Paper Menu >>
Journal Menu >>
 Pharmacology & Pharmacy, 2010, 1, 18-26 10.4236/pp.2010.11003 Published Online July 2010 (http://www.SciRP.org/journal/pp) Copyright © 2010 SciRes. PP Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex ——Rapidly Disintegrating Fast Release Tablet Tapan Kumar Giri, Biswanath Sa* Centre for Advanced Research in Pharmaceutical Sciences, Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India. Email: biswanathsa2003@yahoo.com Received June 1st, 2010; accepted July 8th, 2010. ABSTRACT This study was undertaken to develop tablets of diazepam-hydroxypropyl-β-cyclodextrin inclusion complex that disinte- grate within 3 minutes and release 85% of drug within 30 minutes to provide rapid action of the drug through oro-mucosal route. Formation of inclusion complex was verified using X-ray diffraction and differential scanning calo- rimetric studies. Enhanced of aqueous solubility, as evident from phase solubility study, and dissolution of the drug were related with the formation of inclusion complex. Among the various formulations, tablet containing inclusion complex of drug/hydroxypropyl-β-cyclodextrin in a molar ratio of 1:2, and a combination of microcrystalline cellu- lose/lactose in a ratio of 4:1 disintegrated in 13 seconds and released 85% drug within 9 minutes. Addition of 10% w/w polyvinyl pyrrolidone in the tablet formulation further enhanced the drug release. Accelerated stability study indicated that mean dissolution time of the drug from the tablet did not change significantly within 6 months. Keywords: X-Ray Diffraction, Phase Solubity, Dissolution Efficiency, Mean Dissolution Time, Stability 1. Introduction Though conventional oral and parenteral routes are used widely to achieve systemic action of drugs, various mu- cosae are being explored as possible alternative routes for drug delivery. Since the invention of nitroglycerin sublingual tablets, the oral mucosal route is drawing at- tention of both academia and industries as a substitute drug delivery approach. Several constraints like difficulty in swallowing experienced by many paediatrics and geri- atrics [1], and in chewing by edentulous [2]; nausea and vomiting experienced with certain drugs when released in stomach [3]; degradation and metabolism of suscepti- ble drugs in gastrointestinal tract [4]; tissue necrosis and irritation from repeated administration of parenterals [5], high expenses due to sterile manufacturing [6] are avoided through oromucosal delivery of drugs. In certain diseases like epilepsy, rapid onset of drug action is nec- essary to suppress convulsion and terminate seizures. Thus early termination of seizures by initiating therapy as soon as possible, preferably at home, has been empha- sized as a key to minimize morbidity of these seizures [7-9]. Benzodiazepines are used for the acute management of severe seizures and have a rapid onset of action once delivered into the central nervous system and are safe. [10] Diazepam, a benzodiazepine, is included in the “WHO Essential Drug list” for the treatment of convul- sion and epileptic seizure [11-14]. Although intravenous therapy is the most rapid way to suppress epileptic con- vulsion, it may produce toxic manifestation due to exces- sive drug concentration [15,16], requires great care and caution to avoid thrombophlebitis and irritation [17] and may not be feasible where adequate medical facilities are not available in the immediate vicinity. While absorption of diazepam from intramuscular route is poor and erratic  Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Copyright © 2010 SciRes. PP 19 [18], the time to reach peak plasma concentration fol- lowing oral administration is 1-2 hours [19] and is ac- companied with acid hydrolysis and extensive liver me- tabolism [10]. If diazepam is formulated in a rapidly disintegrating fast dissolving tablet dosage form, the high vascularity and rich blood supply of oral mucosa [20] may provide rapid absorption and faster onset of action [21] and could enable a patient for self medication even without the aid of water in a situation where onset of convulsion is ap- prehended. Two principle criteria appear to be important for de- veloping rapidly disintegrating fast dissolving tablets: 1) disintegration time preferably < 3 minutes [22] and 2) rapid drug dissolution: time required for 85% dissolution (t85%) less than 30 minutes [23]. Valuable research re- ports for formulation of rapidly disintegrating tablets are available [24]; also, various technologies for improving dissolution property of poorly water soluble drugs have been documented to enhance bioavailability following oral absorption [25]. Among the various strategies, for- mulation of solid dispersions with hydrophilic carriers especially polyethylene glycols (PEGS) have been suc- cessfully used for enhancing dissolution of poorly water soluble drugs [26-29]. However, development of dosage forms like tablet and capsule using the solid dispersion encounters problems in pulverization/sifting