 Pharmacology & Pharmacy, 2012, 3, 397-403 http://dx.doi.org/10.4236/pp.2012.34053 Published Online October 2012 (http://www.SciRP.org/journal/pp) 1 Local Peripheral Effects of β-Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice Hikari Kuwahata1,2, Soh Katsuyama2,3, Takaaki Komatsu2, Hitoshi Nakamura3, Maria Tiziana Corasaniti4, Giacinto Bagetta5, Shinobu Sakurada6, Tsukasa Sakurada2*, Kazuo Takahama1 1Department of Environmental and Molecular Health and Sciences, Graduated School of Pharmaceutical Sciences, Kumamoto Uni- versity, Kumamoto, Japan; 2Department of Pharmacology, Daiichi College of Pharmaceutical Sciences, Fukuoka, Japan; 3Department of Clinical Pharmaceutics, Tohoku Pharmaceutical University, Sendai, Japan; 4Department of Pharmacobiological Sciences, Univer- sity of Magna Gracia of Catanzaro, Catanzaro, Italy; 5Department of Pharmacobiology, University Consortium for Adaptive Disor- ders and Headach (UCADH), Section of Neuropharmacology of Normal and Pathological Neuronal Plasticity, University of Calabria, Arcavacata di Rende, Italy; 6Department of Physiology and Anatomy, Tohoku Pharmaceutical University, Sendai, Japan. Email: *tsukasa@daiichi-cps.ac.jp Received July 13th, 2012; revised August 15th, 2012; accepted September 10th, 2012 ABSTRACT β-Caryophyllene (BCP) is known as a common constitute of the essential oils of numerous food plants and primary component in Cannabis. In this study, we investigated the effect of local intraplantar (i.pl.) injection of BCP on me- chanical hypersensitivity induced by partial sciatic nerve ligation (PSNL) in mice. Relative to sham operation controls, mice with the PSNL displayed a maximum level of hyperresponsiveness to von Frey metallic filament on post-operative day 7. PSNL-induced allodynia was seen in the ipsilateral side of nerve ligation, but not in the contralateral side. The i.pl. injection of BCP into the ipsilateral hindpaw to PSNL attenuated mechanical allodynia in a dose-dependent manner. BCP injection into the contralateral hindpaw did not produce anti-allodynic effects, suggesting a local peripheral anti-allodynic effect of BCP. Anti-allodynic effects induced by i.pl. injection of BCP were prevented by pretreatment with the cannabinoid (CB)2 receptor antagonist AM630, but not by the CB1 receptor antagonist AM251. These data suggest that i.pl. injection of BCP could produce anti-allodynia by activating peripheral CB2 receptors, but not CB1 re- ceptors in a mouse model of neuropathic pain. Taken together, these results suggest that peripheral CB2 receptors may contribute to the effectiveness of BCP in the treatment of neuropathic pain disorders. Keywords: β-Caryophyllene (BCP); Neuropathic Pain; Partial Sciatic Nerve Ligation (PSNL); Peripheral Cannabinoid (CB) Receptor 1. Introduction Plants are used for various purposes including their cos- metic, nutritive, and biomedical properties. Plant essen- tial oils are typically composed of volatile aromatic ter- penes and phenylpropanoids. The natural sesquiterpene β-caryophyllene (BCP) is found in many essential oils of different spice and food plants, such as clove, oregano, thyme, black pepper and cinnamon [1,2], all of which have been used as natural remedies and also as fra- grances. This compound is also known to be anti-micro- bial [3], anti-oxidant [3,4], and anti-carcinogenic [5] and to possess skin penetration-enhancing properties [6]. Moreover, BCP is also a major component in the essen- tial oil of Cannabis sativa L [7]. BCP showed marked anti-inflammatory activity against carrageenan- and pro- staglandin E1-induced edema in rats as well as anti-ar- thritic activity [8-10]. Oral administration of BCP sig- nificantly reduced the inflammation of colon [11], the carrageenan-induced inflammatory response in wild-type mice but not in mice lacking cannabinoid (CB)2 receptors [12]. However, the antinociceptive efficacy of intraplan- tar (i.pl.) BCP on partial sciatic nerve ligation (PSNL)- induced mechanical allodynia in mice is unknown. Can- nabinoid CB receptors, CB1 and CB2 receptors, at the peripheral and central sites have been proposed to medi- ate the CB-induced