American Journal of Plant Sciences
Vol.5 No.9(2014), Article ID:44882,9 pages DOI:10.4236/ajps.2014.59147
Allelopathic Effects of Argemone mexicana to Growth of Native Plant Species
Hassan S. Namkeleja, Mokiti T. C. Tarimo, Patrick A. Ndakidemi*
School of Life Sciences and Bioengineering, The Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
Email: *ndakidemipa@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 8 January 2014; revised 15 March 2014; accepted 3 April 2014
ABSTRACT
Argemone mexicana is known to have significant effects on cultivated agricultural fields. However, there is little information about allelopathic effect of A. mexicana on the growth of wild plant species such as those found in wildlife protected areas. This review presents evidence that allelochemicals present in A. mexicana may affect the overall growth of other plant species.
Keywords
Allelochemicals; Chlorophyll; Ecosystems; Growth

1. Introduction
Invasive alien species are broadly defined as those species that are not native to an area and that may displace or otherwise adversely affect native plant and animal species [1] . Invasive alien species have become a major threat to global biodiversity and this is ranked second position to habitat destruction [2] . The threat posed by invasive species affects natural and managed ecosystem globally with Tanzania not being an exception.
Alien invasive plant species threaten the integrity of agricultural and natural ecosystems throughout the world by displacing native species and establishing mono-species in new habitat [3] -[5] . Competitive ability of invasive species over native species is explained by various hypothesis which includes release from natural enemies that hold them in check and make them free to utilize their full competitive potential [3] [6] , evolution of increased competitive ability [7] [8] , phenotypic plasticity that helps invasive plants to adapt to novel environments and compete against native plants in recipient communities [9] and the production of allelopathic compounds [3] [4] [10] . Plants can affect neighbouring plants by producing and releasing chemicals into the environment [11] . The Austrian plant physiologist Hans Molish named this phenomenon, “allelopathy” in 1937. Allelopathy refers to the effects of one plant on another plant or organisms through the release of chemicals into the environment [4] [12] . These chemicals (allelochemicals) are classified as secondary metabolites and are produced as offshoots of the primary metabolic pathways in plants [13] . Allelopathic effect of some invasive species is stronger on other species in introduced ranges than in native ranges because in new habitat species may not be as adapted to specific allelochemicals of invaders as species do in the native range [10] . Most of invasive plant species has competitive and defensive characteristics which accounts to allelopathic impact [3] [10] [14] . Allelochemicals also affect native species through different pathways that includes interruption of plants nutrients uptake, change in membrane permeability [15] , interference in cell division and elongation in roots and shoots [16] -[18] , interference in chlorophyll formation [19] , protein synthesis inhibition [15] [20] and change or inactivate the activity and functions of certain hormones and enzymes [15] [21] .
Allelochemicals from plants are released into the environment by exudation from roots, leaching from stems and leaves or decomposition of plant material [22] . Allelopathic effects can be stimulatory or inhibitory, depending on the identity of the active compound on the static and dynamic availability, persistence and fate of organics in the environment and on the particular target species [23] . Allelopathy has been increasingly recognized as an important ecological mechanism which influences plant dominance, succession, formation of plant communities and climax vegetation and crop productivity [24] . Although A. mexicana is allelopathic and invasive alien plant in Tanzania but there is little information about its effect on germination, growth and chlorophyll content of wild plant species used for pasture by livestock and wildlife in agricultural fields and natural ecosystems.
2. Allelopathic Potential of Argemone mexicana
2.1. Description of Argemone mexicana
Argemone mexicana L. a weed native to Central America (Mexico) is one of invasive alien plant species in Tanzania with allelopathic behaviour [25] . A. mexicana is a common herb plant found in most places by road sides, agricultural fields and natural ecosystem in Tanzania. A. mexicana belonging to the family papaveraceae is a widely distributed plant throughout the tropical and subtropical regions of the world. A. mexicana is an annual herb, up to 150 cm tall with a slightly branched tap root [26] . The stem is erect, branched, usually prickly, pale bluish-green and exudes an unpleasant smelling yellow sap when cut. Leaves are alternate, without petioles, more or less sheathing the stem, up to 15 cm long, deeply lobed with irregularly toothed, spiny margins; greyish white veins are conspicuous on the bluish green upper surface of the leaves [27] .