of the solid dispersion which are usually soft and tacky and exhibits poor flow properties. In recent years, inclusion com- plexes of poorly water soluble drugs with cyclodextrins especially hydroxypropyl-β-cyclodextrins (HPβCD) have become popular to enhance the solubility and bioavail- ability of drugs. Loftsson [30] reported that the solubility of diazepam and various poorly water soluble drugs improved in solu- tion with the natural cyclodextrins and their derivatives. Subsequent studies also shows that the incorporation of hydrophilic polymer such as sodium carboxymethyl cel- lulose, hydroxypropyl methyl cellulose and polyvinyl pyrrolidone increase the solubilizing effect of the cyclo- dextrins and reduce the amount of cyclodextrin required. The objective of this study was to develop a rapidly disintegrating fast dissolving tablet of diazepam that can disintegrate in less than 3 minutes and release/dissolve 85% of the drug within 30 minutes in the oral cavity. The initial part of this work involved preparation of diaze- pam-HPβCD inclusion complex by kneading method and characterization of the complex using X-ray diffrac- tion(XRD) and differential scanning calorimetric (DSC) studies. The subsequent phase involved the preparation of tablets of diazepam-HPβCD complex by direct com- pression method to meet the specified time limits of dis- integration and drug dissolution. Finally the optimized tablets were subjected to accelerated stability study. 2. Experimental 2.1 Materials Diazepam (East India Pharmaceutical Works Ltd., Kol- kata, India), Saccharin-Na, Crosscarmellose sodium (Ac-Disol), Microcrystalline Cellulose (Avicel, PH-102) [Dey’s Medical Stores (Mfg.) Ltd., Kolkata, India], Hy- droxypropyl beta cyclodextrin (HPβCD) [Dr Reddy’s Lab, Hyderabad, India] were obtained as gift samples. Mannitol, lactose monohydrate and sorbitol (Merc, India), PVP-K30 (Qualigens, Mumbai, India), magnesium stearate and all other ingredients were obtained commercially and used as received. 2.2 Preparation of Solid Complexes Solid inclusion complexes of diazepam-HPβCD were prepared in 1:1 and 1:2 ratio by kneading method with and without the addition of polyvinyl pyrrolidone (PVP). PVP was added at a concentration of 10% (w/w) of the solid complex. Mixture was triturated for one hour in a mortar with a small volume of water to obtain a homo- geneous paste. During the process, the water content of the paste was empirically adjusted to maintain the con- sistency of the paste. The paste was dried at 45°C for 48 hours, pulverized and passed through sieve # 100. 2.3 Thermal Analysis Differential thermal analysis of diazepam, HPβCD, di- azepam/HPβCD physical mixture, and inclusion complex were carried out using Perkin-Elmer instrument (Pyris Diamond TG/DTA, Singapore) equipped with a liquid nitrogen subambient accessory. About 4 mg samples were kept in aluminum pan, hermetically sealed and scanned at a rate of 5°/min between 30°-210°C under ni- trogen atmosphere. 2.4 X-Ray Diffraction Study X-ray powder diffraction patterns of diazepam, HPβCD, diazepam/HPβCD physical mixture, and inclusion com- plex were conducted with a X-ray powder diffractometer (Rigaku-MiniFlax, Tokyo, Japan.) using a copper Kα target with a nickel filter at 30 kV voltage, 15 mA cur- rent and at scanning speed of 1°/min over a 2θ range of 5°-60°. 2.5 Phase Solubility Study Phase solubility studies were carried out by adding ex- cess amounts of drug to 10 ml of USP phosphate buffer solution (pH 5.8) containing various concentrations of HPβCD (3-15 mM) in stoppered conical flasks. The flasks were shaken at 50 revolutions per minute in a shaking incubator (Model KMC 8480 SL, Vision Scien- tific Company, Ltd., Seoul, South Korea) at 37 ± 0.5°C 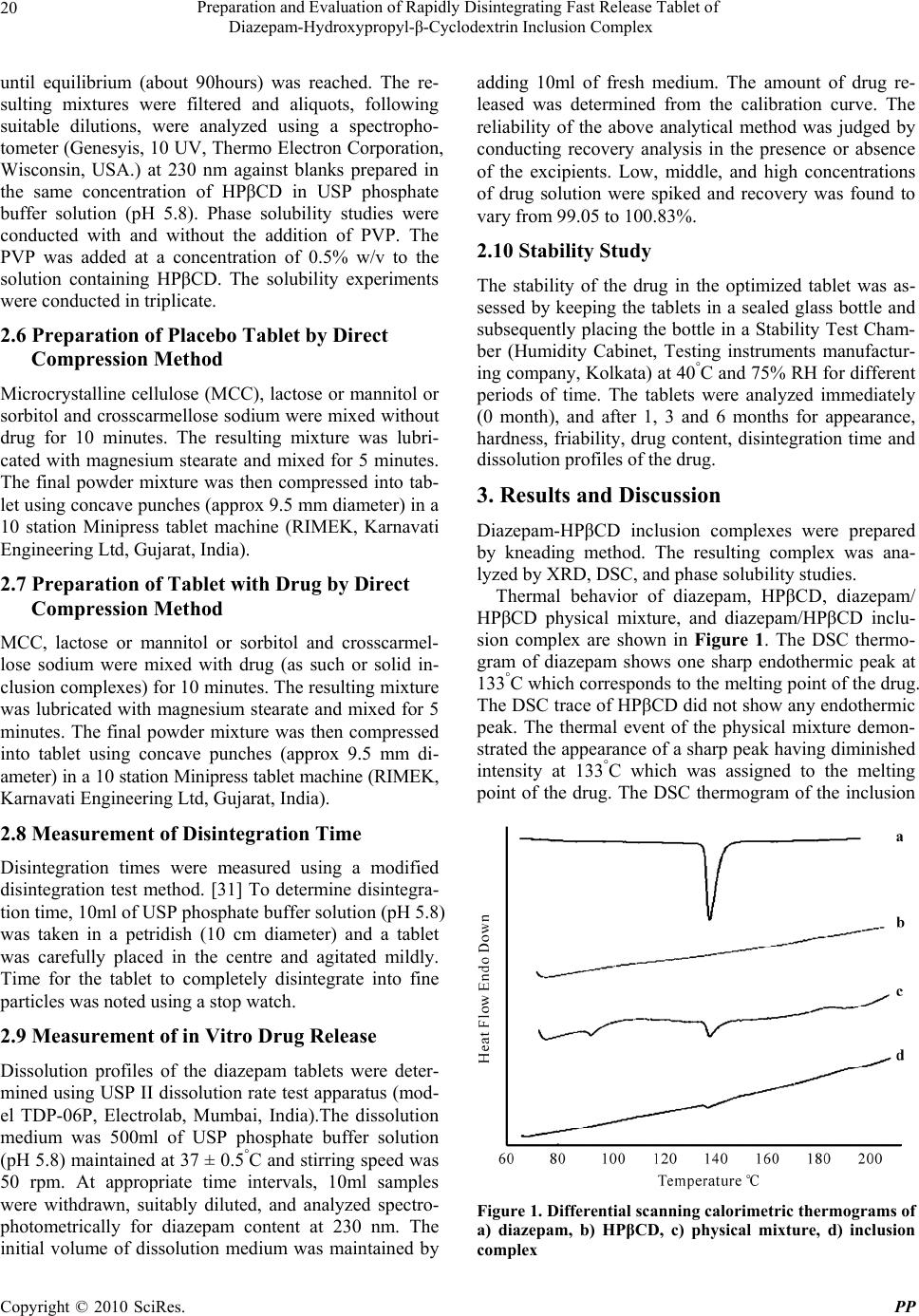 Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Copyright © 2010 SciRes. PP 20 until equilibrium (about 90hours) was reached. The re- sulting mixtures were filtered and aliquots, following suitable dilutions, were analyzed using a spectropho- tometer (Genesyis, 10 UV, Thermo Electron Corporation, Wisconsin, USA.) at 230 nm against blanks prepared in the same concentration of HPβCD in USP phosphate buffer solution (pH 5.8). Phase solubility studies were conducted with and without the addition of PVP. The PVP was added at a concentration of 0.5% w/v to the solution containing HPβCD. The solubility experiments were conducted in triplicate. 2.6 Preparation of Placebo Tablet by Direct Compression Method Microcrystalline cellulose (MCC), lactose or mannitol or sorbitol and crosscarmellose sodium were mixed without drug for 10 minutes. The resulting mixture was lubri- cated with magnesium stearate and mixed for 5 minutes. The final powder mixture was then compressed into tab- let using concave punches (approx 9.5 mm diameter) in a 10 station Minipress tablet machine (RIMEK, Karnavati Engineering Ltd, Gujarat, India). 