antinociceptive effects [13-17]. CB2 receptors are not found in the central nervous system (CNS), but are predominantly expressed in immune cells, their roles including the modulation of cytokine release and immune cell migration [18,19]. CB2 receptor selec- tive agonists produced peripheral antinociception [15, 20,21], but do not cause the effects of CNS [15,22], sug- *Corresponding author. Copyright © 2012 SciRes. PP  Local Peripheral Effects of β-Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice 398 gesting that selective activation of CB2 receptors may achieve the goal of peripheral pain relief without CNS effects. Therefore, in our study we used local injections to the injured paw in order to exclude the role of central effects, and validate the role of peripheral CB receptors in neuropathic pain. Peripheral nerve damage can result in long-lasting anomalous pain conditions referred to as neuropathic pain. These abnormal pain states are often manifested by an increased sensitivity to nociceptive stimuli, termed hyperalgesia, as well as the perception of typical innocuous stimuli being painful, a state referred to as allodynia [23]. CB2 receptor selective agonists have been also known to produce antinociception without overt behavioral effects in neuropathic pain [20,22,24, 25]. The aims of this work was 1) to investigate whether i.pl. injection of BCP would produce antinociception in PSNL-induced mechanical allodynia model in mice; and 2) to determine a possible role of peripheral CB recap- tors in BCP-induced anti-allodynic effects. 2. Materials and Methods 2.1. Animals and Neuropathic Surgery Male mice of ddY strain weighing 22 - 24 g were pur- chased from Kyudo Industries, Kumamoto, Japan. They were housed in cages of 15 - 20 animals matched for weight and placed in a colony room. Animals were al- lowed free access to standard food (Clea Japan, Inc., Osaka, Japan) and tap water in an air-conditioned room under a constant 12:12 h light/dark cycle (light on 08:00 h) at a room temperature of 22˚C - 24˚C and 50% - 60% relative humidity. All experiments followed the Guide- lines on Ethical Standards for Investigation of Experi- mental Pain in Animals [26]. Additionally, the study was approved by the Committee of Animal Experiments in Daiichi College of Pharmaceutical Sciences. Partial ligation of the sciatic nerve of mice was per- formed under pentobarbital anesthesia (50 mg/kg, i.p.) following the methods Malmberg and Basbaum [27]. Briefly, the common sciatic nerve of the right hand leg of mice was exposed at high thigh level through a small incision and dorsal 13 to 12 of the nerve thickness was tightly ligated with a silk suture. The wound was closed with a single muscle suture, and antibiotic powder was dusted over the wound area following surgery. For sham surgery, the sciatic nerve was exposed as described above, but no contact was made with the nerve. To minimize differences in technique, all operations were done by the same person. Immediately following surgery, the animals were kept in a soft bag cage with some food inside so that they could feed themselves without having difficulty in standing. The wound healed within 1 to 2 days, and the mice behaved normally. The behavior of the mice was monitored carefully for any visual indica- tion of motor disorders or change in weight. Testing procedures were conducted on day 7 after PSNL, except for the time-course experiment of PSNL-induced allody- nia. 2.2. Mechanical Threshold Behavioral testing was conducted from 10:00 to 16:00 in a quiet room. Each animal received drugs only once and was used in only one experiment. The mice were weighed and placed individually in a Plexiglas chamber (11.0 × 17.0 × 14.0 cm, wire mesh floor) and allowed to acclimatize for at least 1 hour. The threshold for nocicep- tive responsiveness to mechanical stimuli applied to the hindpaw was assessed using an electronic version of the von Frey test (dynamic plantar anesthesiometer, model 37400; Ugo Basile, Milan, Italy). The servo-controlled mechanical stimulus (a pointed metallic filament) was applied to the plantar surface, which exerted a progres- sively increasing punctate pressure, reaching up to 3.0 g within 5.0 s. The pressure evoking a clear voluntary hind- paw withdrawal response (usually close to 3.0 g) was recorded automatically and taken as the mechanical no- ciceptive thereshold index. 