It has varying physiology of seed production and germination whereby it can produce seed at an average of 60 to 90 capsules per plant with 300 to 400 seeds in each capsule [28] . Seeds are dormant when shed and after ripening for several weeks or months [29] . Most seeds fall around the base of the parent plant where they form a carpet of seedlings. Most seeds do not normally germinate the year after shedding. Instead they enter the seed bank and seedlings establish, even in well maintained field, probably for many years [25] [29] . Dispersal occurs in surface water and in mud adhering to farm machinery and the feet of man and livestock [30] . Chemical investigations of this plant have revealed the presence of alkaloids, amino acids, phenolics and fatty acids [31] . Therefore some of chemicals in A. mexicana might have allelopathic potential that may affect other plants in their vicinity.
2.2. Allelochemicals in Argemone mexicana
Interaction between plants can be facilitated through release of allelochemicals from donor plants which then influence germination, growth, development, and establishment of receptor plants [16] . These processes play an important role on the determination of vegetation pattern and it is an invasive strategy used by many invasive plant species. In natural ecosystems, allelochemicals produced by invasive plants can inhibit the growth of competing native species through direct or indirect means, thereby providing the invader with a competitive advantage [3] [32] [33] . Phenolic compounds including p-hydroxybenzoic acid, vanillic acid and salicylic acid (Figure 1) are the major allelochemicals of A. mexicana [34] . Phenolic compounds are amongst the most widespread plant secondary metabolites which are of great significance in plant metabolism like defence against ultraviolet radiation or aggression by pathogens [21] . Phenolic allelochemicals have been observed in both natural and managed ecosystems, where they cause ecological and economic problems, such as decline in crop yield due to soil sickness, regeneration failure of natural forests, and replanting problems in orchards [15] .
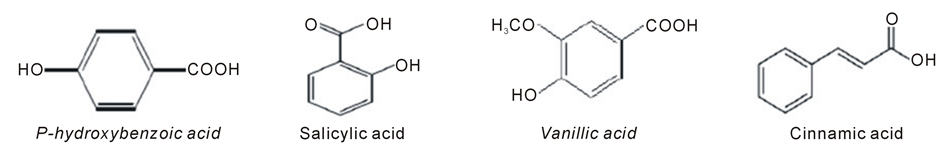
Figure 1. Chemical structures of phenolic allelochemicals found in Argemone mexicana [35] .
2.2.1. 4-Hydroxybenzoic Acid (p-Hydroxybenzoic Acid)
p-hydroxybenzoic acid is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. The benzoic acid derivatives produced by higher plants have been frequently implicated in allelopathy [36]. p-hydroxybenzoic interferes plant-water balance as one mechanism to reduce plant growth. According to Chen et al. [19] p-hydroxybenzoic acid affects physiological characteristic of plants in their vicinity and hence reduces chlorophyll content, rate of photosynthesis and root activity. Barkosky and Einhellig [37] reported that soybean growth, stomatal conductance and water potential were reduced with application of high concentrations of p-hydroxybenzoic acid.
2.2.2. 2-Hydroxybenzoic Acid (Salicylic Acid)
Salicylic acid (SA) is a phenolic phytohormone and is found in plants with roles in plant growth and development, photosynthesis, transpiration, ion uptake, transport and induces specific changes in leaf anatomy and chloroplast structure [38] [39] . Salicylic acid plays many roles in plant physiology including pathogenesis related resistance response to abiotic stress [40] by inducing the production of pathogenesis related proteins [41] such as antioxidant enzymes and heat shock protein [42] . It is involved in the Systemic Acquired Resistance (SAR) in which a pathogenic attack on one part of the plant induces resistance in other parts [43] . The signal can also move to nearby plants by salicylic acid being converted to the volatile ester, methyl salicylate. Despite Salicylic acid being one of the important phenolic compound in plants it has also been reported as allelopathic chemical [15] . Effects of salicylic acid on seed germination, seedling growth, and flowering and biochemical activities were studied out in four cowpea (Vigna unguiculata) genotypes by Chandra et al. [44] and found that both germination and seedling growth were negatively affected. Salicylic acid inhibited growth of soyabean (Glycine max L.) seedling and stable carbon isotope ratio (13C:12C) in tissue of treated plants was higher than control, indicating that salicylic acid caused a water stress [45] . Hence, interference with plant-water relationships is one mechanism whereby this allelochemical inhibits plant growth. In some cases salicylic acid is used as stimulator to decrease adverse effect of allelopathic components of some plant species on germination properties of another species. Saberi et al. [46] studied the influence of salicylic acid in decreasing of allelopathic effect of Eucalyptus camaldulensis on germination properties of Onobrychis sativa and concluded that early seedling growth of Onobrychis sativa increased by pre-treatment of seeds in salicylic acid.