2.7 Preparation of Tablet with Drug by Direct Compression Method MCC, lactose or mannitol or sorbitol and crosscarmel- lose sodium were mixed with drug (as such or solid in- clusion complexes) for 10 minutes. The resulting mixture was lubricated with magnesium stearate and mixed for 5 minutes. The final powder mixture was then compressed into tablet using concave punches (approx 9.5 mm di- ameter) in a 10 station Minipress tablet machine (RIMEK, Karnavati Engineering Ltd, Gujarat, India). 2.8 Measurement of Disintegration Time Disintegration times were measured using a modified disintegration test method. [31] To determine disintegra- tion time, 10ml of USP phosphate buffer solution (pH 5.8) was taken in a petridish (10 cm diameter) and a tablet was carefully placed in the centre and agitated mildly. Time for the tablet to completely disintegrate into fine particles was noted using a stop watch. 2.9 Measurement of in Vitro Drug Release Dissolution profiles of the diazepam tablets were deter- mined using USP II dissolution rate test apparatus (mod- el TDP-06P, Electrolab, Mumbai, India).The dissolution medium was 500ml of USP phosphate buffer solution (pH 5.8) maintained at 37 ± 0.5°C and stirring speed was 50 rpm. At appropriate time intervals, 10ml samples were withdrawn, suitably diluted, and analyzed spectro- photometrically for diazepam content at 230 nm. The initial volume of dissolution medium was maintained by adding 10ml of fresh medium. The amount of drug re- leased was determined from the calibration curve. The reliability of the above analytical method was judged by conducting recovery analysis in the presence or absence of the excipients. Low, middle, and high concentrations of drug solution were spiked and recovery was found to vary from 99.05 to 100.83%. 2.10 Stability Study The stability of the drug in the optimized tablet was as- sessed by keeping the tablets in a sealed glass bottle and subsequently placing the bottle in a Stability Test Cham- ber (Humidity Cabinet, Testing instruments manufactur- ing company, Kolkata) at 40°C and 75% RH for different periods of time. The tablets were analyzed immediately (0 month), and after 1, 3 and 6 months for appearance, hardness, friability, drug content, disintegration time and dissolution profiles of the drug. 3. Results and Discussion Diazepam-HPβCD inclusion complexes were prepared by kneading method. The resulting complex was ana- lyzed by XRD, DSC, and phase solubility studies. Thermal behavior of diazepam, HPβCD, diazepam/ HPβCD physical mixture, and diazepam/HPβCD inclu- sion complex are shown in Figure 1. The DSC thermo- gram of diazepam shows one sharp endothermic peak at 133°C which corresponds to the melting point of the drug. The DSC trace of HPβCD did not show any endothermic peak. The thermal event of the physical mixture demon- strated the appearance of a sharp peak having diminished intensity at 133°C which was assigned to the melting point of the drug. The DSC thermogram of the inclusion Figure 1. Differential scanning calorimetric thermograms of a) diazepam, b) HPβCD, c) physical mixture, d) inclusion complex 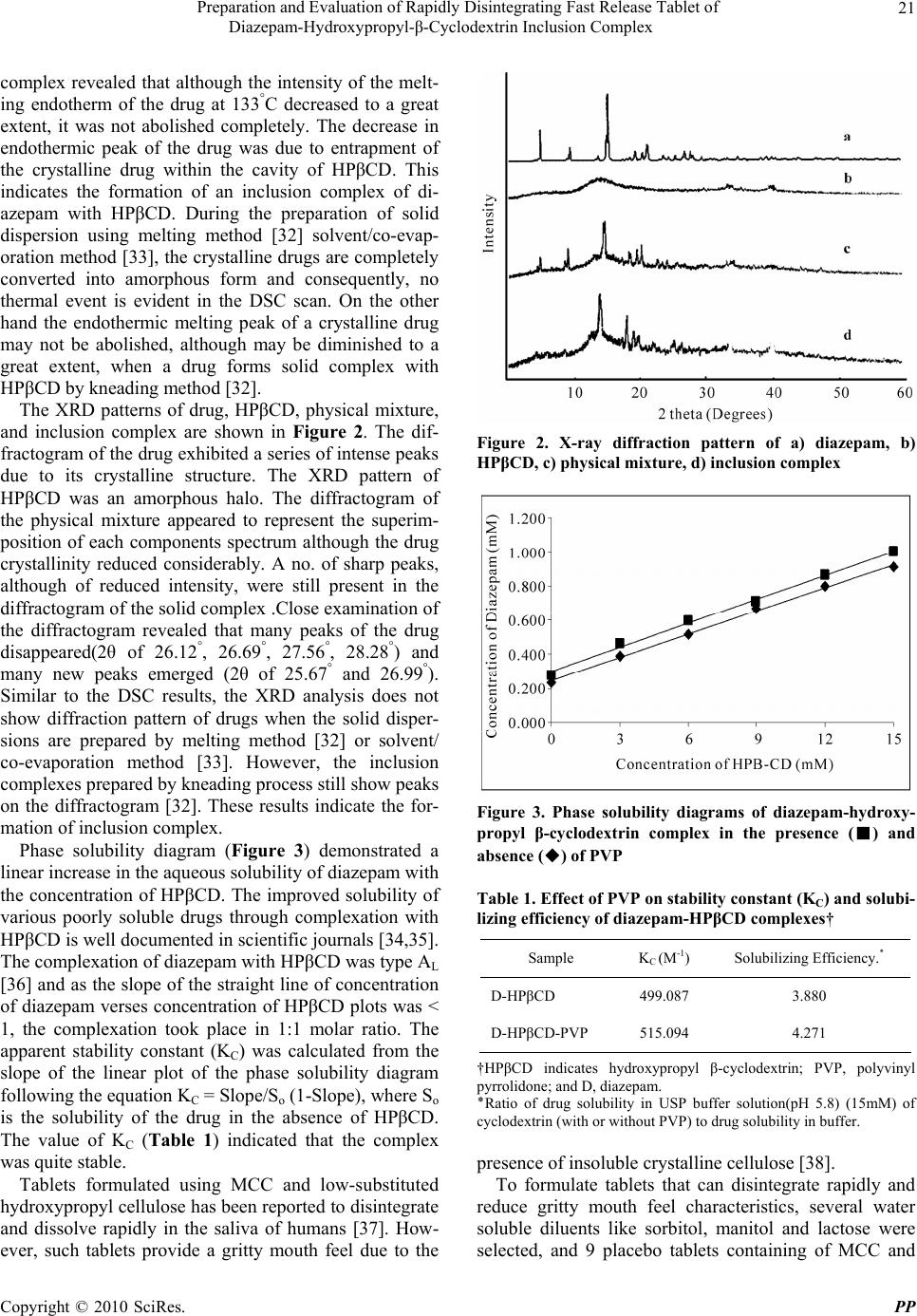 Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Copyright © 2010 SciRes. PP 21 complex revealed that although the intensity of the melt- ing endotherm of the drug at 133°C decreased to a great extent, it was not abolished completely. The decrease in endothermic peak of the drug was due to entrapment of the crystalline drug within the cavity of HPβCD. This indicates the formation of an inclusion complex of di- azepam with HPβCD. During the preparation of solid dispersion using melting method [32] solvent/co-evap- oration method [33], the crystalline drugs are completely converted into amorphous form and consequently, no thermal event is evident in the DSC scan. On the other hand the endothermic melting peak of a crystalline drug may not be abolished, although may be diminished to a great extent, when a drug forms solid complex with HPβCD by kneading method [32]. The XRD patterns of drug, HPβCD, physical mixture, and inclusion complex are shown in Figure 2. The dif- fractogram of the drug exhibited a series of intense peaks due to its crystalline structure. The XRD pattern of HPβCD was an amorphous halo. The diffractogram of the physical mixture appeared to represent the superim- position of each components spectrum although the drug crystallinity reduced considerably. A no. of sharp peaks, although of reduced intensity, were still present in the diffractogram of the solid complex .Close examination of the diffractogram revealed that many peaks of the drug disappeared(2θ of 26.12°, 26.69°, 27.56°, 28.28°) and many new peaks emerged (2θ of 25.67° and 26.99°). Similar to the DSC results, the XRD analysis does not show diffraction pattern of drugs when the solid disper- sions are prepared by melting method [32] or solvent/ co-evaporation method [33]. However, the inclusion complexes prepared by kneading process still show peaks on the diffractogram [32]. These results indicate the for- mation of inclusion complex. Phase