American Journal of Plant Sciences
Vol.07 No.03(2016), Article ID:65100,14 pages
10.4236/ajps.2016.73058
Germination Biology and Occurrence of Polyembryony in Two Forms of Cats Claw Creeper Vine, Dolichandra unguis-cati (Bignoniaceae): Implications for Its Invasiveness and Management
Joshua C. Buru1*, Kunjithapatham Dhileepan2, Olusegun O. Osunkoya2,3, Tanya Scharaschkin1*
1Earth, Environmental and Biological Sciences, Science and Engineering Faculty, Queensland University of Technology, Brisbane, Australia
2Department of Agriculture and Fisheries, Biosecurity Queensland, Eco-Sciences Precinct, Brisbane, Australia
3College of Marine and Environmental Sciences, James Cook University, Cairns, Australia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 11 February 2016; accepted 25 March 2016; published 29 March 2016
ABSTRACT
Cat’s claw creeper vine, Dolichandra unguis-cati (L.) Lohmann (syn. Macfadyena unguis-cati (L.) Gentry), is a major environmental weed in Australia. Two forms (“long” and “short” pod) of the weed occur in Australia. This investigation aimed to evaluate and compare germination behavior and occurrence of polyembryony (production of multiple seedlings from a single seed) in the two forms of the weed. Seeds were germinated in growth chambers set to 10/20˚C, 15/25˚C, 20/30˚C, 30/45˚C and 25˚C, representing ambient temperature conditions of the region. Germination and polyembryony were monitored over a period of 12 weeks. For all the treatments in this study, seeds from the short pod form exhibited significantly higher germination rates and higher occurrence of polyembryony than those from the long pod form. Seeds from the long pod form did not germinate at the lowest temperature of 10/20˚C; in contrast, those of the short pod form germinated under this condition, albeit at a lower rate. Results from this study could explain why the short pod form of D. unguis-cati is the more widely distributed form in Australia, while the long pod form is confined to a few localities. The results have implication in predicting future ranges of both forms of the invasive D. unguis-cati, as well as inform management decisions for control of the weed.
Keywords:
Macfadyena unguis-cati, Plant Sexual Reproduction, Plant Invasion, Propagule Pressure, Seed Ecology, Woody Vine

1. Introduction
Plant invasions result in environmental degradation [1] , heavy financial costs [2] and loss of biodiversity [3] . Understanding plant traits contributing to invasiveness may thus help in determining the best way to manage invasive species [4] . Many biotic and abiotic hypotheses have been proposed to explain why some species become invasive [5] - [7] . While species-specific traits (e.g. high specific leaf area, competitiveness, greater morphological and physiological plasticity than co-occurring non-invasive species, niche pre-emption, and release from natural enemies in the novel environment [8] [9] ) may be important in determining invasiveness, there is an increasing evidence that propagule pressure (size, number of individuals introduced, temporal and spatial patterns of arrival and establishment in a novel ecosystem) plays a major role in driving invasion success [10] - [12] .
Reproductive strategies of invasive plants also play a significant role at all the stages of the invasion process. Versatility in reproductive strategies ensures variable range of environments in which the invasive plants can spread and proliferate [6] . Time-to-germination initiation and rate of germination are measurable characteristics that can be used to predict the success of any species in a given environment [13] . Most plant species germinate optimally within a narrow range of environmental conditions, but the ability to germinate under different environmental conditions (i.e., germination plasticity) can be an adaptation to maximize fitness, especially for invasive species in novel environments [6] [12] . An important cue for seed germination is the ambient temperature, especially during periods of soil water availability [14] . The interactive effects of temperature and light conditions may also substantially influence germination and thus enhance the survival and establishment of the seedling stage [15] , perhaps through provision of synergistic environmental resources.
Some plant species exhibit a rare phenomenon of polyembryony, i.e., the formation of extranumerary embryos in single seeds [16] [17] . Polyembryony has been shown to further increase the propagule pressure of a species in novel environments [18] . Such embryos arise from either apomictic (asexual) or amphimictic (sexual) processes [19] . The occurrence of polyembryony is ascertained through emergence of multiple seedlings from a single seed during germination [20] . Although little is known about the ecological consequences of polyembryony [18] , any process that increases the number of individuals to the next generation is advantageous as it adds to the propagule pressure [10] . However, some evidence suggests that polyembryony may be disadvantageous due to competition between polyembryonic siblings from early developmental stages to seedling establishment (eg. [19] ). Although polyembryony is widely reported in angiosperms, it is prevalent in only a few families, including Myrtaceae, Cactaceae, Rutaceae, Anacardiaceae and Bignoniaceae [21] . In the family Bignoniaceae, polyembryony has been reported in Handroanthus ochraceus, H. chrysotrichus [22] , Anemopaegma acutifolium, A. arvense, A. glaucum and A. scabriusculum [20] .
Cat’s claw creeper vine, Dolichandra unguis-cati (L.) Lohmann (syn. Macfadyena unguis-cati (L.) Gentry) (Bignoniaceae) is a native of the Greater and Lesser Antilles, Mexico, South and Central America to Argentina, including Trinidad and Tobago [23] . It was introduced to Australia as an ornamental plant in the late 1800s, but has since naturalised and is considered as a major environmental weed [24] . D. unguis-cati has recently been listed as a Weed of National Significance (WoNS) in Australia [25] . It is regarded as an environmental weed in other parts of the world, such as southern and central Africa, Asia, North America and parts of Europe [26] and is included in the Global Invasive Species Database (GISD) [27] .
D. unguis-cati is a woody vine (liana) of riparian areas, where its mothers the tree canopies and can cause trees to collapse due to its immense biomass [28] . It also creates thick mats on forest floors that smother low vegetation and hamper seedling recruitment [24] . This growth pattern transforms natural habitats into monospecific stands, resulting in loss of floral biodiversity and changes in soil biota and chemistry [29] [30] . D. unguis-cati regenerates sexually, through the production of numerous papery seeds, and asexually (vegetatively) by production of subterranean tubers [24] [31] .