2.2.3. 4-Hydroxy-3-Methoxybenzoic Acid (Vanillic Acid)
Allelopathic potential of vanillic acid reduced soybean growth, stomatal conductance and water potential [37] . Ghareib et al. [47] evaluated allelopathic potential of vanillic acid on tomato and found that at low concentrations of vanillic acid stimulated the germination and growth of tomato and had stimulating effects on the activity of some antioxidant enzymes while at highest concentrations exerted negative effects on all the measured parameters. Inhibition effect on seed germination and seedling growth by vanillic acid on Barnyardgrass (Echinochloa crus-galli L) eggplant were also reported by Esmaeili et al. [48] and Chen et al., [49] . Since A. mexicana contain vanillic acid, there is a possibility of inhibiting germination and growth of some grass and leguminous species growing in natural habitats.
2.2.4. (d) (E)-3-Phenylprop-2-Enoic Acid (Cinnamic Acid)
Cinnamic Acid (CA) is a widespread phenolic acid released into soil by root exudates, leaf leachates and decomposed plant tissues of different plants such as cucumber [50] and alfalfa [51] . Cinnamic acid is among of phenolic compounds with allelopathic effect that inhibits the germination and growth when applied exogenously [52] . Cinnamic Acid is an allelochemical responsible for allelopathy in root growth in cucumber [53] ; shoot and root length, fresh and dry weight of Cabbage (Brassica oleracea var. capitata) seedlings [52] . At high concentration cinnamic acid posed allelopathic effect on verticillium wilt (V. dahliae) and eggplant seedling growth [49] . Cinnamic acid is also found in A. mexicana and might affect the germination and growth of other plants in their vicinity.
2.3. Allelopathic Mechanisms of Phenolics
Allelochemicals changes membrane permeability and inhibit plant nutrient uptake [15] . Cell membrane permeability can be increased by phenolic allelochemicals resulting to spill of cell contents, increased lipid peroxidation and hence slow growth or death of plant tissue [16] . Inhibition of plants from absorbing nutrients from surroundings affects the normal growth of plants. Phenolic allelochemicals may inhibit cell division, elongation, and sub microscopic structure and consequently interferes with the normal growth and development of the whole plant [17] [54] . Disruption of amino acid metabolism is another important mode of action for some allelochemicals [15] [20] . On another hand, phenolic allelochemicals can change the activity and functions of certain enzymes [15] . Some of phenolic allelochemicals reduce or inactivate the physiological activity of plant hormones which may then inhibit the normal physiological process of plants [21] . Hence, the contribution of phenolic compounds to allelopathy is probably not due to a single substance; there are a series of physiological and biochemical changes in plants induced by phenolic compounds. Hence phenolic compounds present in A. mexicana may affect germination, seedling growth, fresh weight and chlorophyll contents of target species.