solubility diagram (Figure 3) demonstrated a linear increase in the aqueous solubility of diazepam with the concentration of HPβCD. The improved solubility of various poorly soluble drugs through complexation with HPβCD is well documented in scientific journals [34,35]. The complexation of diazepam with HPβCD was type AL [36] and as the slope of the straight line of concentration of diazepam verses concentration of HPβCD plots was < 1, the complexation took place in 1:1 molar ratio. The apparent stability constant (KC) was calculated from the slope of the linear plot of the phase solubility diagram following the equation KC = Slope/So (1-Slope), where So is the solubility of the drug in the absence of HPβCD. The value of KC (Table 1) indicated that the complex was quite stable. Tablets formulated using MCC and low-substituted hydroxypropyl cellulose has been reported to disintegrate and dissolve rapidly in the saliva of humans [37]. How- ever, such tablets provide a gritty mouth feel due to the Figure 2. X-ray diffraction pattern of a) diazepam, b) HPβCD, c) physical mixture, d) inclusion complex Figure 3. Phase solubility diagrams of diazepam-hydroxy- propyl β-cyclodextrin complex in the presence (■) and absence (◆) of PVP Table 1. Effect of PVP on stability constant (KC) and solubi- lizing efficiency of diazepam-HPβCD complexes† Sample KC (M-1) Solubilizing Efficiency٭ . D-HPβCD 499.087 3.880 D-HPβCD-PVP 515.094 4.271 †HPβCD indicates hydroxypropyl β-cyclodextrin; PVP, polyvinyl pyrrolidone; and D, diazepam. ٭Ratio of drug solubility in USP buffer solution(pH 5.8) (15mM) of cyclodextrin (with or without PVP) to drug solubility in buffer. presence of insoluble crystalline cellulose [38]. To formulate tablets that can disintegrate rapidly and reduce gritty mouth feel characteristics, several water soluble diluents like sorbitol, manitol and lactose were selected, and 9 placebo tablets containing of MCC and  Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Copyright © 2010 SciRes. PP 22 either sorbitol, manitol or lactose in the ratios of 1:1, 2:1, and 4:1 were prepared by direct compression method. The compositions of the placebo tablets are shown in Table 2. The blended powder of each formulation exhibited good flowability as evident from the measurement of angle of repose (measured by conventional method) that varied from about 31.3° to 34° (Table 3). Angle of repose below 40° is an indication of good flowability of pow- der/granules [39]. The compression force during tablet- ting was adjusted in such a way that the hardness (meas- ured using Monsanto type hardness tester) of the tablets, each weighing 300 mg, was 2 Kg-F. The friability of the tablets (determined using Friabilator, Veego instrument, Mumbai, India) was found between 0.03 to 0.29% that was below 1% indicating sufficient mechanical integrity and strength of the placebo tablets. The disintegration time of tablets (P1) which were prepared using MCC and sorbitol in 1:1 ratio was 42.85 seconds. The tablets P2 and P3 which consisted of MCC and sorbitol in a ratio of 2:1 and 4:1 respectively disinte- grated in 29.03 and 27.34 seconds. The result indicates that increase in MCC/sorbitol ratio decreased the disin- tegration time of the tablets and this decrease was found significant at 95% confidence limit (p < 0.05). MCC is considered as one of the most versatile excipients in tab- let manufacturing. In addition to its performance as dilu- ents and dry binder, it is also regarded as an excellent disintegrant for tablets prepared by direct compression method. This property is related to its wicking action due to which water penetrates into the tablet and the devel- oped hydrostatic pressure causes break down of the tablet. The rate and extent of water penetration is related to the porosity (determined using laboratory pycnometer) it provides in the tablets. The greater the amount of MCC, the greater will be the porosity in the tablet matrix. Table 3 shows that as the ratio of MCC/sorbitol increased, the porosity of the tablets increased significantly (p < 0.05) that provided faster penetration of water. Determination of wetting time (determined by the method described by Bi et al. [40]) demonstrated that the wetting time of the tablets decreased significantly (p < 0.05) with increase in the amount of MCC (Table 3) indicating faster penetra- tion of water into the tablets. Similar observations were noted for the tablets prepared using MCC/manitol (tab- lets P4, P5, P6) and MCC/lactose (tablets P7, P8, P9). Table 2. Composition of placebo tablets (without drug) prepared by direct compression method Ingredients(mg/tablet) P1 P2 P3 P4 P5 P6 P7 P8 P9 MCC 144.25 193 230.8 144.25 193 230.8 144.25 193 230.8 Lactose - - - - - - 144.25 96.5 57.7 Mannitol - - - 144.25 96.5 57.7 - - - Sorbitol 144.25 96.5 57.7 - - - - - - Crosscarmellose-Na 7.5 7.5 7.5 7.5 7.5 7.5 7.5 7.5 7.5 Mg-St. 1 1 1 1 1 1 1 1 1 Saccharin-Na 3 3 3 3 3 3 3 3 3 Total 300 300 300 300 300 300 300 300 300 Table 3. Physical characteristics