2.3. Drugs β-Caryophyllene(tra ns-(1R,9S)-8-Methylene-4,11,11-tri- methylbicyclo [7.2.0] undec-4-ene; BCP) (Sigma, St. Louis, MO, USA) diluted in jojoba wax (Simmondsia chinensis) (KSA International, Co. Ltd., Kanagawa, Ja- pan), was injected to the plantar surface of the hindpaw in mice. N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlo- rophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) and 6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-in- dol-3-yl] (4-methoxyphenyl) methanone (AM630) (Toc- ris Cookson, Bristol, UK), dissolved in physiological saline was administered i.pl. or subcutaneous (s.c.) 30 min before BCP. I.pl. and s.c. injections were given in a volume of 20 μL/site and 0.1 mL/10 g of body weight, respectively. 2.4. Analyses of Data All data are expressed as means ± SEM. Statistical dif- ferences between groups were assessed with a two-way ANOVA followed by Bonferroni’s test. The 5% (P < 0.05) level of statistical significance was set in all ex- periments. 3. Results 3.1. The Effects of BCP on Mechanical Allodynia The responsiveness to mechanical stimuli was deter- mined on days 1 - 35 after PSNL. Compared with the sham-operated mice, PSNL resulted in mechanical allo- Copyright © 2012 SciRes. PP 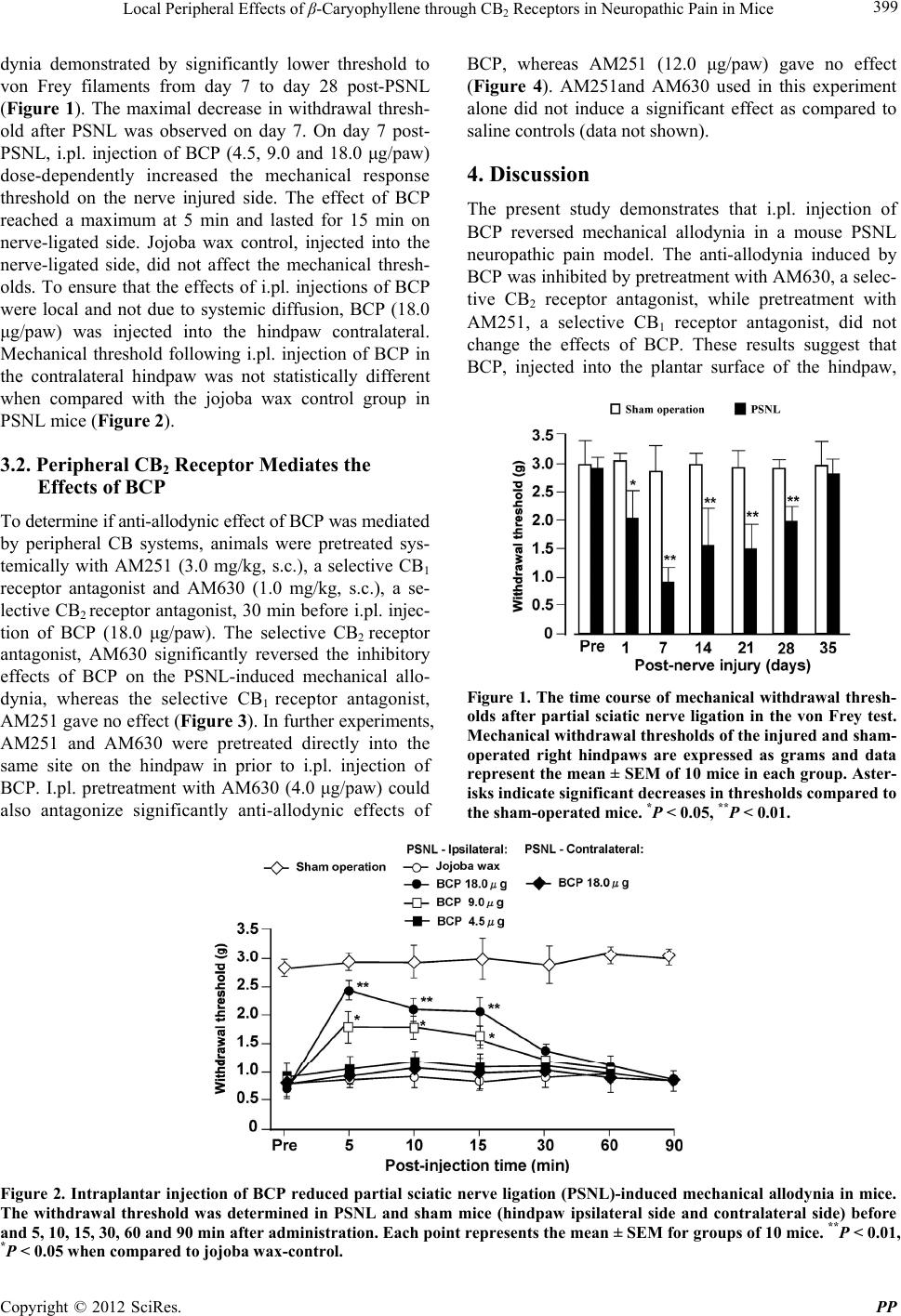 Local Peripheral Effects of β-Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice Copyright © 2012 SciRes. PP 399 BCP, whereas AM251 (12.0 μg/paw) gave no effect (Figure 4). AM251and AM630 used in this experiment alone did not induce a significant effect as compared to saline controls (data not shown). dynia demonstrated by significantly lower threshold to von Frey filaments from day 7 to day 28 post-PSNL (Figure 1). The maximal decrease in withdrawal thresh- old after PSNL was observed on day 7. On day 7 post- PSNL, i.pl. injection of BCP (4.5, 9.0 and 18.0 μg/paw) dose-dependently increased the mechanical response threshold on the nerve injured side. The effect of BCP reached a maximum at 5 min and lasted for 15 min on nerve-ligated side. Jojoba wax control, injected into the nerve-ligated side, did not affect the mechanical thresh- olds. To ensure that the effects of i.pl. injections of BCP were local and not due to systemic diffusion, BCP (18.0 μg/paw) was injected into the hindpaw contralateral. Mechanical threshold following i.pl. injection of BCP in the contralateral hindpaw was not statistically different when compared with the jojoba wax control group in PSNL mice (Figure 2). 4. Discussion The present study demonstrates that i.pl. injection of BCP reversed mechanical allodynia in a mouse PSNL neuropathic pain model. The anti-allodynia induced by BCP was inhibited by pretreatment with AM630, a selec- tive CB2 receptor antagonist, while pretreatment with AM251, a selective CB1 receptor antagonist, did not change the effects of BCP. These results suggest that BCP, injected into the plantar surface of the hindpaw, 3.2. Peripheral CB2 Receptor Mediates the Effects of BCP To determine if anti-allodynic effect of BCP was mediated by peripheral CB systems, animals were pretreated sys- temically with AM251 (3.0 mg/kg, s.c.), a selective CB1 receptor antagonist and AM630 (1.0 mg/kg, s.c.), a se- lective CB2 receptor antagonist, 30 min before i.pl. injec- tion of BCP (18.0 μg/paw). The selective CB2 receptor antagonist, AM630 significantly reversed the inhibitory effects of BCP on the PSNL-induced mechanical allo- dynia, whereas the selective CB1 receptor antagonist, AM251 gave no effect (Figure 3). In further experiments, AM251 and AM630 were pretreated directly into the same site on the hindpaw in prior to i.pl. injection of BCP. I.pl. pretreatment with AM630 (4.0 μg/paw) could also antagonize significantly anti-allodynic effects of Figure 1. The time course of mechanical withdrawal thresh- olds after partial sciatic nerve ligation in the von Frey test. Mechanical withdrawal