3. Effects of Allelochemicals on Seed Germination, Seedling Growth and Chlorophyll Contents
3.1. Effects on Seed Germination
Many allelopathic chemicals have more dramatic effects on seed germination than on the growth and viability of matured plants [20] . The allelopathic effects on seed germination are related to the types and concentrations of allelochemicals, species of recipient plants and environmental conditions [55] . Seed germination inhibition by allelochemicals is associated with changes in physiological and biochemical processes necessary for seed germination. Disruption of mitochondrial respiration is one of the mechanisms used by seed to inhibit seed germination [20] . During seed germination, there is a rapid increase in glycolytic activity (Glycolysis) linked to an increased rate of respiration [56] . Glycolytic activity is necessary to mobilize stored carbohydrates to provide the seed with the reducing power, ATP, and carbon products required for the biosynthesis of the roots and aerial parts of the emerging seedling [20] . Therefore, allelochemicals may disrupt activity of metabolic enzymes that are involved in glycolysis [57] . However, all kinds of allelochemicals can affect seed germination through affecting seed cell membrane permeability, cell division and differentiation, protein synthesis, gene expression, and hormone synthesis and equilibrium [55] . Paul and Begum [58] reported that aqueous extracts of A. mexicana inhibited germination of Lentil (Lens culinaris). Therefore A. mexicana may similarly affect the germination of several plant species growing in the ecosystem.
3.2. Effects on Root and Shoot Length
Root growth is characterized by high metabolic rates and, for this reason; roots are highly susceptible to environmental stresses such as allelochemicals in soils [18] . Normal plant cell growth and morphogenesis requires regulation of the concentrations of hormones such as auxins and gibberellins [59] . Some of allelochemicals disrupts hormone equilibrium. For instance they may inhibit polar auxin transport leading to a disturbance in normal auxin levels and resulting in the induction of lateral roots and the suppression of ageotropic growth [60] . The contact of phenolic acids with the root cell membrane leads to depolarization, an efflux of ions, and a reduction of hydrolic conductivity, water uptake and net nutrient uptake [61] . Moreover, allelochemical inhibit root elongation and cell division and enlarge thickness of roots due to the inhibition of root longitudinal growth [51] . The allelochemical also initiates a series of reactive oxygen species (ROS) in root meristem which inactivate enzymes, damage vital cellular organelles in plants, and destroy membranes by inducing the degradation of pigments, proteins, lipids and nucleic acids which ultimately results in death of the root system [62] [63] . It were also significantly decreased with the increase of both root and leaf extracts concentration of A. mexicana [34] [64] -[66] . Therefore, A. mexicana is known to possess allelochemicals that might induce reduced root and shoot growth in different plant species growing in their vicinity. However, the extent of such damage in different plant species requires scientific studies to substantiate.
3.3. Effects of Allelochemicals on Plants Fresh and Dry Weights
Decrease in plant growth caused by allelochemicals subsequently decreases fresh and dry weights. Allelochemicals reduce plant water potential [37] and inhibits minerals and ion uptake by plants [20] and reduces fresh weight. Interference with plant water balance is one of the mechanisms of action of p-hydroxybenzoic acid causing a reduction in plant growth. Studies have shown that soybean growth was reduced and water potential lowered with high concentrations of p-hydroxybenzoic acid [37] . Allelochemicals inhibits protein and carbohydrates synthesis, which in-turn reduce plant growth and weight [15] . Moreover, Einhellig and Rasmussen [67] found that decrease in biomass of treated soybean by phenolic acids were associated with reduced chlorophyll content in leaves. Similarly, Alagesaboopathi [65] also reported the decrease in fresh and dry weights of sorghum upon treatment with different concentrations of A. mexicana leaf aqueous extracts. Thus, allelochemicals in A. mexicana may inhibit growth of plants.
3.4. Influence of Allelochemicals on Photosynthesis and Chlorophyll Contents in Plants
Allelochemicals inhibits photosynthesis and oxygen evolution through interactions with components of photosystem II (PSII) [38] [68] . Allelochemicals can affect the performance of the three main processes of photosynthesis: stomatal control of carbon dioxide supply, thylakoid electron transport (light reaction), and the carbon reduction cycle (dark reaction) [38] . Chlorophyll is among the molecules in photosystem involved in light reaction which can be affected by allelochemicals. Chlorophyll is the most important pigment for photosynthesis [69] . The chlorophyll a and b are essential pigments for the conversion of light energy (solar radiation) to stored chemical energy [70] . Photosynthetic potential of a plant is determined by chlorophyll contents and any changes are expected to bring about change in photosynthesis [71] [72] . Chlorophyll also gives estimation of the plant nutrient status because much of leaf nitrogen is incorporated in chlorophyll [72] -[74] . The amount of chlorophyll per unit leaf area in plant is an important indicator of the overall plant healthy condition [72] . Healthy plants capable of maximum growth are generally expected to have larger amounts of chlorophyll than unhealthy ones [73] . Allelochemicals may reduce chlorophyll accumulation by inhibiting chlorophyll synthesis and/or stimulating chlorophyll degradation [75] . Several studies reported the decrease in chlorophyll contents with increase in concentration of allelopathic phenolics (vanillic acid, o-hydroxyphenyl acetic, p-hydroxybenzoic acid, camminic acid, ferulic and p-coumaric acids) in rice cabbage, Chinese fir, Echinochloa crus-galliand Chenopodium album seedlings [19] [52] [76] [78] .