Two morphologically and phenologically distinct forms of D. unguis-cati occur in Australia [32] . These forms have been informally referred to as long pod (LP) and short pod (SP) plants based on their average fruit length at maturity (LP 70 cm; SP 30 cm). LP and SP have, on average, 120 and 61 seeds per fruit at maturity, respectively. The fruits are capsules but have been informally referred to as pods. Seeds of both forms are two-winged, papery and flattened/oblong in shape, 10 - 18 mm long, 4 - 6 mm wide. The average seed biomass is not significantly different between the two forms [32] . SP is the more prevalent form in Australia and occurs in eastern Queensland and northeast New South Wales, while LP is only known from a few isolated localities in southeast Queensland (Dhileepan K, per. observation; [33] ). SP is the form of D. unguis-cati that regarded as an environmental weed in different parts of the world [33] [34] . LP does not appear to be as invasive as SP as it occurs in only a few localities in southeast Queensland. However, the cause for this difference in the level of prevalence between the two forms is not known, and one potential cause could be differences in their seed biology.
Seed germination dynamics of both forms of D. unguis-cati have not been adequately studied. The only study on the seed bank ecology of the SP [35] found it to have low seed longevity, usually less than 12% and 1 year for soil-surface (<1 cm depth) and 1% for buried seeds (5 cm depth), respectively. The same study also inferred the occurrence of polyembryony to be approximately 40% due to emergence of multiple seedlings from single seeds [35] , but did not confirm the presence of polyembryony using established methods (e.g., radicle emergence and seedling separation) nor differentiate between different classes of polyembryony (e.g., twins, triplets). It is not known whether polyembryony occurs in LP and at what frequency. Interestingly a study from the native range, concluded that D. unguis-cati did not exhibit any polyembryony [20] .
The aims of this study were to determine whether there are differences in seed germination behaviour of the two forms of D. unguis-cati by 1) documenting the range of temperature and photo-regime over which seeds of will germinate; 2) confirming if polyembryony occurs in both forms; and 3) determining the frequency and classes of polyembryony in the two forms of the weed.
2. Materials and Methods
2.1. Acquisition and Storage of Seeds
Seeds of the long pod (LP) and the short pod (SP) forms of D. unguis-cati were collected during the fruiting months of 2013 from various sites around the greater Brisbane area in southeast Queensland, Australia. SP seeds were obtained from the following infestation sites: South Bank (27˚55'S, 153˚01'E), Ipswich Forest Reserve (27˚32'S, 152˚42'E), Chelmer (27˚47'S, 152˚58'E), Bardon (27˚30'S, 152˚41'E) and Boonah (27˚60'S, 152˚41'E). LP seeds plants were collected from Carindale (27˚30'S, 152˚41'E), Bardon (27˚30'S, 152˚41'E) and Sherwood (27˚30'S, 152˚59'E). Fewer sites were sampled for LP because this form is less prevalent than SP, and (and consequently fruit formation) does not occur every year [32] . Seeds were collected from fruits at the time of dehiscence to maturity of the seeds [24] . Once collected, seeds were stored for two weeks at room temperature in paper envelopes placed in containers with silica gel to ensure they remained dry before the commencement of germination assays. For the purposes of this experiment, seeds from different sites were pooled together to ensure adequate sample sizes, although we recognise that there could be differences in germination behaviour between individual plants and amongst sites for a given form.
2.2. Experimental Design
Seed germination: Seeds were physically screened to ensure they were firm and intact. Those that appeared not to have viable embryonic content and/or were damaged by insects were not included in the germination assays. Seeds were sterilised by soaking in 1% sodium hypochlorite (NaOCl) for 5 minutes, then rinsed in water for 3 minutes [14] . Sterilised seeds were placed in 15 cm diameter petri dishes lined with 2 - 3 layers of 15 cm Whatman filter paper (No. 1) moistened with distilled water. They were subsequently exposed to varying temperature regimes in growth chambers (model ADAPTIS A1000; Conviron Ltd., USA) at the Queensland University of Technology (QUT) in Brisbane, QLD-Australia. Germination conditions were set to 1) cool (10/20˚C), 2) moderate (15/ 25˚C), 3) warm (20/30˚C) and 4) hot (30/45˚C) temperature regimes for 12 hours at each alternate temperature. Seed germination took place in light/dark conditions (12-hour photo-period) or constant 24 hours dark. When a 12-hour photo-period was applied, the higher temperature corresponded with the presence of light. Most of the temperature regimes followed the conditions applied by Vivian-Smith and Panetta [35] and also reflect the night-day temperature fluctuations in the distribution range of D. unguis-catiin Australia. Two additional treatments included constant room temperature (25˚C) with 5) 12 hour light/dark and 24-hour dark. Fifteen (15) replicates of 20 seeds each were used for each pod form in each treatment. Germination data were recorded every seven (7) days for 12 weeks after which the assay was terminated due to logistic reasons, and because no more appreciable germination was observed. After the 12th week, the ungerminated seeds were physically checked and found to be mostly rotten with no visible viable embryo; testing the embryo of ungerminated seeds confirms loss of viability, except for those in the low temperature (10/20˚C). A dim light was used to examine the seed germination in the continuous darkness treatment.
Each seed was considered to have germinated with the emergence of one or more radicles [15] . Total germination percentage was calculated from the total number of germinated seeds divided by the total number of seeds. Germination rate index (GRI) was calculated following the Equation (1) of Maguire [36] ,
 , (1)
, (1)
where Ni represents germinated seeds on the ith day and Di is the number of days from the commencement of the germination assay to the ith day (see also [14] ). GRI was determined at six and 12 weeks for each treatment. Cumulative mean germination data (%) were plotted against time (weeks) from which the following indices were extracted: time-to-initiation of germination (T1), time to 50% (T50), and time to maximum germination (65% (T65)) in this study (see [13] ).