of placebo tablets (without drug) prepared by direct compression method P1 P2 P3 P4 P5 P6 P7 P8 P9 Angle of Repose 34.03 (0.33) 33.56 (0.39) 31.47 (1.4) 33.99 (0.29) 32.66 (0.69) 32.49 (1.13) 35.38 (0.8) 32.37 (1.02) 31.29 (0.96) Porosity 9.99 (0.13) 15.21 (0.21) 22.39 (0.21) 17.26 (0.14) 21.28 (0.16) 26.17 (0.28) 19.31 (0.19) 24.34 (0.42) 31.87 (0.7) Disintegration time, seconds 42.85 (1.89) 29.03 (0.67) 27.34 (0.62) 43.29 (0.77) 18.76 (0.25) 15.3 (0.9) 26.49 (1.36) 17.25 (0.17) 13.23 (0.46) Wetting time, seconds 119.92 (1.63) 92.69 (2.1) 84.78 (1.85) 82.56 (1.86) 52.69 (2.81) 42.53 (1.7) 80.67 (1.78) 50.3 (1.07) 35.43 (1.62) Figures in parentheses indicate ± SD; n = 6 for disintegration time and ± SD, n = 3 for Angle of Repose, porosity and wetting time 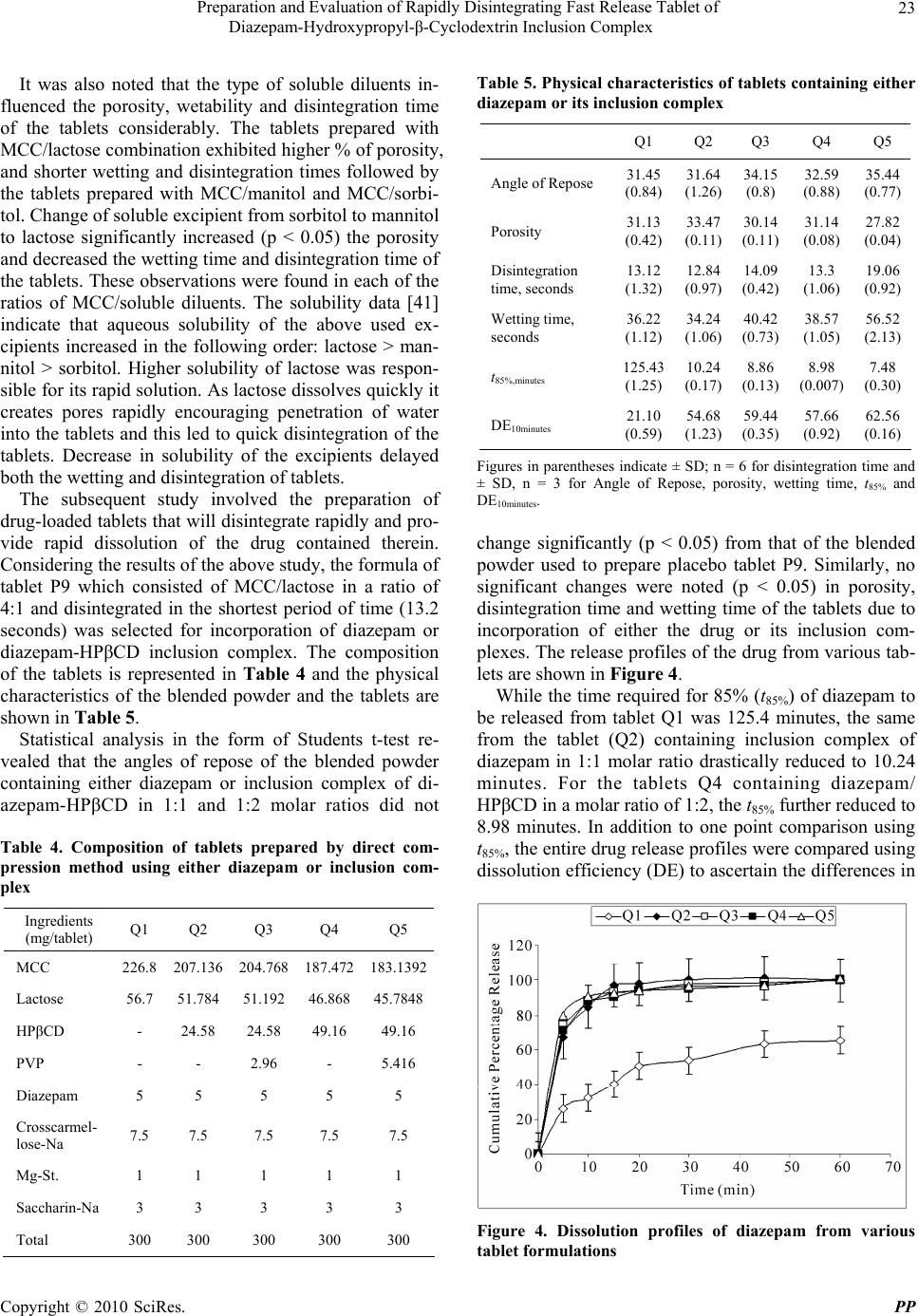 Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Copyright © 2010 SciRes. PP 23 It was also noted that the type of soluble diluents in- fluenced the porosity, wetability and disintegration time of the tablets considerably. The tablets prepared with MCC/lactose combination exhibited higher % of porosity, and shorter wetting and disintegration times followed by the tablets prepared with MCC/manitol and MCC/sorbi- tol. Change of soluble excipient from sorbitol to mannitol to lactose significantly increased (p < 0.05) the porosity and decreased the wetting time and disintegration time of the tablets. These observations were found in each of the ratios of MCC/soluble diluents. The solubility data [41] indicate that aqueous solubility of the above used ex- cipients increased in the following order: lactose > man- nitol > sorbitol. Higher solubility of lactose was respon- sible for its rapid solution. As lactose dissolves quickly it creates pores rapidly encouraging penetration of water into the tablets and this led to quick disintegration of the tablets. Decrease in solubility of the excipients delayed both the wetting and disintegration of tablets. The subsequent study involved the preparation of drug-loaded tablets that will disintegrate rapidly and pro- vide rapid dissolution of the drug contained therein. Considering the results of the above study, the formula of tablet P9 which consisted of MCC/lactose in a ratio of 4:1 and disintegrated in the shortest period of time (13.2 seconds) was selected for incorporation of diazepam or diazepam-HPβCD inclusion complex. The composition of the tablets is represented in Table 4 and the physical characteristics of the blended powder and the tablets are shown in Table 5. Statistical analysis in the form of Students t-test re- vealed that the angles of repose of the blended powder containing either diazepam or inclusion complex of di- azepam-HPβCD in 1:1 and 1:2 molar ratios did not Table 4. Composition of tablets prepared by direct com- pression method using either diazepam or inclusion com- plex Ingredients (mg/tablet) Q1 Q2 Q3 Q4 Q5 MCC 226.8 207.136 204.768 187.472 183.1392 Lactose 56.7 51.784 51.192 46.868 45.7848 HPβCD - 24.58 24.58 49.16 49.16 PVP - - 2.96 - 5.416 Diazepam 5 5 5 5 5 Crosscarmel- lose-Na 7.5 7.5 7.5 7.5 7.5 Mg-St. 1 1 1 1 1 Saccharin-Na 3 3 3 3 3 Total 300 300 300 300 300 Table 5. Physical characteristics of tablets containing either diazepam or its inclusion complex Q1 Q2 Q3 Q4 Q5 Angle of Repose 31.45 (0.84) 31.64 (1.26) 34.15 (0.8) 32.59 (0.88) 35.44 (0.77) Porosity 31.13 (0.42) 33.47 (0.11) 30.14 (0.11) 31.14 (0.08) 27.82 (0.04) Disintegration time, seconds 13.12 (1.32) 12.84 (0.97) 14.09 (0.42) 13.3 (1.06) 19.06 (0.92) Wetting time, seconds 36.22 (1.12) 34.24 (1.06) 40.42 (0.73) 38.57 (1.05) 56.52 (2.13) t85%,minutes 125.43 (1.25) 10.24 (0.17) 8.86 (0.13) 8.98 (0.007) 7.48 (0.30) DE10minutes 21.10 (0.59) 54.68 (1.23) 59.44 (0.35) 57.66 (0.92) 62.56 (0.16) Figures in parentheses indicate ± SD; n = 6 for disintegration time and ± SD, n = 3 for Angle of Repose, porosity, wetting time, t85% and DE10minutes. change significantly (p < 0.05) from that of the blended powder used to prepare placebo tablet P9. Similarly, no significant changes were noted (p < 0.05) in porosity, disintegration time and wetting time of the tablets due to incorporation of either the drug or its inclusion com- plexes. The release profiles of the drug from various tab- lets are shown in Figure 4. While the time required for 85% (t85%) of diazepam to be released from tablet Q1 was 125.4 minutes, the same from the tablet (Q2) containing inclusion complex of diazepam in 1:1 molar ratio drastically reduced to 10.24 minutes. For the tablets Q4 containing diazepam/ HPβCD in a molar ratio of 1:2, the t85% further reduced to 8.98 minutes. In addition to one point comparison using t85%, the entire drug release profiles were compared using dissolution efficiency (DE) to ascertain the differences in Figure 4. Dissolution profiles of diazepam from various tablet formulations  Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Copyright © 2010 SciRes. PP 24 the release of the drug from various tablets. The DE is defined as the area under the dissolution curve upto a certain time, t, expressed as a percentage of the area of the rectangle described by 100% dissolution in the same time [42]. 