4. Conclusion
Little information is available about the allelopatic effects of A. mexicana on the growth of important wild plant species such as those grown in the protected areas. However, allelochemicals from A. Mexicana may affect germination, growth and chlorophyll content of wild plant species. Therefore, as proposed by Makoi and Ndakidemi [79] , there is a need to explore the allelopathic potential present in diverse plant species growing in mixture so as to get full benefits of allelopathy in the ecosystem.
Acknowledgements
This study was funded by Tanzania Commission for Science and Technology (COSTECH) through the Nelson Mandela African Institution of Science and Technology.
References
- Drake, S.J., Weltzin, J.F. and Parr, P.D. (2003) Assessment of Non-Native Invasive Plant Species on the United States Department of Energy Oak Ridge National Environmental Research Park. Castanea, 68, 15-30.
- CBD (2005) Invasive Alien Species. Convention on Biological Diversity. http://www.biodiv.org/programmes/cross-cutting/alien/
- Callaway, R.M. and Aschehoug, E.T. (2000) Invasive Plants versus Their New and Old Neighbors: A Mechanism for Exotic Invasion. Science, 290, 521-523. http://dx.doi.org/10.1126/science.290.5491.521
- Bais, H.P., Vepachedu, R., Gilroy, S., Callaway, R.M. and Vivanco, J.M. (2003) Allelopathy and Exotic Plant Invasion: From Molecules and Genes to Species Interactions. Science, 301, 1377-1380. http://dx.doi.org/10.1126/science.1083245
- Seastedt, T.R., Callaway, R.M., Pollock, J.L. and Kaur, J. (2008) Allelopathy and Plant Invasions: Traditional, Congeneric, and Bio-geographical Approaches. Biological Invasions, 10, 875-890. http://dx.doi.org/10.1007/s10530-008-9239-9
- Maron, J.L., Vila, M., Bommarco, R., Elmendorf, S. and Beardsley, P. (2004) Rapid Evolution of an Invasive plant. Ecological Monograph, 74, 261-280. http://dx.doi.org/10.1890/03-4027
- Stockwell, C.A., Hendry, A.P. and Kinnison, M.T. (2003) Contemporary Evolution Meets Conservation Biology. Trends in Ecology & Evolution, 18, 94-101. http://dx.doi.org/10.1016/s0169-5347(02)00044-7
- Blossey, B. and Notzold, R. (1995) Evolution of Increased Competitive Ability in Invasive Nonindigenous Plants: A Hypothesis. Journal of Ecology, 83, 887-889. http://dx.doi.org/10.2307/2261425
- Yuan, Y., Wang, B., Zhang, S., Tang, J., Tu, C., Hu, S., Yong, J.W. and Chen, X. (2013) Enhanced Allelopathy and Competitive Ability of Invasive Plant Solidago canadensis in its Introduced Range. Journal of Plant Ecology, 6, 253- 263. http://dx.doi.org/10.1093/jpe/rts033
- Callaway, R.M. and Ridenour, W.M. (2004) Novel weapons: Invasive Success and the Evolution of Increased Competitive Ability. Frontiers in Ecology and the Environment, 2, 436-443. http://dx.doi.org/10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2
- He, H.B., Wang, H.B., Fang, C.X., Lin, Z.H., Yu, Z.M. and Lin, W.X. (2012) Separation of Allelopathy from Resource Competition Using Rice/Barnyardgrass Mixed-Cultures. PloS ONE, 7, e37201. http://dx.doi.org/10.1371/journal.pone.0037201
- Callaway, R.M. (2002) The Detection of Neighbors by Plants. Trends in Ecology & Evolution, 17, 104-105. http://dx.doi.org/10.1016/S0169-5347(01)02438-7