2.3. Estimation of Occurrence of Polyembryony
If only one radicle emerged during germination, seeds were considered mono-embryonic, but if two or more radicles were observed then the seeds were considered polyembryonic [20] . Total percentage polyembryony was calculated from the proportion of seeds showing two or more radicles at germination for a given treatment. Seeds showing polyembryony were grouped into classes (twins, triplets and quadruplets) depending on the number of radicles that emerged during germination [19] .
2.4. Confirmation of Polyembryony
Confirmation of polyembryony in the two forms of D. unguis-cati was further determined through seedling establishment [19] [20] . After germination, polyembryonic siblings with at least one pair of leaves were taken out of the germination petri-dishes and transferred into 20 cm plastic pots filled with locally available commercial soil (Osmocote Multi-Purpose Potting Mix with trace elements). Seedlings were left to grow in a light environment (range: 60 - 250 µmol∙m−2∙s−1) over a 1 year period to ascertain if the individual seedlings would establish independent of other siblings, and whether each sibling would develop separate roots and tubers or not. The same process was repeated for polyembryonic seedlings in which the siblings were physically separated.
2.5. Data Analysis
Germination percentage data were arcsine transformed before analysis to improve normality of residuals. Analysis of variance (ANOVA) was used to test the effects of light, temperature regimes, and pod form of D. unguis-cati, as well as their interactions on germination indices and occurrence of polyembryony. The form of D. unguis-cati, light, and temperature regimes were used as fixed effects on the ANOVA model. A Tukey HSD post-hoc test was performed to assess the germination and polyembryony differences between the temperature treatments. When no significant interactions were detected, a Pearson’s c2 statistical test was used to compare the frequency of polyembryonic seeds of LP and SP plant forms. All statistical tests were carried out at α < 0.05 using the R statistical program on R version 3.1.0 [37] .
3. Results
3.1. Temperature and Light Effects on Time-to-Start and Rate of Germination
At all the temperature regimes, the seeds of the short pod (SP) plant form showed rapid germination whilst those of the long pod (LP) form were gradual and slower (Table 1; Figure 1). Across the temperature range and photoperiod cycles tested, it took seeds of SP plants an average of 11.5 days to initiation of germination (T1), except for the cool 10/20˚C regime where up to 28 days was required to T1 (Table 1). It took seeds of LP plants significantly longer period (an average of 20.2 days) to initiation of germination (T1) across temperatures, other than 10/20˚C in which no germination was recorded (Figure 1(a)). The inhibitory effect of low temperatures (10/20˚C) on germination was more pronounced on seeds of the LP form than those of SP plants, since the former did not germinate at all at this temperature. When the experiment was terminated, seed germination in SP at 10/20˚C had reached about 42.9% (Figure 1(a)). However, the gradient of the curve indicate that had the experiment continued, more seeds would have germinated with time, and therefore a higher total germination percentage could have been attained at this temperature too.
Time to 50% germination (T50) was also significantly lower for seeds of SP plants (range: 14 - 34 days) when compared to those of LP plants (range: 30 - 84 days) across the temperature regimes tested (Table 1). Light did not have any significant effect on T1, T50 or T65 for either LP or SP plants at 20/30˚C (Figure 1(c)), but that was not the case at constant 25˚C for LP plants (Figure 1(d)). Light had a significant germination effect on T50 on LP plants at 25˚C, but not on seeds of SP plants. At 25˚C, it took LP 30 days to reach T50 under light conditions but >84 days to reach T50 in constant darkness (Table 1 and Figure 1(d)). In contrast, at 25˚C, T50 for SP was 14 - 16 days, irrespective of the light conditions.
The rate (speed) of germination estimated by Maguire’s germination rate index (GRI) showed significantly higher germination rates for seeds of SP plants than LP plants at all temperature regimes (Figure 2). The magnitude of the difference in germination rate between the two pod forms was also greater at 6 weeks compared to 12 weeks, especially for the moderate (15/25˚C) and warm (25˚C and 20/30˚C) temperature regimes (Figure 2(a), Figure 2(b) and Table 2). At 6 weeks, GRI values for all interaction effects (pod form x light; pod form x temperature, light x temperature, and form x light x temperature) were significant (P < 0.05; Table 2), suggesting that GRI response values for seeds of each plant pod form varied significantly depending on light and/or temperature conditions. In contrast, at 12-weeks, only the interaction effect of form x light was significant (F4, 218 = 9.05; P < 0.0001) (Table 2), implying a greater role of light than temperature regime on this germination index.
3.2. Occurrence and Frequency of Polyembryony in LP and SP
Polyembryony occurs in both plant forms of D. unguis-cati as demonstrated by emergence of two or more radicles from individual seeds during germination (Figure 3(a)). However, there was a significant difference in the
Table 1. Effect of temperature and light regimes on the time (days) to initiation of germination (T1), time to 50% (T50) and time to final (65% (T65)) germination for two forms of D. unguis-cati. These data were extracted from cumulative mean germination percentage curves plotted as a function of time. LL: Low light = 24 hour or constant darkness; HL: high light = 12 hour photoperiod; SP: short pod plants; LP: long pod. Data from the 30/45˚C temperature regime were not included in the table because germination incidents were minimal.
Figure 1. Cumulative mean germination percentage as a function of time for two forms of D. unguis-cati seeds at different regimes of 12 hour low/high temperature and photoperiod. LP: long pod; SP: short pod; LL: Low light = constant darkness; HL: High light conditions = 12 hour photoperiod. (a): 10/20˚C with constant darkness; (b): 15/25˚C with constant darkness; (c): 20/30˚C with both constant darkness and light conditions; (d): room temperature (25˚C) with both constant darkness and light conditions. Cumulative germination curves for the 30/45˚C temperature regime were not included because of insignificant germination incidents.