thresholds of the injured and sham- operated right hindpaws are expressed as grams and data represent the mean ± SEM of 10 mice in each group. Aster- isks indicate significant decreases in thresholds compared to the sham-operated mice. *P < 0.05, **P < 0.01. Figure 2. Intraplantar injection of BCP reduced partial sciatic nerve ligation (PSNL)-induced mechanical allodynia in mice. The withdrawal threshold was determined in PSNL and sham mice (hindpaw ipsilateral side and contralateral side) before and 5, 10, 15, 30, 60 and 90 min after administration. Each point represents the mean ± SEM for groups of 10 mice. **P < 0.01, *P < 0.05 when compared to jojoba wax-control. 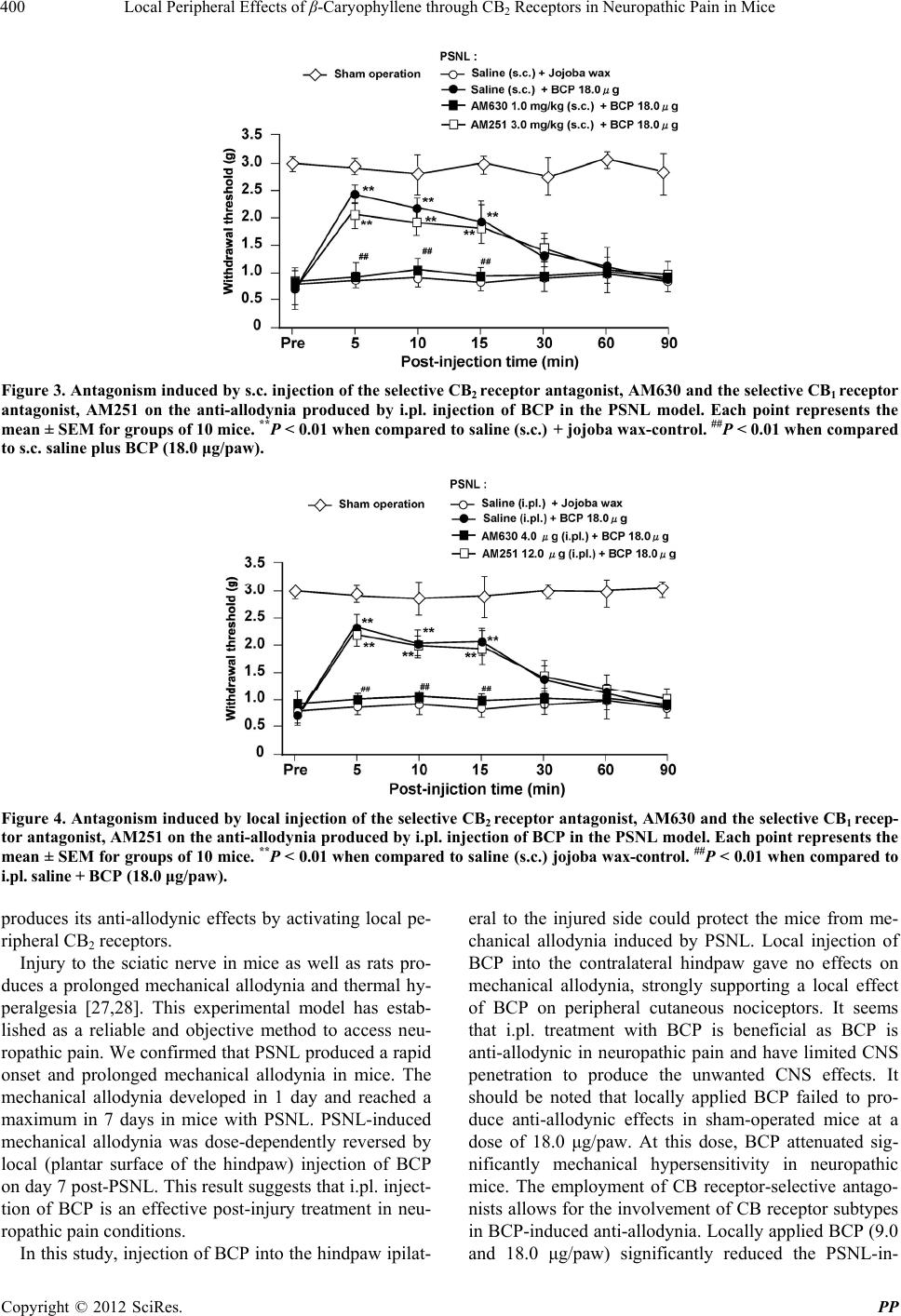 Local Peripheral Effects of β-Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice 400 Figure 3. Antagonism induced by s.c. injection of the selective CB2 receptor antagonist, AM630 and the selective CB1 receptor antagonist, AM251 on the anti-allodynia produced by i.pl. injection of BCP in the PSNL model. Each point represents the mean ± SEM for groups of 10 mice. **P < 0.01 when compared to saline (s.c.) + jojoba wax-control. ##P < 0.01 when compared to s.c. saline plus BCP (18.0 μg/paw). Figure 4. Antagonism induced by local injection of the selective CB2 receptor antagonist, AM630 and the selective CB1 recep- tor antagonist, AM251 on the anti-allodynia produced by i.pl. injection of BCP in the PSNL model. Each point represents the mean ± SEM for groups of 10 mice. **P < 0.01 when compared to saline (s.c.) jojoba wax-control. ##P < 0.01 when compared to i.pl. saline + BCP (18.0 μg/paw). produces its anti-allodynic effects by activating local pe- ripheral CB2 receptors. Injury to the sciatic nerve in mice as well as rats pro- duces a prolonged mechanical allodynia and thermal hy- peralgesia [27,28]. This experimental model has estab- lished as a reliable and objective method to access neu- ropathic pain. We confirmed that PSNL produced a rapid onset and prolonged mechanical allodynia in mice. The mechanical allodynia developed in 1 day and reached a maximum in 7 days in mice with PSNL. PSNL-induced mechanical allodynia was dose-dependently reversed by local (plantar surface of the hindpaw) injection of BCP on day 7 post-PSNL. This result suggests that i.pl. inject- tion of BCP is an effective post-injury treatment in neu- ropathic pain conditions. In this study, injection of BCP into the hindpaw ipilat- eral to the injured side could protect the mice from me- chanical allodynia induced by PSNL. Local injection of BCP into the contralateral hindpaw gave no effects on mechanical allodynia, strongly supporting a local effect of BCP on peripheral cutaneous nociceptors. It seems that i.pl. treatment with BCP is beneficial as BCP is anti-allodynic in neuropathic pain and have limited CNS penetration to produce the unwanted CNS effects. It should be noted that locally applied BCP failed to pro- duce anti-allodynic effects in sham-operated mice at a dose of 18.0 μg/paw. At this dose, BCP attenuated sig- nificantly mechanical hypersensitivity in neuropathic mice. The employment of CB receptor-selective antago- nists allows for the involvement of CB receptor subtypes in BCP-induced anti-allodynia. Locally applied BCP (9.0 and 18.0 μg/paw) significantly reduced the PSNL-in- Copyright © 2012 SciRes. PP  Local Peripheral Effects of β-Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice 401 duced neuropathic pain in mice. The effects of BCP were inhibited by pretreatment with the selective CB2 receptor antagonist, AM630 (1.0 mg/kg, s.c. and 4.0 μg, i.pl.) but not by pretreatment with the selective CB1 receptor an- tagonist, AM251 (3.0 mg/kg, s.c. and 12.0 μg, i.pl.). The doses AM630 (1 mg/kg) and AM251 (3 mg/kg) used in the present experiment have been previously shown to inhibit the effect of WIN 55,212-2, a CB receptor agonist in vivo [18,29]. In binding assays, AM630 is CB2 selec- tive with 165-fold lower Ki value in CB2 transfected CHO cell membranes than in membranes of CB1 trans- fected cells [30], and AM251 is a high degree selectivity (306-fold) for CB1 receptors [31]. Therefore, the