- M. An (2005) Mathematical Modelling of Dose-response Relationship (Hormesis) in Allelopathy and its Application. Nonlinearity in Biology, Toxicology, and Medicine, 3, 153-172. http://dx.doi.org/10.2201/nonlin.003.02.001
- Jarchow, M.E. and Cook, B.J. (2009) Allelopathy as a Mechanism for the Invasion of Typha angustifolia. Plant Ecology, 204, 113-124. http://dx.doi.org/10.1007/s11258-009-9573-8
- Li, Z.-H., Wang, Q., Ruan, X., Pan, C.-D. and Jiang, D.-A. (2010) Phenolics and Plant Allelopathy. Molecules, 15, 8933-8952. http://dx.doi.org/10.3390/molecules15128933
- Cruz-Ortega, R., Lara-Núñez, A. and Anaya, A.L. (2007) Allelochemical Stress Can Trigger Oxidative Damage in Receptor Plants: Mode of Action of Phytotoxicity. Plant Signaling & Behavior, 2, 269-270. http://dx.doi.org/10.4161/psb.2.4.3895
- Colpas, F.T., Ono, E.O., Rodrigues, J.D. and de Souza Passos, J.R. (2003) Effects of Some Phenolic Compounds on Soybean Seed Germination and on Seed-borne Fungi. Brazilian Archives of Biology and Technology, 46, 155-161. http://dx.doi.org/10.1590/S1516-89132003000200003
- Cruz-Ortega, R., Anaya, A.L., Hernández-Bautista, B.E. and Laguna-Hernández, G. (1998) Effects of Allelochemical Stress Produced by Sicyos deppei on Seedling Root Ultrastructure of Phaseolus vulgaris and Cucurbita ficifolia. Journal of Chemical Ecology, 24, 2039-2057. http://dx.doi.org/10.1023/A:1020733625727
- Chen, L., Liao, L., Wang, S., Huang, Z. and Xiao, F. (2002) Effect of Vanillin and P-hydroxybenzoic acid on Physiological Characteristics of Chinese Fir Seedlings. The journal of Applied Ecology, 13, 1291.
- Weir, T.L., Park, S.-W. and Vivanco, J.M. (2004) Biochemical and Physiological Mechanisms Mediated by Allelochemicals. Current Opinion in Plant Biology, 7, 472-479. http://dx.doi.org/10.1016/j.pbi.2004.05.007
- Muzaffar, S., Ali, B. and Wani, N.A. (2012) Effect of Catechol, Gallic Acid and Pyrogallic Acid and the Germination, Seedling Growth and the Level of Endogenous Phenolics in Cucumber (Cucumis sativus L.). International Journal of Life Science Biotechnology and Pharma Research, 1, 51-59.
- El-Darier, S.M., Nasrine, S. and El-Taher, H.M. (2011) Allelopathic Effect from Some Medicinal Plants and Their Potential Uses as Control of Weed. http://www.ipcbee.com/vol24/4-ICBEC2011-C00010.pdf
- Weston, L.A. (1996) Utilization of Allelopathy for Weed Management in Agroecosystems. Agronomy Journal, 88, 860-866. http://dx.doi.org/10.2134/agronj1996.00021962003600060004x
- Narwal, S., Palaniraj, R. and Sati, S. (2005) Role of Allelopathy in Crop Production. Herbologia, 6, 327-332.
- Sanaa, A. (2012) Perspectives on the Relationship between Invisibility, Richness, Plant Size, Seed Production, Seed Bank and Community Productivity of Invasive Argemone ochroleuca Sweet in Taif, Saudi Arabia. Life Science Journal, 9, 953-958.
- Rajvaidhya, S.A. (2012) Review on Argemone mexicana Linn—An Indian Medicinal Plant. International Journal, 3. 2494-2501.
- Dash, G. and Murthy, P. (2011) Evaluation of Argemone mexicana Linn. Leaves for Wound Healing Activity. Journal of Natural Product and Plant Resources, 1, 46-56.