Figure 2. The effects of temperature on germination rate index (mean ± s.e.m) on two forms of D. unguis-cati, long pod (LP) and short pod (SP) plants. (a) Germination rate index at 6 weeks since start of germination; (b) Germination rate index at the end of the germination assay, i.e., 12 weeks since start of germination. Data for light and darkness levels were combined at each temperature regime because they were not significantly different. Arrows indicate no germination incidents by LP plants at 10/20˚C.
frequency of polyembryony between LP and SP plants (χ2 = 71.730, df = 1, p < 0.002). SP plants displayed a significantly higher frequency of polyembryony than LP plants (SP plant: 38.52% ± 2.74%; LP plant: 4.68% ± 1.13%) (see Figure 4 and Figure 5). The polyembryonic seedlings lack any connections and were easily separated from each other, with each shoot system detaching with a corresponding radicle or root system (Figure 6).
Table 2. Summary results of ANOVA showing effects of the form of D. unguis-cati, temperature and light regimes on the germination rate index (GRI) and total germination %. Significant effects are shown in bold.
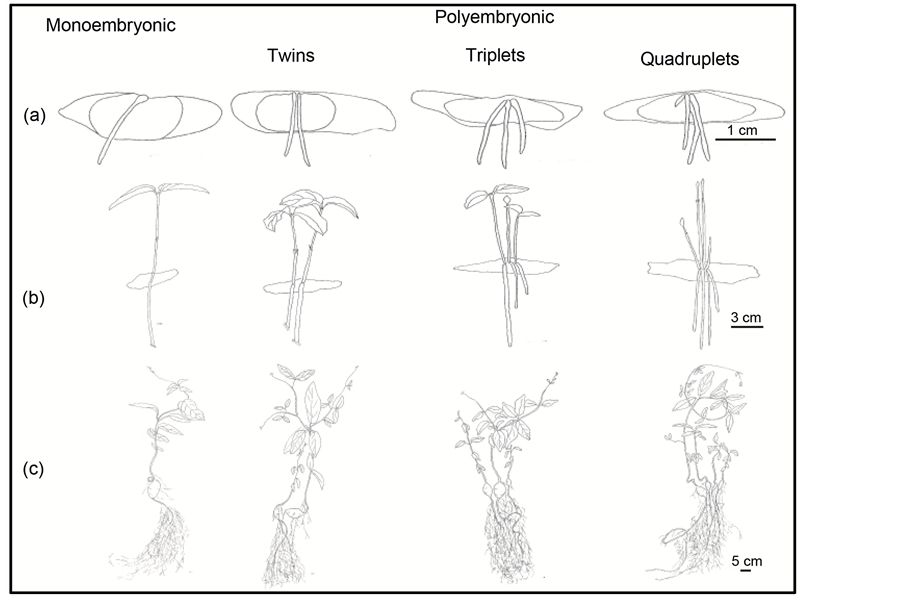
Figure 3. Illustrations of monoembryonic and polyembryonic seedlings of D. unguis-cati at different stages of development. Top row (a): Seeds showing emergence of radicles one week since start of germination. Middle row (b): Seedlings at 4 - 6 week since start of germination. Bottom row (c): One year old seedlings, with polyembryonic seedlings clearly showing independent development of tubers and root systems (Illustrations by Tanya Scharaschkin).
Figure 4. Frequency of different classes of polyembryony in the two forms of D. unguis-cati, long pod (LP) and short pod (SP) plants. Only twin seedlings were observed in LP polyembryonic seeds whereas SP also had triplet and quadruplet seedlings.
Figure 5. Comparison of the proportion of germination percentage (darkercolor) and polyembryony percentage (diagonal lines) of LP and SP plants for the same temperature regimes: 10/20˚C, 15/25˚C, 20/30˚C and room temperature (25˚C). Differences in polyembryony frequency between temperature regimes were not significant within each form of Dolichandra unguis-cati (F-value = 0.902, df = 1, p value = 0.345).
3.3. Classes of Polyembryony in LP and SP
Three classes (twin, triplets and quadruplets) of polyembryonic seedlings were observed in this study (Figure 3). All classes were observed in SP: single (60.4%; N = 628/1040), twin (25.5%; N = 265/1040), triplet (11.7%; N = 122/1040) and quadruplet (2.01%; N = 21/1040) (Figure 4). All polyembryonic seedlings in LP were twins, and constituted only 4.68% (N = 42/897) of germinated seeds (Figure 4).
3.4. Seedling Establishment of Polyembryonic Siblings
Polyembryonic seedlings were easily separated from each other, with each shoot system detaching with a corre
Figure 6. Multiple seedlings emerging from a single seed of D. unguis-cati four weeks since start of germination; (a)-(c): short pod (SP) and (d)-(f): long pod (LP). Intact seeds showing emergence of twin seedlings (a) (d); (Partial separation of seeds into two halves to separate the two seedlings from the same seed (b) (e); Complete separation of seedlings emerging from the same polyembryonic seed (c) (f). Scale bars represent 1 cm.
sponding radicle or root system (Figure 6). Separated polyembryonic seedlings were able to develop and establish individually when transplanted into growth media and developed their own root system with tubers (Figure 3(c)). When left intact and transferred to growth media, polyembryonic siblings still developed individually with independent root and tuber systems. We also observed that for each set of polyembryonic siblings, one seedling was more robust and had a larger subterranean tuber than that of other siblings (Figure 3).
3.5. Polyembryony and Germination Rates of LP and SP
This study established that SP plants exhibited higher germination indices and higher frequency of polyembryony than LP plants, irrespective of temperature regimes (Figure 5). There were no significant interaction effects of temperature and/or light regime on the occurrence, frequency or classes of polyembryony (temperature x plant form: F-ratio = 0.594, df = 1, p = 0.443; light x plant form: F-ratio = 0.021, df = 1, p = 0.886), implying that these environmental resources did not influence the dynamics of polyembryony. Nonetheless, both LP and SP plants showed their highest frequency of polyembryony (15.9% and 41.2%, respectively) at 15/25˚C (Figure 5). At 30/45˚C, only a few incidents (0.3%) of germination (and no incidence of polyembryony) was observed for SP and no germination whatsoever for LP.