data suggests that the anti-hyperalgesic effects of BCP in neuropathic mice are mediated through activation of pe- ripheral CB2 receptors. In line with our study, systemic or local administration of CB2 receptor selective agonists have been shown to produce antinociception without overt behavioral effects in several pain models [15,20,32,33]. Besides peripherally mediated anti-allodynic effect, peripheral administration of BCP or its containing essen- tial oil also indicates anti-inflammatory and anti-arthritic potential [8-11,34]. Moreover, oral administration of BCP inhibits potent anti-inflammatory effects in wild- type mice but not in mice lacking CB2 receptors [12]. This is consistent with our present study, suggesting the involvement of specific CB receptor subtype, a CB2 re- ceptor, in BCP-induced peripheral anti-allodynia. Inter- estingly, the essential oil component (E)-BCP selectively binds to the CB2 receptor, leading to the cellular activa- tion as a CB2 agonist and anti-inflammatory effects [12]. Thus, the anti-allodynic effect of BCP points to the pe- ripheral CB2 receptor as an interesting target in searching for new peripherally active analgesics for chronic pain therapy. The action site of BCP is of particular interest since neuropathy observed in patients is often coupled with not only neuropathic pain but also inflammatory symptoms [35,36]. The peripheral mechanism by which BCP produces anti-allodynic effect in the present findings are unclear. However, there are various lines of evidence that selec- tive activation of peripheral CB2 receptors is sufficient to display antinociception in models of acute, inflammatory and nerve injury-induced nociception [15,21,22,32,37- 39]. Indeed, CB2 receptors are expressed primarily on mast cells and immune system such as B cells, T cells and macrophages [19,40,41]. Activation of CB2 receptors on mast or immune cells could inhibit the release of molecules that sensitize the peripheral nociceptor (e.g. histamine, serotonin, prostaglandins, interleukin-1β, tu- mor necrosis factor-α, and nerve growth factor). A recent study have demonstrated that the oral treatment of BCP causes a significant reduction of prostaglandin E2 and tumor necrosis factor-α generation in carrageenan injected paw [34]. Therefore, it seems that activation of periph- eral CB2 receptors might decrease the sensitivity of pri- mary afferent neurons by inhibiting the release of sensi- tizing substances from neighboring mast and immune cells. Moreover, there is evidence for the presence of CB2 receptors on peripheral nerve terminals [42,43]. In- deed, Walczak et al. (2005) have shown that the saphe- nous partial ligation-induced neuropathic pain model in- creases the expression of CB2 receptors in the paw skin [44]. Another action mechanism of BCP is that i.pl. BCP may stimulate CB2 receptors and inhibit the responsive- ness of primary afferent neurons by stimulating local release of β-endorphin, an endogenous opioid peptide, from keratinocytes, which are very abundant in skin and have been reported to express CB2 receptors [35]. How- ever, further studies are required to explain the specific mechanisms underlying the observed BCP effect. 5. Conclusion In conclusion, i.pl. injection of BCP reduced the me- chanical allodynia by the PSNL model in mice. The anti- allodynic effects of BCP was antagonized by s.c. and i.pl. pretreatment with the selective CB2 receptor antagonist, AM630, but not by the selective CB1 receptor antagonist, AM251. The results suggest that a local effect of BCP on cutaneous nociceptors is mediated through CB2 receptors. BCP is predicted to be effective in treating allodynia without the central side effects of cannabinoids-based drugs retaining activity at the CB1 receptor. REFERENCES [1] A. Di Sotto, M. G. Evandri and G. Mazzanti, “An- timutagenic and Mutagenic Activities of Some Terpenes in the Bacterial Reverse Mutation Assay,” Mutation Re- search/Genetic Toxicology and Environmental Mutagene- sis, Vol. 653, No. 1-2, 2008, pp. 130-133. doi:10.1016/j.mrgentox.2008.04.004 [2] J. Legault and A. Pichette, “Potentiating Effect of Beta- Caryophyllene on