- Holm, L., Pancho, J.V., Herberger, J.P. and Plucknett, D.L. (1977) A Geographical Atlas of World Weeds. John Wiley and Sons, Hoboken.
- Karlsson, L.M., Tamado, T. and Milberg, P. (2003) Seed Dormancy Pattern of the Annuals Argemone ochroleuca and A. mexicana (Papaveraceae). Flora-Morphology, Distribution, Functional Ecology of Plants, 198, 329-339. http://dx.doi.org/10.1078/0367-2530-00104
- Parsons, W.W.T. and Cuthbertson, E.E.G. (2001) Noxious Weeds of Australia. Csiro Publishing, Canberra, 534-536.
- Hussain, S.F., Nakkady, S., Khan, L. and Shamma, M. (1983) Oxyhydrastinine, an Isoquinolone Alkaloid from the Papaveraceae. Phytochemistry, 22, 319-320. http://dx.doi.org/10.1016/S0031-9422(00)80125-9
- Fernandez, C., Santonja, M., Gros, R., Monnier, Y., Chomel, M., Baldy, V. and Bousquet-Mélou, A. (2013) Allelochemicals of Pinus halepensis as Drivers of Biodiversity in Mediterranean Open Mosaic Habitats during the Colonization Stage of Secondary Succession. Journal of Chemical Ecology, 39, 298-311. http://dx.doi.org/10.1007/s10886-013-0239-6
- Ridenour, W.M. and Callaway, R.M. (2001) The Relative Importance of Allelopathy in Interference: The Effects of an Invasive Weed on a Native Bunchgrass. Oecologia, 126, 444-450. http://dx.doi.org/10.1007/s004420000533
- Burhan, N. and Shaukat, S.S. (1999) Allelopathic Potential of Argemone mexicana L—A Tropical Weed. Pakstan Journal of Biological Sciences, 2, 1268-1273. http://dx.doi.org/10.3923/pjbs.1999.1268.1273
- Wang, Q., Ruan, X. and Li, Z. (2007) Autotoxicity of Plants and Research of Coniferous Forest Autotoxicity. Scientia Silvae Sinicae, 43, 134-142.
- Rice, E.L. (1984) Allelopathy. Academic Press, London.
- Barkosky, R.R. and Einhellig, F.A. (2003) Allelopathic Interference of Plant-Water Relationships by Para-Hydroxybenzoic Acid. Botanical Bulletin of Academia Sinica, 44, 53-58.
- Einhellig, F.A., Rasmussen, J.A., Hejl, A.M. and Souza, I.F. (1993) Effects of Root Exudate Sorgoleone on Photosynthesis. Journal of Chemical Ecology, 19, 369-375. http://dx.doi.org/10.1007/BF00993702
- Durner, J., Shah, J. and Klessig, D.F. (1997) Salicylic Acid and Disease Resistance in Plants. Trends in Plant Science, 2, 266-274. http://dx.doi.org/10.1016/S1360-1385(97)86349-2
- Yuan, S. and Lin, H. (2008) Role of Salicylic Acid in Plant Abiotic Stress. Zeitschrift fur Naturforschung c, 63, 313.
- Van Huijsduijnen, R.H., Alblas, S., De Rijk, R. and Bol, J. (1986) Induction by Salicylic Acid of Pathogenesis-Related Proteins and Resistance to Alfalfa mosaic virus Infection in Various Plant Species. Journal of General Virology, 67, 2135-2143. http://dx.doi.org/10.1099/0022-1317-67-10-2135
- Clarke, S.M., Mur, L.A., Wood, J.E. and Scott, I.M. (2004) Salicylic Acid Dependent Signaling Promotes Basal Thermotolerance But Is Not Essential for Acquired Thermotolerance in Arabidopsis thaliana. The Plant Journal, 38, 432- 447. http://dx.doi.org/10.1111/j.1365-313X.2004.02054.x
- Durrant, W. and Dong, X. (2004) Systemic Acquired Resistance. Annual Reviews Phytopathol, 42, 185-209. http://dx.doi.org/10.1146/annurev.phyto.42.040803.140421
- Chandra, A., Anand, A. and Dubey, A. (2007) Effect of Salicylic Acid on Morphological and Biochemical Attributes in Cowpea. Journal of Environmental Biology, 28, 193-196.