0 100 . D.E 100% . t ydt yt where y is the drug percent dissolved in time t. DE can have a range of values depending on the time interval chosen. However, while comparing a set of data, a constant time interval should be selected. In the present study, DE10minutes (dissolution efficiency upto 10 minutes) were calculated from the dissolution profile of each tab- let and used for comparison. It was found that in com- parison to DE10minutes obtained from tablet containing diazepam, a 2.59 and 2.73 fold increase in DE10minutes were obtained from the tablets prepared using diazepam/ HPβCD inclusion complex in molar ratios of 1:1 and 1:2 respectively. These increase in DE10minutes were found to be statistically significant (p < 0.05). Faster release of diazepam from the tablets prepared using its inclusion complexes was related to the enhanced solubility of the drug because of the formation of complex with HPβCD. In another two batches of tablets namely Q3 and Q5 containing respectively 1:1 and 1:2 diazepam-HPβCD complex, PVP, a hydrophilic polymer was added at a concentration of 10% w/w of the solid complexes (Table 4) to investigate its effect on the various physical char- acteristics which are shown in Table 5. Incorporation of PVP in the formula increased the angle of repose of the blended powders and decreased the porosity of the tab- lets significantly (P < 0.05). Moreover, PVP increased both the disintegration time and wetting time of the tab- lets significantly (p < 0.05). The larger the amount of PVP, the higher the disintegration time and the wetting time. PVP which acts as a binder densified the powder resulting in decreased flowability of the powder blend and reduced the porosity of the tablets. Reduced porosity, in turn, protracted the wetting and the disintegration of the tablets. Addition of PVP in the tablet formulations, however, further reduced t85% and increased DE indicat- ing that drug dissolution took place faster than that from the tablets without containing PVP. Addition of PVP in the complex also increased the solubility linearly having a slope < 1 as evident from phase solubility diagram (Figure 3). At each point of determination, the solubility of the drug was higher than that produced by the com- plex in absence of PVP. The stability constant was also found to be higher (Table 1). The potentiated solubility of diazepam in complex form in the presence of PVP was evaluated by determining the solubilization efficiency, which is defined as ratio of solubility of drug in USP phosphate buffer solution (pH 5.8) of 15 mM HPβCD (with or without PVP) and the solubility of the drug in buffer solution. Table 1 show that while HPβCD without PVP produced 3.88 fold increases, the same in presence of PVP produced 4.27 fold increases in the solubility of diazepam. It, therefore, appears that presence of hydro- philic polymer like PVP enhances the solubilizing effi- ciency of HPβCD. Accelerated stability test on the tablets (Q5) was con- ducted at 40°C and 75% RH in accelerated stability test chamber (Humidity Cabinet, Testing instruments manu- facturing company, Kolkata) and the physical character- istics of the tablets observed after different periods of time are shown in Table 6. The physical characteristics such as appearance, fri- ability and drug content did not change and were con- fined within the specified limits upto 6 months of time. The disintegration time and t85% of the tablets upto one month storage did not change significantly when com- pared to those of the fresh tablets. However, marginal Table 6. Various characteristics of Diazepam tablets stored at 40°C and 70% RH Storage Time (month) Conditions Appearance Friability (%)Content (mg)DT (seconds)t85% (minutes) MDT (minutes) Specifications White,round-flat tablet < 1% 95-105% <3 minutes < 30 minutes - Initial Complies 0.140 (0.010) 4.96 (0.015) 19.53 (0.395) 7.56 (0.15) 2.85 (0.045) 1 Complies 0.147 (0.012) 4.98 (0.015) 20.26 (0.352) 7.90 (0.17) 2.848 (0.049) 3 Complies 0.177 (0.012) 4.94 (0.006) 20.84 (0.40) 8.22 (0.16) 2.847 (0.050) 6 40°C /75% RH Complies 0.157 (0.025) 4.97 (0.006) 21.71 (0.262) 8.21 (0.11) 2.677 (0.12)  Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Copyright © 2010 SciRes. PP 25 changes in the two characteristics were observed after 3 and 6 months storage. Although, the changes were statis- tically significant, the tablets complied with the specified limits even after 6 months. To further investigate the effect of storage conditions on drug dissolution, mean dissolution time (MDT) were calculated from the release profiles of the tablets kept under stressed condition for different periods of time and were compared with that from freshly prepared tablet. MDT was calculated from the following equation: 1 1 n mid i n i tM MDT M where tmid is the time at the midpoint between i and i-1, and M is the additional amount of drug dissolved be- tween i and i-1. Table 6 shows that MDT values of the tablets did not differ significantly (p < 0.05). This indicates that the drug dissolution from the tablets (Q5) which were kept under stressed condition were similar to that from the freshly prepared tablets upto 6 months. 