Anticancer Activity of Alpha-Humulene, Isocaryophyllene and Paclitaxel,” Journal of Pharmacy and Pharmacolology, Vol. 59, No. 12, 2007, pp. 1643-1647. doi:10.1211/jpp.59.12.0005 [3] A. C. Lourens, D. Reddy, K. H. Baser, A. M. Viljoen and S. F. Van Vuuren, “In Vitro Biological Activity and Es- sential Oil Composition of Four Indigenous South Afri- can Helichrysum Species,” Journal of Ethnopharmacol- ogy, Vol. 95, No. 2-3, 2004, pp. 253-258. doi:10.1016/j.jep.2004.07.027 [4] G. Singh, P. Marimuthu, C. S. De Heluani and C. A. Catalan, “Antioxidation and Biocidal Activities of Carum nigrum (Seed) Essential Oil, Oleoresin, Their Selected Components,” Journal of Agricultural and Food Chemis- try, Vol. 54, No. 1, 2006, pp. 174-181. doi:10.1021/jf0518610 [5] I. Kubo, S. K. Chaudhuri, Y. Kubo, Y. Sanchez, T. Ogura, Copyright © 2012 SciRes. PP  Local Peripheral Effects of β-Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice 402 T. Saito, H. Ishikawa and H. Haraguchi, “Cytotoxic and Antioxidation Sesquiterpenoids from Heterotheca inu- loides,” Planta Medica, Vol. 62, No. 5, 1996, pp. 427-430. doi:10.1055/s-2006-957932 [6] P. A. Cornwell and B. W. Barry, “Sesquiterpene Compo- nents of Volatile Oils as Skin Penetration Enhancers for the Hydrophilic Permeant 5-Fluorouracil,” Journal of Phar- macy and Pharmacolology, Vol. 46, No. 6, 1994, pp. 261- 269. doi:10.1111/j.2042-7158.1994.tb03791.x [7] H. Hendriks, T. Malingre, S. Battermann and R. Boss, “Mono- and Sesquiterpene Hydrocarbons of Essential Oil of Cannabis sativa,” Phytochemistry, Vol. 14, No. 3, 1975, pp. 814-815. doi:10.1016/0031-9422(75)83045-7 [8] R. B. Agarwal and V. D. Rangari, “Phytochemical Inves- tigation and Evaluation of Anti-Inflammatory and Anti- Arthritic Activities of Essential Oil of Strobilanthus ixio- cephala Benth,” Indian Journal of Experimental Biology, Vol. 41, No. 8, 2003, pp. 890-894. [9] D. Baricevic, S. Sosa, R. Della Loggia, A. Tubaro, B. Simonovska, A. Krasna and A. Zupancic, “Topical Anti- Inflammatory Activity of Salvia officinalis L. Leaves: The Relevance of Ursolic Acid,” Journal of Ethnophar- macology, Vol. 75, No. 2, 2001, pp. 125-132. doi:10.1016/S0378-8741(00)00396-2 [10] S. Martin, E. Padilla, M. A. Ocete, J. Galvez, J. Jimenez and A. Zarzuelo, “Anti-Inflammatory Activity of the Es- sential Oil of Bupleurum fruticescens,” Planta Medica, Vol. 59, No. 6, 1993, pp. 533-536. doi:10.1055/s-2006-959755 [11] J. Y. Cho, H.-J. Chang, S.-K. Lee, H.-J. Kim, J.-K. Hwang and H. S. Chun, “Amelioration of Dextran Sulfate So- dium-Induced Colitis in Mice by Oral Administration of β-Caryophyllene, a Sesquiterpene,” Life Sciences, Vol. 80, No. 10, 2007, pp. 932-939. doi:10.1016/j.lfs.2006.11.038 [12] J. Gertsch, M. Leonti, S. Raduner, I. Racz, J.-Z. Chen, X.-Q. Xie, K.-H. Altmann, M. Karsak and A. Zimmer, “Beta-Caryophyllene Is a Dietary Cannabinoid,” Proceed- ing of the National Academy of Sciences, Vol. 105, No. 26, 2008, pp. 9099-9104. doi:10.1073/pnas.0803601105 [13] A. Fox, A. Kesingland, C. Gentry, K. McNair, S. Patel, L. Urban and I. James, “The Role of Central and Peripheral Cannabinoid 1 Receptors in the Antihyperalgesic Activity of Cannabinoids in a Model of Neuropathic Pain,” Pain, Vol. 92, No. 1-2, 2001, pp. 91-100. doi:10.1016/S0304-3959(00)00474-7 [14] U. Herzberg, E. Eliav, G. J. Bennett and I. J. Kopin, “The Analgesic Effects of R(+)-WIN 55,212-2 Mesylate, a High Affinity Cannabinoid Agonist, in a Rat Model of Neuropathic Pain,” Neuroscience Letters, Vol. 221, No. 2-3, 1997, pp. 157-160. doi:10.1016/S0304-3940(96)13308-5 [15] T. P. Malan Jr, M. M. Ibrahim, H. Deng, Q. Liu, H. P. Mata, T. Vanderah, F. Porreca and A. Makriyannis, “ CB2 Cannabinoid Receptor-Mediated Peripheral Antinocicep- tion,” Pain, Vol. 93, No. 3, 2001, pp. 239-245. doi:10.1016/S0304-3959(01)00321-9 [16] E. Palazzo, I. Marabese, V. de Novellis, P. Oliva, F. Rossi, L. Berrino and S. Maione, “Metabotropic and NMDA Glutamate Receptors Participate in the Cannabinoid-In- duced Antinociception.” Neuropharmacology, Vol. 40, No. 3, 2001, pp. 319-326. doi:10.1016/S0028-3908(00)00160-X [17] D. R. Sagar, S. Kelly, P. J. Mills, C. T. O’Shaughnessey, D. A. Kendall