4. Discussion
Our results indicate that, on average, there are significant differences in the seed germination responses of the two forms of the invasive woody vine, D. unguis-cati under varying environmental resources of temperature and light regimes. Seeds of SP exhibit higher mean value as well greater variation in its germination niche (from cool to warm temperatures) than those of LP plants (warm temperatures only) (Figure 1 and Figure 2). The polyembryony results confirm previous findings by [35] of occurrence of multiple seedlings from single seeds in SP. However, the two forms exhibit different frequencies of polyembryony: about 40% in SP plants and a much lower frequency (often <5%) in LP.
4.1. Predicting Invasiveness Potential of the Two Forms of D. unguis-cati
Our results suggest that germination niche requirements of SP are broad and non-specific, while LP germinates optimally only under warmer temperature conditions (20/30˚C and 25˚C; Figure 1; Table 1). Flexible germination cues enhance the invasive capacity of plants by enabling them to spread and establish in novel climatic conditions of recipient communities [38] [39] . SP plants had a much higher germination rate (compared to LP plants) at the cooler temperature regime of 10/20˚C. Equally, although of low frequency, seeds of SP plants showed evidence of germination incidents at hot, 30/45˚C temperature regime while there was no germination at all for LP plants under this scenario. These wider germination amplitudes may indicate greater resilience in SP seeds, and may suggest a potential for this form of D. unguis-cati to spread further into the cooler state of New South Wales and Victoria as well as into the warmer/hotter areas of Australia (e.g., Northern Territory and western Queensland), especially under a climate change scenario. In general, the rapid germination behaviour of seeds of SP seeds (Figure 1) is typical of invasive species [38] [40] . Whilst the longevity of seeds of SP seeds is low (<12% by 1 year) after dispersal [35] , its rapid germination under a wider range of temperatures may confer a fitness advantage in terms of seedling establishment and spread.
The higher frequency of polyembryony in seeds of SP plants could also allow it to proliferate successfully in a variable environment in contrast with those of LP plants (Figure 4 and Figure 5). Thus for the same number of initial seeds introduced in a new environment, there is a greater likelihood that more SP rather than LP plants would establish. Considering that twins, triplets and quadruplets occur in polyembryonic SP seeds, while only twins occur in LP (and at a lower frequency), it can be assumed that a higher propagule pressure would be exerted by SP than LP plants upon introduction. Polyembryony may also increase invasiveness potentials by the bet-hedging strategy, which ensures that at least one individual seedling from a polyembryonic seed survives [41] [42] . Although mature LP fruits are known to have twice as many seeds per fruit as SP fruits [32] , higher germination and polyembryony exhibited by SP may be likely to increase propagule pressure leading to SP plants being the more invasive form than LP. Propagule pressure has previously been correlated with plant invasiveness [43] - [45] . Nonetheless, caution is needed in the interpretation of this finding because the polyembryony phenomenon may not necessarily be adaptive as it puts siblings into direct competition for environmental resources [18] [42] .
Some evidence suggests that polyembryony reduces seed germinability significantly [19] , but our results do not support this position. SP consistently had higher germination rates than LP at all temperature regimes whilst also exhibiting higher frequency of polyembryony than LP. Temperature did not significantly affect expression of polyembryony, suggesting a genetic basis for the phenomenon [46] , but both LP and SP appeared to show relatively higher polyembryony frequencies at 15/25˚C (their optimal growth condition) than at other temperature regimes. Our preliminary observations of 1-year old siblings from polyembryonic seeds indicate equal survival rates, but slower mean growth rates per individual as the number of siblings increase (Figure 3; JC Buru, unpublished data). However, it remains to be seen how differences in germination and polyembryony rates will translate to initial biomass gain per individual, and ultimately offspring fitness.
The differences in the invaded range distributions of the two forms could be a product of colonization events, with the LP form being a more recent arrival in Australia compared to the SP form. There may also be an underlying genetic basis responsible for the observed pattern [47] . Although largely untested, and therefore speculative, the prevalence of SP in Australia may potentially be a classic case of (i) recent whole genome duplication followed by diploidization during which genes are lost/modified/rearranged [48] [49] , and/or (ii) release from natural enemies in the novel environment. Both scenarios have the tendency to increase competitiveness, niche pre-emption, and ultimately spread and distribution [5] [50] [51] .
4.2. LP and SP: Are They the Same Species?
The differences in germination dynamics and frequency of polyembryony between SP and LP plants lends further credence to suggestions that the two forms of D. unguis-cati could be two extremes of the same species or even different species [33] . Anecdotal evidence suggests that the predominant form of D. unguis-cati in the native range is similar in appearance to the form being referred to as LP plants in Australia (Dhileepan K. personal comm.). Interestingly, an earlier study conducted within the native range found that D. unguis-cati did not exhibit any polyembryony [20] . This is in sharp contrast to what we have observed in SP plants (40%), but is closer to our results for LP plants (<5%). Whether the two forms are different species, products of two independent introductions from the native range of the weed, or arose from (auto-/allo-) polyploidy remains to be determined. Only a comprehensive phylogenetic study of the different forms of the species and other members of the genus Dolichandra will help clarify the status of the two forms (but see [34] ). Moreover, work on chromosome number (karyotype), level of polyploidy (a trait known to correlate significantly with polyembryony [20] [47] ), as well understanding the interplay of their breeding systems (sexual vs. asexual) variation [31] , will also help our understanding of their invasiveness potential.