4. Conclusions Tablets of diazepam were prepared by direct compres- sion method that disintegrated quickly (in 13.3 seconds) and released 85% drug rapidly (in 8.98 minutes). Rapid disintegration was achieved using MCC and lactose as excipients in a ratio of 4:1. Rapid drug dissolution was obtained through the formulation of inclusion complex of the drug with HPβCD. Inclusion of PVP further in- creased the drug dissolution. Such tablets seem to be suitable for achieving rapid onset of action of the drug through oro-mucosal route. REFERENCES [1] J. S. Schindler and J. H. Kelly, “Swallowing Disorders in the Elderly,” Laryngoscope, Vol. 112, No. 4, 2002, pp. 589-602. [2] J. C. Ofstehage and K. Magilvy, “Oral Health and Ag- ing,” Geriatric Nursing, Vol. 81, No. 7, 1986, pp. 238- 241. [3] G. Motola, F. Russo, F. Mazzeo, B. Rinaldi, A. Capuano, F. Rossi and A. Filipelli, “Over-the-Counter Oral Non- steroidal Anti-Inflammatory Drugs: A Pharmacoepidemi- ologic Study in Southern Italy,” Advances in Therapy, Vol. 18, No. 5, 2001, pp. 216-222. [4] L. Yang, S. C. James and A. Joseph, “Colon-Specific Drug Delivery: New Approaches and in Vitro/in Vivo Evaluation,” International Journal of Pharmaceutics, Vol. 235, No. 1-2, 2002, pp. 1-15. [5] K. D. Tripathi, “Essentials of Medical Pharmacology,” 5th Edition, Jaypee Brothers Medical Publishers (P) LTD, New Delhi, 2003. [6] L. Li, S. Gorukanti, Y. M. Choi and K. H. Kim, “Rap- id-Onset Intranasal Delivery of Anticonvulsants: Phar- macokinetic and Pharmacodynamic Evaluation in Rab- bits,” International Journal of Pharmaceutics, Vol. 199, No. 1, 2000, pp. 65-76. [7] B. Alldredge, A. Gelb, S. Isaacs, M. Corry, F. Allen, S. Ulrich, M. Gottwald, N. O’Neil, J. Neuhaus, M. Segal and D. A. Lowenstein, “Comparison of Lorazepam, DZ, and Placebo for the Treatment of Out-of-Hospital Status Epilepticus,” New England Journal of Medicine, Vol. 345, No. 9, 2001, pp. 631-637. [8] C. O’Dell, “What Do We Tell Parents of a Child with Simple or Complex Febrile Seizures?” In: T. Z. Baram, and S. Shinnar, Eds., Febrile Seizures, San Diego, CAS Academic Press, 2002, pp. 305-316. [9] C. O’Dell, S. Shinnar, K. Ballaban-Gil, M. Hornick, M. Sigalova, H. Kang and S. Moshe, “Rectal DZ Gel in the Home Management of Seizures in Children,” Pediatric Neurology, Vol. 33, No. 3, 2005, pp. 166-172. [10] G. Abdelbary and R. H. Fahmy, “Diazepam-Loaded Solid lipid Nanoparticles: Design and Characterization,” AAPS PharmSciTech, Vol. 10, No. 1, 2009, pp. 211-219. [11] E. Bechgaard, S. Gizurarason and R. K. Hjortkjaer, “Pharmacokinetic and Pharmacodynamic Response after Intranasal Administration of Diazepam to Rabbits,” Journal of Pharmacy Pharmacology, Vol. 49, No. 8, 1997, pp. 747-750. [12] S. Gizurarson, F. K. Gudbrandsson, H. Jonsson and E. Bechgaard, “Intranasal Administration of Diazepam Aiming at the Treatment of Acute Seizures: Clinical Tri- als in Healthy Volunteers,” Biological and Pharmaceuti- cal Bulletin, Vol. 22, No. 4, 1999, pp. 425-427. [13] E. Rey, J. M. Treluyer and G. Pons, “Pharmacokinetic Optimization of Benzodiazepine Therapy for Acute Sei- zures. Focus on Delivery Routes,” Clinical Pharmacoki- netics, Vol. 36, No. 6, 1999, pp. 409-424. [14] F. U. Knudsen, “Rectal Administration of Diazepam in Solution in the Acute Treatment of Convulsions in Infants and Children,” Archives of Disease in Childhood, Vol. 54, No. 11, 1979, pp. 855-857. [15] E. Norris, O. Marzouk, A. Nunn, J. Mclntyre and I. Choonara, “Respiratory Depression in Children Receiving Diazepam for Acute Seizures: A Prospective Study,” De- velopmental Medicine and Child Neurology, Vol. 41, No. 5, 1999, pp. 340-343. [16] B. R. Ogutu, C. R. J. C. Newton, J. Crawley, S. N. Mu- chohi, G. O. Otieno, G. Edwards, K. Marsh and G. O. Kokwaro, “Pharmacokinetics and Anticonvulsant Effects of Diazepam in Children with Severe Falciparum Malaria and Convulsions,” British Journal of Clinical Pharma- cology, Vol. 53, No. 1, 2002, pp.49-57. [17] C. W. Graham, R. R. Pagano and J. T. Conner, “Pain and Clinical Thrombophlebitis Following Intravenous Diaze- pam and Lorazepam,” Anaesthesia, Vol. 33, No. 2, 1978, 188-91.  