and V. Chapman, “Inhibitory Effects of CB1 and CB2 Receptor Agonists on Responses of DRG Neurons and Dorsal Horn Neurons in Neuropathic Rats,” European Journal of Neuroscience, Vol. 22, No. 2, 2005, pp. 371-379. doi:10.1111/j.1460-9568.2005.04206.x [18] C. Di Filippo, F. Rossi, S. Rossi and M. D’Amico, “Can- nabinoid CB2 Receptor Reduces Mouse Myocardial Ische- mia-Reperfusion Injury: Involvement of Cytokine/Che- mokines and PMN,” Journal of Leukocyte Biology, Vol. 75, No. 3, 2004, pp. 453-459. doi:10.1189/jlb.0703303 [19] S. Galiegue, S. Mary, J. Marchand, D. Dussossoy, D. Carriere, P. Carayon, M. Bouaboula, D. Shire, G. Le Fur and P. Casellas, “Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations,” European Journal of Bio- chemistry, Vol. 232, No. 1, 1995, pp. 54-61. [20] A. Hervera, R. Negrete, S. Leanez, J. Martin-Campos and O, Pol, “The Role of Nitric Oxide in the Local Antiallo- dynic and Antihyperalgesic Effects and Expression of δ- Opioid and Cannabinoid-2 Receptors during Neuropathic Pain in Mice,” Journal of Pharmacology and Experimen- tal Therapeutics, Vol. 334, No. 3, 2010, pp. 887-896. doi:10.1124/jpet.110.167585 [21] M. M. Ibrahim, M. L. Rude, N. J. Stagg, H. P. Mata, J. Lai, T. W. Vanderah, F. Porreca, N. E. Buckley, A. Makri- yannis and T. P. Malan, “CB2 Cannabinoid Receptor Me- diation of Antinociception,” Pain, Vol. 122, No. 1-2, 2006, pp. 36-42. doi:10.1016/j.pain.2005.12.018 [22] L. Hanus, A. Breuer, S. Tchilibon, S. Shiloah, D. Golden- berg, M. Horowitz, R. G. Pertwee, R. A. Ross, R. Mech- oulam and E. Fride, “HU-308: A Specific Agonist of CB2 a Peripheral Cannabinoid Receptor,” Proceedings of the National Academy of Sciences, Vol. 96, No. 96,1999, pp. 14228-14233. doi:10.1073/pnas.96.25.14228 [23] R. Payne, “Neuropathic Pain Syndromes, with Special Reference to Causalgia and Reflex Sympathetic Dystro- phy,” Clinical Journal of Pain, Vol. 2, No. 1, 1986, pp. 59-73. doi:10.1097/00002508-198602010-00010 [24] S. J. Elmes, M. D. Jhaveri, D. Smart, D. A. Kendall and V. Chapman, “Cannbinoid CB2 Receptor Activation In- hibits Mechanically Evoked Responses of Wide Dynamic Range Dorsal Horn Neurons in Native Rats and in Rat Models of Inflammatory and Neuropathic Pain,” Euro- pean Journal of Neuroscience, Vol. 20, No. 9, 2004, pp. 2311-2320. doi:10.1111/j.1460-9568.2004.03690.x [25] M. M. Ibrahim, F. Porreca, J. Lai, P. J. Albrecht, F. L. Rice, A. Khodoroba, G. Davar, A. Makriyannis, T. W. Vanderah, H. P. Mata and T. P. Malan, “CB2 Cannnabi- noid Receptor Activation Produces Antinociception by Stimulating Peripheral Release of Endogenous Opioids,” Proceedings of the National Academy of Sciences, Vol. 102, No. 102, 2005, pp. 3093-3098. doi:10.1073/pnas.0409888102 [26] M. Zimmermann, “Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals,” Pain, Vol. Copyright © 2012 SciRes. PP  Local Peripheral Effects of β-Caryophyllene through CB2 Receptors in Neuropathic Pain in Mice Copyright © 2012 SciRes. PP 403 16, No. 2, 1983, pp.109-110. doi:10.1016/0304-3959(83)90201-4 [27] A. B. Malmberg and A. I. Basbaum, “Partial Sciatic Nerve Injury in the Mouse as a Model of Neuropathic Pain: Be- havioral and Neuroanatomical Correlates,” Pain, Vol. 76, No. 3, 1998, pp. 215-222. doi:10.1016/S0304-3959(98)00045-1 [28] Z. Seltzer, R. Dubner and Y. Shir, “A Novel Behavioral Model of Neuropathic Pain Disorders Produced in Rats by Partial Sciatic Nerve Injury,” Pain, Vol. 43, No. 2, 1990, 205-218. doi:10.1016/0304-3959(90)91074-S [29] A. T. Hama and J. Sagen, “Cannabinoid Receptor-Medi- ated Antinociception with Acetaminophen Drug Combi- nations in Rats with Neuropathic Spinal Cord Injury Pain,” Neuropharmacology, Vol. 58, No. 4-5, 2010, pp. 758-766. doi:10.1016/j.neuropharm.2009.12.010 [30] R. A. Ross, H. C. Brockie, L. A. Stevenson, V. L. Mur- phy, F. Templeton, A. Makriyannis and R. G. Pertwee, “Agonist-Inverse Agonist Characterization at CB1 and CB2 Cannabinoid Receptors of L759633, L759656 and Am630,” British Journal of Pharmacology, Vol. 126, No. 3, 1999, pp. 665-672. doi:10.1038/sj.bjp.0702351 [31] R. Lan, Q. Liu, P. Fan, S. Lin, S. R. Fernando, D. McCal- lion, R. Pertwee and