5. Conclusions and Recommendations
The current study is one of the first to report on the comparative germination rates of the two forms of D. unguis-cati in Australia. Further germination assays could involve other conditions such as different moisture thresholds and seed burial and retrieval experiments to explore the extent and contribution of above environmental cues to variation in invasiveness of the two plant forms. To ascertain the ecological consequence (fitness) of polyembryony on D. unguis-cati, future studies should consider comparing growth rates of mono- and poly- embryonic embryonic seedlings in intra- and inter-specific competition. Due to the vast differences in the germination behaviour (this study), floral [25] and leaf morphological/physiological traits of the two forms of D. unguis-cati ( [33] ; Buru J, unpublished data), different control strategies should be considered for these two forms. Currently, the same chemical and biological control strategies are used to manage the two forms, thus potentially compromising the efficacy of these control options. Leaf-feeding biocontrol agents have been released [25] [52] [53] , and they are applied to both LP and SP forms. However, whether these agents are equally effective in controlling both forms is yet to be determined. The need for additional fruit- or seed-eating biocontrol agents has been previously highlighted by [31] and is further supported by the current findings. In light of the current study findings, we have demonstrated potential roles of propagule pressure and polyembryony as drivers of spread of D. unguis-cati. The efficacy of fruit- or seed-attacking agents as a biocontrol strategy should be considered for both forms of the weed, but perhaps more so for SP, given the results of this study. It will be well worth investigating if the same germination dynamics and frequency of polyembryony are observed in D. unguis-cati in other parts of its native and invaded ranges, as it could shed light on the role played by propagule pressure in the spread of weeds.
Acknowledgements
We acknowledge technical assistance provided by Mark Crase and Amy Carmichael (QUT) and thank Liz Snow of the Department of Agriculture and Fisheries (DAF) in Brisbane for helping with seed collection. Thanks to the Plant Structure and Systematics Research Group at QUT and Dr. Jennifer Firn for providing valuable feedback on an earlier version of the manuscript. We thank two anonymous reviewers for constructive comments on an earlier version of this manuscript. The first author was funded by a scholarship from the Government of Botswana and DAF (Queensland, Australia) while performing this research.
Cite this paper
Joshua C. Buru,Kunjithapatham Dhileepan,Olusegun O. Osunkoya,Tanya Scharaschkin, (2016) Germination Biology and Occurrence of Polyembryony in Two Forms of Cats Claw Creeper Vine, Dolichandra unguis-cati (Bignoniaceae): Implications for Its Invasiveness and Management. American Journal of Plant Sciences,07,657-670. doi: 10.4236/ajps.2016.73058
References
- 1. Pysek, P. and Richardson, D.M. (2010) Invasive Species, Environmental Change and Management, and Health. Annual Review of Environment and Resources, 35, 25-55.
http://dx.doi.org/10.1146/annurev-environ-033009-095548 - 2. Pimentel, D., Zuniga, R. and Morrison, D. (2005) Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Eco-logical Economics, 52, 273-288.
http://dx.doi.org/10.1016/j.ecolecon.2004.10.002 - 3. Wilson, E.O. (1989) Threats to Biodiversity. Scientific American, 261, 108-116.
http://dx.doi.org/10.1038/scientificamerican0989-108 - 4. Burns, J.H. (2004) A Comparison of Invasive and Non-Invasive Dayflowers (Commelinaceae) across Experimental Nutrient and Water Gradients. Diversity and Distributions, 10, 387-397.
http://dx.doi.org/10.1111/j.1366-9516.2004.00105.x - 5. Keane, R.M. and Crawley, M.J. (2002) Exotic Plant Invasions and the Enemy Release Hypothesis. Trends in Ecology and Evolution, 17, 164-170.
http://dx.doi.org/10.1016/S0169-5347(02)02499-0 - 6. Baker, H.G. (1974) The Evolution of Weeds. Annual Review of Ecology and Systematics, 5, 1-24.
http://dx.doi.org/10.1146/annurev.es.05.110174.000245 - 7. Blossey, B. and Notzold, R. (1995) Evolution of Increased Competitive Ability in Invasive Nonindigenous Plants: A Hypothesis. Journal of Ecology, 83, 887-889.
http://dx.doi.org/10.2307/2261425 - 8. Callaway, R.M. and Ridenour, W.M. (2004) Novel Weapons: Invasive Success and the Evolution of Increased Competitive Ability. Frontiers in Ecology and the Environment, 2, 436-443.
http://dx.doi.org/10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2 - 9. Osunkoya, O.O., Bayliss, D., Panetta, F.D. and Vivian-Smith, G. (2010) Leaf Trait Co-Ordination in Relation to Construction Cost, Carbon Gain and Resource-Use Efficiency in Exotic Invasive and Native Woody Vine Species. Annals of Botany, 106, 371-380.
http://dx.doi.org/10.1093/aob/mcq119 - 10. Catford, J.A., Jansson, R. and Nilsson, C. (2009) Reducing Redundancy in Invasion Ecology by Integrating Hypotheses into a Single Theoretical Framework. Diversity and Distributions, 15, 22-40.
http://dx.doi.org/10.1111/j.1472-4642.2008.00521.x - 11. Simberloff, D. (2009) The Role of Propagule Pressure in Biological Invasions. Annual Review of Ecology, Evolution, and Systematics, 40, 81-102.
http://dx.doi.org/10.1146/annurev.ecolsys.110308.120304 - 12. Lockwood, J.L., Cassey, P. and Blackburn, T. (2005) The Role of Propagule Pressure in Explaining Species Invasions. Trends in Ecology & Evolution, 20, 223-228.
http://dx.doi.org/10.1016/j.tree.2005.02.004 - 13. Soltani, A., Galeshi, S., Zeinali, E. and Latifi, N. (2002) Germination, Seed Reserve Utilization and Seedling Growth of Chickpea as Affected by Salinity and Seed Size. Seed Science and Technology, 30, 51-60.
- 14. Mijani, S., Nasrabadi, S.E., Zarghani, H. and Abadi, M.G. (2013) Seed Germination and Early Growth Responses of Hyssop, Sweet Basil and Oregano to Temperature Levels. Notulae Scientia Biologicae, 5, 462-467.
- 15. Baskin, C.C. and Baskin, J.M. (2001) Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. Elsevier, New York.