Preparation and Evaluation of Rapidly Disintegrating Fast Release Tablet of Diazepam-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Copyright © 2010 SciRes. PP 26 [18] C. Lehmann and G. L. Wannarka, “Bioavailability and Dose Proportionality of Intramuscular Diazepam Admin- istered by Autoinjector,” Journal of Clinical Pharmacol- ogy, Vol. 48, No. 4, 2008, pp. 436-444. [19] J. G. Hardman, L. E. Limbard and A. G. Gilman, “The Pharmacological Basis of Therapeutics,” 10th Edition, McGraw-Hill Medical Publishing Division, New Delhi, 2001. [20] A. J. Hoogstrate, J. C. Verhoef, B. Tuk and A. Pijpers, “In-Vivo Buccal Delivery of Fluorescin Isothiocyanate- Dextran 4400 with Glycodeoxycholate as an Absorption Enhancer in Pig,” Journal of Pharmaceutical Sciences, Vol. 85, No. 5, 1996, pp. 457-460. [21] T. Keiko, O. Yasuko, N. Tsuneji, L. Thorseinn and T. Kozo, “Buccal Absorption of Ergotamine Tartrate Using the Bioadhesive Tablet System in Guinea-Pigs,” Interna- tional Journal of Pharmaceutics, Vol. 238, No. 1-2, 2002, pp. 161-170. [22] B. S. Kuchekar, S. B. Bhise and V. Arumugam, “Design of Fast Dissolving Tablet,” Indian Journal of Pharma- ceutical Education, Vol. 35, No. 4, 2001, pp. 150-152. [23] T. Loftsson, “Cyclodextrins and the Biopharmaceutics Classification System of Drugs,” Journal of Inclusion Phenomenon, Vol. 44, No. 1-4, 2002, pp. 63-67. [24] M. Sugimoto, S. Narisawa, K. Matsubara, H. Yoshino, M. Nakano and T. Handa, “Effect of Formulated Ingredients on Rapidly Disintegrating Oral Tablets Prepared by the Crystalline Transition Method,” Chemical and Pharma- ceutical Bulletin, Vol. 54, No. 2, 2006, pp. 175-180. [25] H. N. Joshi, R. W. Tejwani, M. Davidovich, V. P. Sahas- rabudhe, M. Jemal, M. S. Bathala, S. A. Varia and A. T. M. Serajuddin, “Bioavailability Enhancement of a Poorly Water-Soluble Drug by Solid Dispersion in Polyethylene Glycol-Polysorbate 80 Mixture,” International Journal of Pharmaceutics, Vol. 269, No. 1, 2004, pp. 251-258. [26] P. C. Sheen, V. K. Khetarpal, C. M. Cariola and C. E. Rowlings, “Formulation Studies of a Poorly Wa- ter-Soluble Drug in Solid Dispersions to Improve Bio- availability,” International Journal of Pharmaceutics, Vol. 118, No. 2, 1995, pp. 221-227. [27] P. Mura, A. Manderioli, G. Bramanti and L. Ceccarelli, “The Properties of Solid Dispersions of Naproxen in Various Polyethylene Glycols,” Drug Development and Industrial Pharmacy, Vol. 22, No. 9-10, 1996, pp. 909- 916. [28] T. Kai, Y. Akiama, S. Nomura and M. Sato, “Oral Ab- sorption Improvement of Poorly Soluble Drug Using Solid Dispersion Technique,” Chemical and Pharmaceu- tical Bulletin, Vol. 44, No. 3, 1996, pp. 568-571. [29] C. Leuner and J. Dressman, “Improving Drug Solubility for Oral Delivery Using Solid Dispersions,” European Journal of Pharmaceutical Sciences, Vol. 50, No. 1, 2000, pp. 47-60. [30] T. Loftsson, “Cyclodextrin Complexation,” US Patent, 1995, 5472954. [31] M. Gohel, M. Patel, A. Amin, R. Agarwal, R. Dave and N. Bariya, “Formulation Design and Optimization of Mouth Dissolve Tablets of Nimesulide Using Vacuum Drying Technique,” AAPS PharmSciTech, Vol. 5, No. 3, 2004, Article 36. [32] S. Chutimaworapan, G. C. Ritthidej, E. Yonemochi, T. Oguchi and K. Yamamoto, “Effect of Water-Soluble Car- riers on Dissolution Characteristics of Nifedipine Solid Dispersions,” Drug Development and Industrial Phar- macy, Vol. 26, No. 11, 2000, pp. 1141-1150. [33] H. G. Choi, K. M. Kim, H. W. Jun, B. K. Yoo and C. S. Yong, “Improvement of Dissolution and Bioavailability of Nifedipine by Inclusion in Hydroxypropyl-β-Cyclo- dextrin,” Drug Development and Industrial Pharmacy, Vol. 29, No. 10, 2003, pp. 1085-1094. [34] H. J. Ahn, K. M. Kim, S. J. Choi and C. K. Kim, “Effects of Cyclodextrin Derivatives on Bioavailability of Keto- profen,” Drug Development and Industrial Pharmacy, Vol. 23, No. 4, 1997, pp. 397-401. [35] A. Latrofa, G. Trapani, M. Franco, M. Serra, M. Muggi- roni, F. P. Franizzi, A. Cutrignelli and G. Liso, “Com- plexation of Phenytoin with Some Hydrophilic Cyclodex- trins: Effect on Aqueous Solubility, Dissolution Rate and Anti-Convulsant Activity in Mice,” European Journal of Pharmaceutics and Biopharmaceutics, Vol. 52, No. 1, 2001, pp. 65-73. [36] T. Higuchi and K. A. Connors, “Phase-Solubility Tech- niques,” Advances in Analytical Chemistry and Instru- mentation, Vol. 53, No. 4, 1965, pp. 117-212. [37] Y. Watanable, K. I. Koizumi, Y. Zama, M. Kiriyama and M. Matsumoto, “New Compressed Tablet Rapidly Disin- tegrating in Saliva in the Mouth Using Crystalline Cellu- lose and a Disintegrant,” Biological and Pharmaceutical Bulletin, Vol. 18, No. 9, 1995, pp. 1308-1310. [38] K. I. Koizumi, Y. Watanable, K. Morita, N. Utoguchi and M.Matsumoto, “New method for preparing high-porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a subliming material,” International Jour- nal of Pharmaceutics, Vol. 152, No. 1, 1997, pp. 127- 131. [39] H. A. Lieberman, L. Lachman and J. B. Schwartz, “Pharmaceutical Dosage Forms: Tablets Vol. 2,” 2nd Edition, Marcel Dekker, New York, 1990, pp. 1-71. [40] Y. Bi, H. Sunada, Y. Yonezawa, K. Danjo, A. Otsuka and K. Iida, “Preparation and Evaluation of a Compressed Tablet Rapidly Disintegrating in the Oral Cavity,” Chem- ical and Pharmaceutical Bulletin, Vol. 44, No. 11, 1996, pp. 2121-2127. [41] R. C. Rowe, P. J. Sheskey and P. J. Weller, “Handbook of Pharmaceutical Excipients,” 4th Edition, Pharmaceutical Society of Great Britain, London, 2003. [42] K. A. Khan, “The Concept of Dissolution Efficiency,” Journal of Pharmacy and Pharmacology, Vol. 27, No. 1, 1975, pp. 48-49. |