A. Makriyannis, “Structure-Activity Relationships of Pirazole Derivatives as Cannabinoid Re- ceptor Antagonists,” Journal of Medicinal Chemistry, Vol. 42, No. 4, 1999, pp. 769-776. doi:10.1021/jm980363y [32] A. Quartilho, H. P. Mata, M. M. Ibrahim, T. W. Vanderah, F. Porreca, A. Makriyannis and T. P. Malan, “Inhibition of Inflammatory Hyperalgesia by Activation of Peripheral CB2 Cannnabinoid Receptors,” Anesthesiology, Vol. 99, No. 4, 2003, pp. 955-960. doi:10.1097/00000542-200310000-00031 [33] K. J. Valenzano, L. Tafesse, G. Lee, J. E. Harrison, J. M. Boulet, S. L. Gottshall, L. Mark, M. S. Pearson, W. Mil- ler, S. Shan, L. Rabadi, Y. Rotshteyn, S. M. Chaffer, P. I. Turchin, D. A. Elsemore, M. Toth, L. Koetzner and G. T. Whiteside, “Pharmacological and Pharmacokinetic Char- acterization of Cannabinoid Receptor 2 Agonist, GW405833, Utilizing Rodent Models of Acute and Chronic Pain, Anxiety, Ataxia and Catalepsy,” Neuropharmacology, Vol. 48, No. 5, 2005, pp. 658-672. doi:10.1016/j.neuropharm.2004.12.008 [34] E. S. Fernandes, G. F. Passos, R. Medeiros, F. M. da Cunha, J. Ferreria, M. M. Campos, L. F. Pianowski and J. B. Calixto, “Anti-Inflammatory Effects of Compounds Alpha-Humulene and (-)-Trans-caryophyllene Isolated from the Essential Oil Cordia verbenacea,” European Journal of Phamacology, Vol. 569, No. 3, 2007, pp. 228- 236. doi:10.1016/j.ejphar.2007.04.059 [35] R. Baron, “Mechanisms of Disease: Neuropathic Pain—A Clinical Perspective,” Nature Clinical Practice Neurol- ogy, Vol. 2, No. 2, 2006, pp. 95-106. doi:10.1038/ncpneuro0113 [36] K. Moriwaki, O. Yuge, H. Tanaka, H. Sasaki, H. Izumi and K. Kaneko, “Neuropathic Pain and Prolonged Re- gional Inflammation as Two Distinct Symptomatological Components in Complex Regional Pain Syndrome with Patchy Osteoporosis—A Pilot Study,” Pain, Vol. 72, No. 1, 1997, pp. 277-282. doi:10.1016/S0304-3959(97)00029-8 [37] N. Clayton, F. H. Marshall, C. Bountra and C. T. O’Shaugh- nessy, “CB1 and CB2 Cannabinoid Receptors Are Impli- cated in Inflammatory Pain,” Pain, Vol. 96, No. 8, 2002, pp. 253-260. doi:10.1016/S0304-3959(01)00454-7 [38] M. M. Ibrahim, H. Deng, A. Zvonok, D. A. Cockayne, J. Kwan, H. P. Mata, T. W. Vanderah, J. Lai, F. Porreca, A. Makriyannis and T. P. Malan, “Activation of CB2 Can- nabinoid Receptors by AM1241 Inhibits Experimental Neuropathic Pain: Pain Inhibition by Receptors Not Pre- sent in the CNS,” Proceedings of the National Academy of Sciences, Vol. 100, No. 18, 2003, pp. 10529-10533. doi:10.1073/pnas.1834309100 [39] A. G. Nackley, A. Makriyannis and A. G. Hohmann, “Se- lective Activation of Cannabinoid CB2 Receptors Sup- presses Spinal Fos Protein Expression and Pain Behavior in a Rat Model of Inflammation,” Neuroscience, Vol. 119, No. 3, 2003, pp. 747-757. doi:10.1016/S0306-4522(03)00126-X [40] L. Facci, R. Dal Toso, S. Romanello, A. Buriani, S. D. Skaper and A. Leon, “Mast Cells Express a Peripheral Cannabinoid Receptor with Differential Sensitivity to Anandamide and Palmitoylethanolamide,” Proceedings of the National Academy of Sciences, Vol. 92, No. 8, 1995, pp. 3376-3380. doi:10.1073/pnas.92.8.3376 [41] S. Munro, K. L. Thomas and M. Abu-Sharr, “Molecular Characterization of a Peripheral Receptor for Cannabi- noids,” Nature, Vol. 365, No. 2, 1993, pp. 61-65. doi:10.1038/365061a0 [42] G. Griffin, S. R. Fernando, R. A. Ros, N. G. McKay, M. L. Ashfold, D. Shire, J. W. Huffman, S. Yu and J. A. Kaintin, “Evidence for the Presence of CB2-Like Can- nabinoid Receptors on Peripheral Nerve Terminal,” Euro- pean Journal of Phramacology, Vol. 339, No. 1, 1997, pp. 53-61. doi:10.1016/S0014-2999(97)01336-8 [43] R. G. Pertwee, “Evidence for the Presence of CB1 Can- nabinoid Receptors on Peripheral Neurons and for the Existence of Neuronal Non-CB1 Cannabinoid Receptors,” Life Sciences, Vol. 65, No. 6-7, 1999, pp. 597-605. doi:10.1016/S0024-3205(99)00282-9 [44] J. S. Walczak, V. Pichette, F. Leblond, K. Desbiens and P. Beauileu, “Behavioral, Pharmacological and Molecular Characterization of the Saphenous Nerve Partial Ligation: A New Model of Neuropathic Pain,” Neuroscience, Vol. 132, No. 4, 2005, pp. 1093-1102. doi:10.1016/j.neuroscience.2005.02.010
|