- 16. Trapero, C., Barranco, D., Martín, A. and Díez, C.M. (2014) Occurrence and Variability of Sexual Polyembryony in Olive Cultivars. Scientia Horticulturae, 177, 43-46.
http://dx.doi.org/10.1016/j.scienta.2014.07.015 - 17. Webber, J. (1940) Polyembryony. The Botanical Review, 6, 575-598.
http://dx.doi.org/10.1007/BF02919556 - 18. Blanchard, M.L., Barney, J.N., Averill, K.M., Mohler, C.L. and DiTommaso, A. (2010) Does Polyembryony Confer a Competitive Advantage to the Invasive Perennial Vine Vincetoxicum rossicum (Apocynaceae)? American Journal of Botany, 97, 251-260.
http://dx.doi.org/10.3732/ajb.0900232 - 19. Mendes-Rodrigues, C., Sampaio, D.S., Costa, M.E., de Souza Caetano, A.P., Ranal, M.A., Júnior, N.S.B. and Oliveira, P.E. (2012) Polyembryony Increases Embryo and Seedling Mortality but Also Enhances Seed Individual Survival in Handroanthus Species (Bignoniaceae). Flora-Morphology, Distribution, Functional Ecology of Plants, 207, 264-274.
http://dx.doi.org/10.1016/j.flora.2011.10.008 - 20. Firetti-Leggieri, F., Lohmann, L., Alcantara, S., Costa, I. and Semir, J. (2013) Polyploidy and Polyembryony in Anemopaegma (Bignonieae, Bignoniaceae). Plant Reproduction, 26, 43-53.
http://dx.doi.org/10.1007/s00497-012-0206-3 - 21. Ganeshaiah, K., Shaanker, R.U. and Joshi, N.V. (1991) Evolution of Polyembryony: Consequences to the Fitness of Mother and Offspring. Journal of Genetics, 70, 103-127.
http://dx.doi.org/10.1007/BF02927810 - 22. Bittencourt Jr., N.S. and Moraes, C.I.G. (2010) Self-Fertility and Polyembryony in South American Yellow Trumpet Trees (Handroanthus chrysotrichus and H. ochraceus, Bignoniaceae): A Histological Study of Postpollination Events. Plant Systematics and Evolution, 288, 59-76.
http://dx.doi.org/10.1007/s00606-010-0313-2 - 23. Gentry, A.H. (1976) Bignoniaceae of Southern Central America: Distribution and Ecological Specificity. Biotropica, 8, 117-131.
http://dx.doi.org/10.2307/2989632 - 24. Downey, P. and Turnbull, I. (2007) The Biology of Australian Weeds. 48. Macfadyena unguis-cati (L.) Ah Gentry. Plant Protection Quarterly, 22, 82-91.
- 25. Dhileepan, K. (2012) Macfadyena unguis-cati (L.) Ah Gentry-Cat’s Claw Creeper. In: Julien, M., McFadyen, R. and Cullen, J., Eds., Biological Control of Weeds in Australia, CSIRO Publishing, Melbourne, 351-359.
- 26. Dhileepan, K., Taylor, D.B., Lockett, C. and Treviño, M. (2013) Cat’s Claw Creeper Leaf-Mining Jewel Beetle Hylaeogena Jureceki Obenberger (Coleoptera: Buprestidae), a Host-Specific Biological Control Agent for Dolichandra unguis-cati (Bignoniaceae) in Australia. Australian Journal of Entomology, 52, 175-181.
http://dx.doi.org/10.1111/aen.12014 - 27. De Poorter, M. and Browne, M. (2005) The Global Invasive Species Database (Gisd) and International Information Exchange: Using Global Expertise to Help in the Fights against Invasive Alien Species. Plant Protection and Plant Health in Europe: Introduction and Spread of Invasive Species, Berlin, 9-11 June 2005, 49-54.
- 28. Osunkoya, O.O., Pyle, K., Scharaschkin, T. and Dhileepan, K. (2009) What Lies Beneath? The Pattern and Abundance of the Subterranean Tuber Bank of the Invasive Liana Cat’s Claw Creeper, Macfadyena unguis-cati (Bignoniaceae). Australian Journal of Botany, 57, 132-138.
http://dx.doi.org/10.1071/BT09033 - 29. Batianoff, G. and Butler, D. (2003) Impact Assessment and Analysis of Sixty-Six Priority Invasive Weeds in South-East Queensland. Plant Protection Quarterly, 18, 11-17.
- 30. Osunkoya, O.O., Polo, C. and Andersen, A.N. (2011) Invasion Impacts on Biodiversity: Responses of Ant Communities to Infestation by Cat’s Claw Creeper Vine, Macfadyena unguis-cati (Bignoniaceae) in Subtropical Australia. Biological Invasions, 13, 2289-2302.
http://dx.doi.org/10.1007/s10530-011-0040-9 - 31. Perrett, C., Osunkoya, O.O. and Clark, C. (2012) Cat’s Claw Creeper Vine, Macfadyena unguis-cati (Bignoniaceae), Invasion Impacts: Comparative Leaf Nutrient Content and Effects on Soil Physicochemical Properties. Australian Journal of Botany, 60, 539-548.
http://dx.doi.org/10.1071/BT12055 - 32. Shortus, M. and Dhileepan, K. (2011) Two Varieties of the Invasive Cat’s Claw Creeper, Macfadyena unguis-cati (Bignoniaceae) in Queensland, Australia. Proceedings of the Royal Society of Queensland, 116, 13-20.
