepatocyte growth factor-induced invasiveness of human hepatoma cells. Hepatology, 35, 1117-1124. doi:10.1053/jhep.2002.32676 [12] Kern, M.A., Schoneweiss, M.M., Sahi, D., Bahlo, M., Haugg, A.M., Kasper, H.U., Dienes, H.P., Käferstein, H., Breuhahn, K. and Schirmacher, P. (2004) Cyclooxy- genase-2 inhibitors suppress the growth of human heap- tocellular carcinoma implants in nude mice. Carcino- genesis, 25, 1193-1199. doi:10.1093/carcin/bgh110 [13] Fodera, D., D’Alessandro, N., Cusimano, A., Poma, P., Notarbartolo, M., Lampiasi, N., Montalto, G. and Cer- vello, M. (2004) Induction of apoptosis and inhibition of cell growth in human hepatocellular carcinoma cells by COX-2 inhibitors. Annals of the New York Academy of Sciences, 1028, 440-449. doi:10.1196/annals.1322.052 [14] Li, S., Tong, Q., Zhang, W., Wang, Q., Chen, Z. and Wu, Q. (2008) Mechanism of growth inhibitory effects of cyclooxygenase-2 inhibitor-NS398 on cancer cells. Can- cer Investigation, 26, 333-337. doi:10.1080/07357900701788056 [15] Nakamoto, N., Higuchi, H., Kanamori, H., Kurita, S., Tada, S., Takaishi, H., Toda, K., Yamada, T., Kumagai, N., Saito, H. and Hibi, T. (2006) Cyclooxygenase-2 in- hibitor and interferon-beta synergistically induce apop- tosis in human hepatoma cells in vitro and in vivo. Inter- national Journal of Oncology, 29, 625-635. [16] Xie, H., Gao, L., Chai, N., Song, J., Wang, J., Song, Z., Chen, C., Pan, Y., Zhao, L., Sun, S., Wu, K., Feitelson, M.A., Liu, J. and Fan, D. (2009) Potent cell growth in- hibitory effects in hepatitis B virus X protein positive hepatocellular carcinoma cells by the selective cyclooxy- genase-2 inhibitor celecoxib. Molecular Carcinogenesis, 48, 56-65. doi:10.1002/mc.20455 [17] Yamanaka, Y., Shiraki, K., Inoue, T., Miyashita, K., Fuke, H., Yamaguchi, Y., Yamamoto, N., Ito, K., Sugimoto, K. and Nakano, T. (2006) COX-2 inhibitors sensitize human hepatocellular carcinoma cells to TRAIL-induced apop- tosis. International Journal of Molecular Medicine, 18, 41-47. [18] Kern, M.A., Haugg, A.M., Koch, A.F., Schilling, T., Breuhahn, K., Walczak, H., Fleischer, B., Trautwein, C., Michalski, C., Schulze-Bergkamen, H., Friess, H., Strem- mel, W., Krammer, P.H., Schirmacher, P. and Müller, M. (2006) Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in heap- tocellular carcinoma. Cancer Research, 66, 7059-7066. doi:10.1158/0008-5472.CAN-06-0325 [19] Okada, H. and Mak, T.W. (2004) Pathways of apoptotic and non-apoptotic death in tumour cells. Nature Reviews Cancer, 4, 592-603. doi:10.1038/nrc1412 [20] Budihardjo, I., Oliver, H., Lutter, M., Luo, X. and Wang, X. (1999) Biochemical pathways of caspase activation during apoptosis. Annual Review of Cell and Develop- mental Biology, 15, 269-290. doi:10.1146/annurev.cellbio.15.1.269 [21] Salvesen, G.S. and Dixit, V.M. (1997) Caspases: Intra- cellular signaling by proteolysis. Cell, 91, 443-446. doi:10.1016/S0092-8674(00)80430-4 [22] Thornberry, N.A. and Lazebnik, Y. (1998) Caspases: Enemies within. Science, 281, 1312-1316. doi:10.1126/science.281.5381.1312 [23] Goldstein, J.C., Waterhouse, N.J., Juin, P., Evan, G.I. and Green, D.R. (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically in- variant. Nature Cell Biology, 2, 156-162. doi:10.1038/35004029 [24] Simonetti, R.G., Liberati, A., Angiolini, C. and Pagliaro, L. (1997) Treatment of hepatocellular carcinoma: A sys- tematic review of randomized controlled trials. Annals of Oncology, 8, 117-136. doi:10.1023/A:1008285123736 [25] Lee, N.O., Park, J.W., Lee, J.A., Shim, J.H., Kong, S.Y., Kim, K.T. and Lee, Y.S. (2012) Dual action of a selective cyclooxygenase-2 inhibitor on vascular endothelial growth factor expression in human hepatocellular carcinoma cells: Novel involvement of discoidin domain receptor 2. Journal of Cancer Research and Clinical Oncology, 138, 73-84. doi:10.1023/A:1008285123736 [26] Leng, J., Han, C., Demetris, A.J., Michalopoulos, G.K. and Wu, T. (2003) Cyclooxygenase-2 promotes hepato- cellular carcinoma cell growth through Akt activation: Evidence for Akt inhibition in celecoxib-induced apop- tosis. Hepatology, 38, 756-768. doi:10.1053/jhep.2003.50380 [27] Li, H.X., Chang, X.M., Song, Z.J. and He, S.X. (2003) Correlation between expression of cyclooxygenase-2 and angiogenesis in human gastric adenocarcinoma. World Journal of Gastroenterology, 9, 674-677. [28] Ogunwobi, O.O. and Liu, C. (2011) Hepatocyte growth factor upregulation promotes carcinogenesis and epithet- lial-mesenchymal transition in hepatocellular