Preparation and in Vitro Drug Release Evaluation of Once-Daily Metformin Hydrochloride Sustained-Release Tablets 473
024681012
0
20
40
60
80
100
120
MH released (%)
Time (h)
0 month
1 month
2 month
3 month
6 month
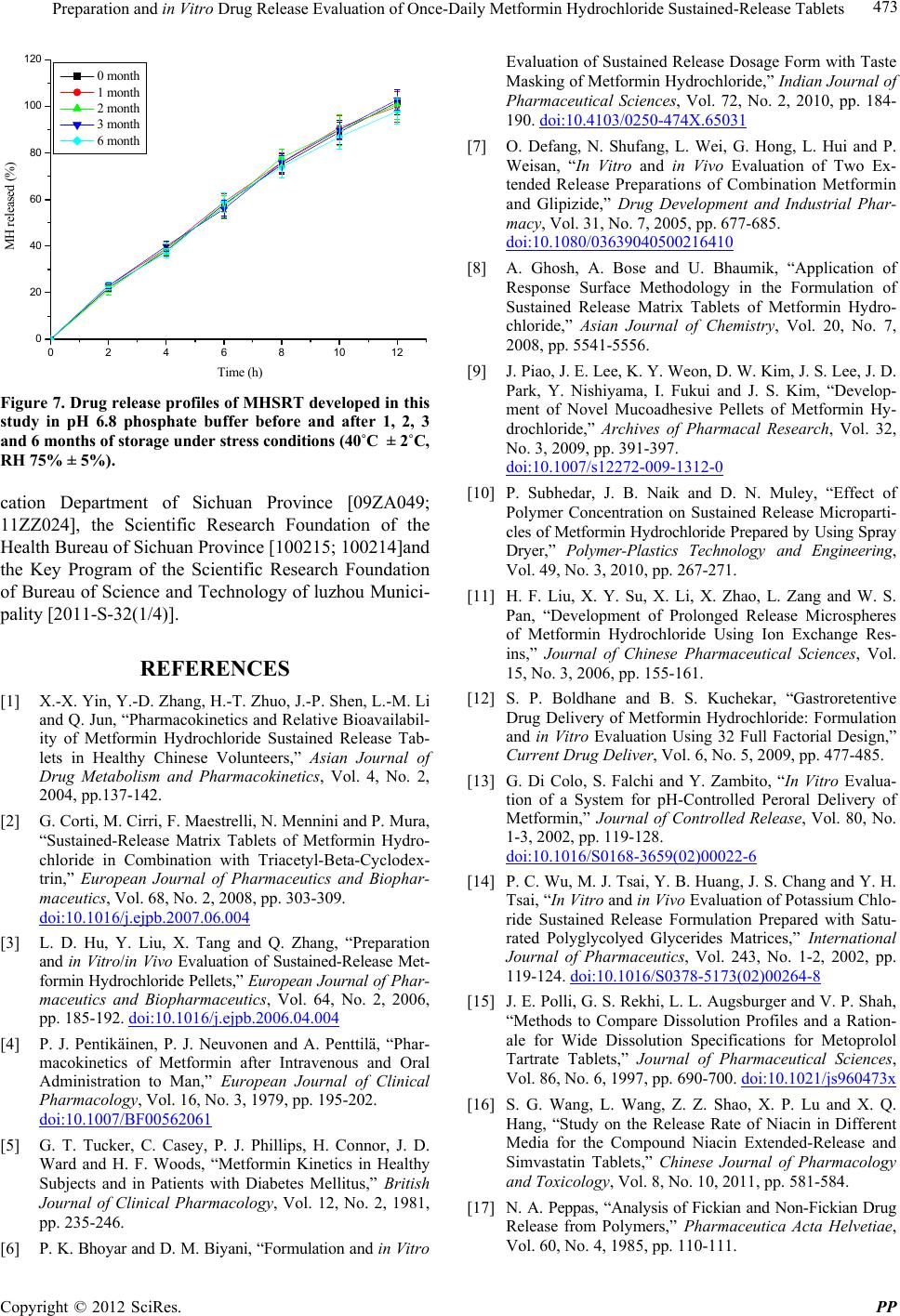
Figure 7. Drug release profiles of MHSRT develope d in this
study in pH 6.8 phosphate buffer before and after 1, 2, 3
and 6 months of storage under stress conditions (40˚C ± 2˚C,
RH 75% ± 5%).
cation Department of Sichuan Province [09ZA049;
11ZZ024], the Scientific Research Foundation of the
Health Bureau of Sichuan Province [100215; 100214]and
the Key Program of the Scientific Research Foundation
of Bureau of Science and Technology of luzhou Munici-
pality [2011-S-32(1/4)].
REFERENCES
[1] X.-X. Yin, Y.-D. Zhang, H.-T. Zhuo, J.-P. Shen, L.-M. Li
and Q. Jun, “Pharmacokinetics and Relative Bioavailabil-
ity of Metformin Hydrochloride Sustained Release Tab-
lets in Healthy Chinese Volunteers,” Asian Journal of
Drug Metabolism and Pharmacokinetics, Vol. 4, No. 2,
2004, pp.137-142.