- 33. Boyne, R.L., Harvey, S.P., Dhileepan, K. and Scharaschkin, T. (2013) Variation in Leaf Morphology of the Invasive Cat’s Claw Creeper, Dolichandra unguis-cati (Bignoniaceae). Australian Journal of Botany, 61, 419-423.
http://dx.doi.org/10.1071/BT13063 - 34. Prentis, P.J., Sigg, D.P., Raghu, S., Dhileepan, K., Pavasovic, A. and Lowe, A.J. (2009) Understanding Invasion History: Genetic Structure and Diversity of Two Globally Invasive Plants and Implications for Their Management. Diversity and Distributions, 15, 822-830.
http://dx.doi.org/10.1111/j.1472-4642.2009.00592.x - 35. Vivian-Smith, G. and Panetta, F.D. (2004) Seedbank Ecology of the Invasive Vine, Cat’s Claw Creeper (Macfadyena Unguis-Cati (L.) Gentry). In: Sindel, B.M. and Johnson, S.B., Eds., Proceedings of the 14th Australian Weeds Conference, Weed Society of New South Wales, Sydney, 531-537.
- 36. Maguire, J.D. (1962) Speed of Germination—Aid in Selection and Evaluation for Seedling Emergence and Vigor. Crop Science, 2, 176-177.
http://dx.doi.org/10.2135/cropsci1962.0011183X000200020033x - 37. R Development Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
http://www.r-project.org - 38. Wainwright, C.E. and Cleland, E.E. (2013) Exotic Species Display Greater Germination Plasticity and Higher Germination Rates than Native Species across Multiple Cues. Biological Invasions, 15, 2253-2264.
http://dx.doi.org/10.1007/s10530-013-0449-4 - 39. Dechoum, M., Zenni, R., Castellani, T., Zalba, S. and Rejmánek, M. (2015) Invasions across Secondary Forest Successional Stages: Effects of Local Plant Community, Soil, Litter, and Herbivory on Hovenia Dulcis Seed Germination and Seedling Establishment. Plant Ecology, 216, 823-833.
http://dx.doi.org/10.1007/s11258-015-0470-z - 40. Ferreras, A.E., Funes, G. and Galetto, L. (2015) The Role of Seed Germination in the Invasion Process of Honey Locust (Gleditsia Triacanthos L., Fabaceae): Comparison with a Native Confamilial. Plant Species Biology, 30, 126-136.
http://dx.doi.org/10.1111/1442-1984.12041 - 41. Ladd, D. and Cappuccino, N. (2005) A Field Study of Seed Dispersal and Seedling Performance in the Invasive Exotic Vine Vincetoxicum rossicum. Botany, 83, 1181-1188.
- 42. Hotchkiss, E.E., DiTommaso, A., Brainard, D.C. and Mohler, C.L. (2008) Survival and Performance of the Invasive Vine Vincetoxicum rossicum (Apocynaceae) from Seeds of Different Embryo Number under Two Light Environments. American Journal of Botany, 95, 447-453.
http://dx.doi.org/10.3732/ajb.95.4.447 - 43. Moravcová, L., Pysek, P., Jarosík, V. and Pergl, J. (2015) Getting the Right Traits: Reproductive and Dispersal Characteristics Predict the Invasiveness of Herbaceous Plant Species. PLoS ONE, 10, e0123634.
http://dx.doi.org/10.1371/journal.pone.0123634 - 44. Pysek, P. and Richardson, D.M. (2007) Traits Associated with Invasiveness in Alien Plants: Where Do We Stand? In: Nentwig, W., Ed., Biological Invasions, Springer, Berlin, 97-125.
http://dx.doi.org/10.1007/978-3-540-36920-2_7 - 45. van Kleunen, M., Dawson, W. and Maurel, N. (2015) Characteristics of Successful Alien Plants. Molecular Ecology, 24, 1954-1968.
http://dx.doi.org/10.1111/mec.13013 - 46. Batygina, T. and Vinogradova, G.Y. (2007) Phenomenon of Polyembryony. Genetic Heterogeneity of Seeds. Russian Journal of Developmental Biology, 38, 126-151.
http://dx.doi.org/10.1134/S1062360407030022 - 47. Hollister, J.D. (2015) Polyploidy: Adaptation to the Genomic Environment. New Phytologist, 205, 1034-1039.
http://dx.doi.org/10.1111/nph.12939 - 48. Hollister, J.D., Arnold, B.J., Svedin, E., Xue, K.S., Dilkes, B.P. and Bomblies, K. (2012) Genetic Adaptation Associated with Genome-Doubling in Autotetraploid Arabidopsis arenosa. PLoS Genetics, 8, e1003093.
http://dx.doi.org/10.1371/journal.pgen.1003093 - 49. Otto, S.P. and Whitton, J. (2000) Polyploid Incidence and Evolution. Annual Review of Genetics, 34, 401-437.
http://dx.doi.org/10.1146/annurev.genet.34.1.401 - 50. Mitchell, C.E. and Power, A.G. (2003) Release of Invasive Plants from Fungal and Viral Pathogens. Nature, 421, 625-627. http://dx.doi.org/10.1038/nature01317
- 51. Elton, C.S. (1958) The Ecology of Invasions by Plants and Animals. Methuen, London, 18.
http://dx.doi.org/10.1007/978-1-4899-7214-9 - 52. Dhileepan, K., Snow, E., Rafter, M., Treviño, M., McCarthy, J. and Wilmot Senaratne, K. (2007) The Leaf-Tying Moth Hypocosmia pyrochroma (Lep., Pyralidae), a Host-Specific Biological Control Agent for Cat’s Claw Creeper Macfadyena unguis-cati (Bignoniaceae) in Australia. Journal of Applied Entomology, 131, 564-568.
http://dx.doi.org/10.1111/j.1439-0418.2007.01208.x - 53. Dhileepan, K., Treviño, M., Bayliss, D., Saunders, M., Shortus, M., McCarthy, J., Snow, E. and Walter, G. (2010) Introduction and Establishment of Carvalhotingis visenda (Hemiptera: Tingidae) as a Biological Control Agent for Cat’s Claw Creeper Macfadyena unguis-cati (Bignoniaceae) in Australia. Biological Control, 55, 58-62.
http://dx.doi.org/10.1016/j.biocontrol.2010.06.016
NOTES
*Corresponding author.