carcinoma via Akt and COX-2 pathway. Clinical and Experimental Metastasis, 28, 721-731. doi:10.1007/s10585-011-9404-x [29] Kern, M.A., Schubert, D., Sahi, D., Schöneweiss, M.M., Moll, I., Haugg, A.M., Dienes, H.P., Breuhahn, K. and Schirmacher, P. (2002) Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in hu- man liver tumor cells. Hepatology, 36, 885-894. [30] Thun, M.J., Namboodiri, M.M. and Heath Jr., C.W. (1991) Aspirin use and reduced risk of fatal colon cancer. New England Journal of Medicine, 325, 1593-1596. doi:10.1056/NEJM199112053252301 [31] Giovannucci, E., Egan, K.M., Hunter, D.J., Stampfer, M.J., Colditz, G.A., Willett, W.C. and Speizer, F.E. (1995) Aspirin and the risk of colorectal cancer in women. New England Journal of Medicine, 333, 609-614. doi:10.1056/NEJM199509073331001 [32] Labayle, D., Fischer, D., Vielh, P., Drouhin, F., Pariente, A., Bories, C., Duhamel, O., Trousset, M. and Attali, P. (1991) Sulindac causes regression of rectal polyps in fa- milial adenomatous polyposis. Gastroenterology, 101, 635-639. [33] Van Stolk, R., Stoner, G., Hayton, W.L., Chan, K., DeY- oung, B., Kresty, L., Kemmenoe, B.H., Elson, P., Rybicki, L., Church, J., Provencher, K., McLain, D., Hawk, E., Fryer, B., Kelloff, G., Ganapathi, R. and Budd, G.T. (2000) Phase I trial of exisulind (sulindac sulfone, FGN-1) Copyright © 2012 SciRes. OPEN ACCESS  I. H. Song et al. / Advances in Bioscience and Biotechnology 3 (2012) 649-656 Copyright © 2012 SciRes. 656 OPEN ACCESS as a chemopreventive agent in patients with familial ade- nomatous polyposis. Clinical Cancer Research, 6, 78-89. [34] Steinbach, G., Lynch, P.M., Phillips, R.K., Wallace, M.H., Hawk, E., Gordon, G.B., Wakabayashi, N., Saunders, B., Shen, Y., Fujimura, T., Su, L.K. and Levin, B. (2000) The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. New England Journal of Medicine, 342, 1946-1952. doi:10.1056/NEJM200006293422603 [35] Tucker, O.N., Dannenberg, A.J., Yang, E.K., Zhang, F., Teng, L., Daly, J.M., Soslow, R.A., Masferrer, J.L., Wo- erner, B.M., Koki, A.T. and Fahey III, T.J. (1999) Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Research, 59, 987-990. [36] Zimmermann, K.C., Sarbia, M., Weber, A.A., Borchard, F., Gabbert, H.E. and Schror, K. (1999) Cyclooxygenase- 2 expression in human esophageal carcinoma. Cancer Research, 59, 198-204. [37] Qiu, D.K., Ma, X., Peng, Y.S. and Chen, X.Y. (2002) Significance of cyclooxygenase-2 expression in human primary hepatocellular carcinoma. World Journal of Gas- troenterology, 8, 815-817. [38] Cusimano, A., Fodera, D., D’Alessandro, N., Lampiasi, N., Azzolina, A., Montalto, G. and Cervello, M. (2007) Potentiation of the antitumor effects of both selective cyclooxygenase-1 and cyclooxygenase-2 inhibitors in human hepatic cancer cells by inhibition of the MEK/ ERK pathway. Cancer Biology and Therapy, 6, 1461- 1468. doi:10.4161/cbt.6.9.4629 [39] Pyrko, P., Soriano, N., Kardosh, A., Liu, Y.T., Uddin, J., Petasis, N.A., Hofman, F.M., Chen, C.S., Chen, T.C. and Schönthal, A.H. (2006) Downregulation of survivin ex- pression and concomitant induction of apoptosis by cele- coxib and its non-cyclooxygenase-2-inhibitory analog, dimethyl-celecoxib (DMC), in tumor cells in vitro and in vivo. Molecular Cancer, 5, 19. doi:10.1186/1476-4598-5-19 [40] Nagahara, T., Okano, J. and Murawaki, Y. (2007) Mecha- nisms of anti-proliferative effect of JTE-522, a selective cyclooxygenase-2 inhibitor, on human liver cancer cells. Oncology Reports, 18, 1281-1290. [41] Cheng, A.S., Chan, H.L., Leung, W.K., Wong, N., Jo- honson, P.J. and Sung, J.J. (2003) Specific COX-2 in- hibitor. NS-398, suppresses cellular proliferation and in- duces apoptosis in human hepatocellular carcinoma cells. International Journal of Oncology, 23, 113-119. [42] Yu, Y., Gong, R., Mu, Y., Chen, Y., Zhu, C., Sun, Z., Chen, M., Liu, Y., Zhu, Y. and Wu, J. (2011) Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxy- genase-2. Journal of Immunology, 187, 4844-4860. doi:10.4049/jimmunol.1100998 [43] Baek, J.Y., Hur, W., Wang, J.S., Bae, S.H. and Yoon, S.K. (2007) Selective COX-2 inhibitor, NS-398, sup- presses cellular proliferation in human hepatocellular carcinoma cell lines via cell cycle arrest. World Journal of Gastroenterology, 13, 1175-1181. [44] Huang, D.S., Shen, K.Z., Wei, J.F., Liang, T.B., Zheng, S.S. and Xie, H.Y. (2005) Specific COX-2 inhibitor NS398 induces apoptosis in human liver cancer cell line HepG2 through BCL-2. World Journal of Gastroen- terology, 11, 204-207.
|