[2] G. Corti, M. Cirri, F. Maestrelli, N. Mennini and P. Mura,
“Sustained-Release Matrix Tablets of Metformin Hydro-
chloride in Combination with Triacetyl-Beta-Cyclodex-
trin,” European Journal of Pharmaceutics and Biophar-
maceutics, Vol. 68, No. 2, 2008, pp. 303-309.
doi:10.1016/j.ejpb.2007.06.004
[3] L. D. Hu, Y. Liu, X. Tang and Q. Zhang, “Preparation
and in Vitro/in Vivo Evaluation of Sustained-Release Met-
formin Hydrochloride Pellets,” European Journal of P har -
maceutics and Biopharmaceutics, Vol. 64, No. 2, 2006,
pp. 185-192. doi:10.1016/j.ejpb.2006.04.004
[4] P. J. Pentikäinen, P. J. Neuvonen and A. Penttilä, “Phar-
macokinetics of Metformin after Intravenous and Oral
Administration to Man,” European Journal of Clinical
Pharmacology, Vol. 16, No. 3, 1979, pp. 195-202.
doi:10.1007/BF00562061
[5] G. T. Tucker, C. Casey, P. J. Phillips, H. Connor, J. D.
Ward and H. F. Woods, “Metformin Kinetics in Healthy
Subjects and in Patients with Diabetes Mellitus,” British
Journal of Clinical Pharmacology, Vol. 12, No. 2, 1981,
pp. 235-246.
[6] P. K. Bhoyar and D. M. Biyani, “Formulation and in Vitro
Evaluation of Sustained Release Dosage Form with Taste
Masking of Metformin Hydrochloride,” Indian Journal of
Pharmaceutical Sciences, Vol. 72, No. 2, 2010, pp. 184-
190. doi:10.4103/0250-474X.65031
[7] O. Defang, N. Shufang, L. Wei, G. Hong, L. Hui and P.
Weisan, “In Vitro and in Vivo Evaluation of Two Ex-
tended Release Preparations of Combination Metformin
and Glipizide,” Drug Development and Industrial Phar-
macy, Vol. 31, No. 7, 2005, pp. 677-685.
doi:10.1080/03639040500216410
[8] A. Ghosh, A. Bose and U. Bhaumik, “Application of
Response Surface Methodology in the Formulation of
Sustained Release Matrix Tablets of Metformin Hydro-
chloride,” Asian Journal of Chemistry, Vol. 20, No. 7,
2008, pp. 5541-5556.
[9] J. Piao, J. E. Lee, K. Y. Weon, D. W. Kim, J. S. Lee, J. D.
Park, Y. Nishiyama, I. Fukui and J. S. Kim, “Develop-
ment of Novel Mucoadhesive Pellets of Metformin Hy-
drochloride,” Archives of Pharmacal Research, Vol. 32,
No. 3, 2009, pp. 391-397.
doi:10.1007/s12272-009-1312-0
[10] P. Subhedar, J. B. Naik and D. N. Muley, “Effect of
Polymer Concentration on Sustained Release Microparti-
cles of Metformin Hydrochloride Prepared by Using Spray
Dryer,” Polymer-Plastics Technology and Engineering,
Vol. 49, No. 3, 2010, pp. 267-271.
[11] H. F. Liu, X. Y. Su, X. Li, X. Zhao, L. Zang and W. S.
Pan, “Development of Prolonged Release Microspheres
of Metformin Hydrochloride Using Ion Exchange Res-
ins,” Journal of Chinese Pharmaceutical Sciences, Vol.
15, No. 3, 2006, pp. 155-161.
[12] S. P. Boldhane and B. S. Kuchekar, “Gastroretentive
Drug Delivery of Metformin Hydrochloride: Formulation
and in Vitro Evaluation Using 32 Full Factorial Design,”
Current Drug Deliver, Vol. 6, No. 5, 2009, pp. 477-485.
[13] G. Di Colo, S. Falchi and Y. Zambito, “In Vitro Evalua-
tion of a System for pH-Controlled Peroral Delivery of
Metformin,” Journal of Controlled Release, Vol. 80, No.
1-3, 2002, pp. 119-128.
doi:10.1016/S0168-3659(02)00022-6
[14] P. C. Wu, M. J. Tsai, Y. B. Huang, J. S. Chang and Y. H.
Tsai, “In Vitro and in Vivo Evaluation of Potassium Chlo-
ride Sustained Release Formulation Prepared with Satu-
rated Polyglycolyed Glycerides Matrices,” International
Journal of Pharmaceutics, Vol. 243, No. 1-2, 2002, pp.
119-124. doi:10.1016/S0378-5173(02)00264-8
[15] J. E. Polli, G. S. Rekhi, L. L. Augsburger and V. P. Shah,
“Methods to Compare Dissolution Profiles and a Ration-
ale for Wide Dissolution Specifications for Metoprolol
Tartrate Tablets,” Journal of Pharmaceutical Sciences,
Vol. 86, No. 6, 1997, pp. 690-700. doi:10.1021/js960473x
[16] S. G. Wang, L. Wang, Z. Z. Shao, X. P. Lu and X. Q.
Hang, “Study on the Release Rate of Niacin in Different
Media for the Compound Niacin Extended-Release and
Simvastatin Tablets,” Chinese Journal of Pharmacology
and Toxicology, Vol. 8, No. 10, 2011, pp. 581-584.
[17] N. A. Peppas, “Analysis of Fickian and Non-Fickian Drug
Release from Polymers,” Pharmaceutica Acta Helvetiae,
Vol. 60, No. 4, 1985, pp. 110-111.
Copyright © 2012 SciRes. PP