- Barkosky, R.R. and Einhellig, F.A. (1993) Effects of Salicylic Acid on Plant-Water Relationships. Journal of Chemical Ecology, 19, 237-247. http://dx.doi.org/10.1007/BF00993692
- Saberi, M., Tarnian, F., Davari, A., Shahreki, E. and Shahreki, M. (2013) Influence of Chemical Stimulators in Decreasing of Allelopathic Effect of Eucalyptus camaldulensis on Germination Properties of Onobrychis sativa. Annals of Biological Research, 4, 1-7.
- Ghareib, H.R.A., Abdelhamed, M.S. and Ibrahim, O.H. (2010) Antioxidative Effects of the Acetone Fraction and Vanillic Acid from Chenopodium murale on Tomato Plants. Weed Biology and Management, 10, 64-72. http://dx.doi.org/10.1111/j.1445-6664.2010.00368.x
- Esmaeili, M., Heidarzadel, A., Pirdashti, H. and Exmaeili, F. (2012) Inhibitory Activity of Pure Allelochemicals on Barnyard Grass (Echinochloa crus-galli. L) Seed and Seedling Parameters. International Journal of Agriculture and Crop Sciences, 4, 274-279.
- Chen, S., Zhou, B., Lin, S., Li, X. and Ye, X. (2011) Accumulation of Cinnamic Acid and Vanillin in Eggplant Root Exudates and the Relationship with Continuous Cropping Obstacle. African Journal of Biotechnology, 10, 2659-2665.
- Yu, J.Q. and Matsui, Y. (1994) Phytotoxic Substances in Root Exudates of Cucumber (Cucumis sativus L.). Journal of Chemical Ecology, 20, 21-31. http://dx.doi.org/10.1007/BF02065988
- Chon, S.-U., Choi, S.-K., Jung, S., Jang, H.-G., Pyo, B.-S. and Kim, S.-M. (2002) Effects of Alfalfa Leaf Extracts and Phenolic Allelochemicals on Early Seedling Growth and Root Morphology of Alfalfa and Barnyard Grass. Crop Protection, 21, 1077-1082. http://dx.doi.org/10.1016/S0261-2194(02)00092-3
- Singh, N., Yadav, K. and Amist, N. (2013) Phytotoxic Effects of Cinnamic Acid on Cabbage (Brassica oleracea var. Capitata). Journal of Stress Physiology & Biochemistry, 9, 307-317.
- Ding, J., Sun, Y., Xiao, C.L., Shi, K., Zhou, Y.H. and Yu, J.Q. (2007) Physiological Basis of Different Allelopathic Reactions of Cucumber and Figleaf Gourd Plants to Cinnamic Acid. Journal of Experimental Botany, 58, 3765-3773. http://dx.doi.org/10.1093/jxb/erm227
- Makoi, J.H. and Ndakidemi, P.A. (2007) Biological, Ecological and Agronomic Significance of Plant Phenolic Compounds in Rhizosphere of the Symbiotic Legumes. African Journal of Biotechnology, 6, 1358-1368.
- Yang, Q., Ye, W., Liao, F. and Yin, X. (2005) Effects of Allelochemicals on Seed Germination. Chinese Journal of Ecology, 24, 1459-1465.
- Podestá, F.E. and Plaxton, W.C. (1994) Regulation of Cytosolic Carbon Metabolism in Germinating Ricinus communis Cotyledons. Planta, 194, 381-387. http://dx.doi.org/10.1007/BF00197539
- Muscolo, A., Panuccio, M. and Sidari, M. (2001) The Effect of Phenols on Respiratory Enzymes in Seed Germination. Plant Growth Regulation, 35, 31-35. http://dx.doi.org/10.1023/A:1013897321852
- Paul, N. and Begum, N. (2010) Allelopathic Effect of Argemone mexicana L. on Germination and Seedling Growth Characteristics of Lentil (Lens culinaris). Journal of Bio-Science, 18, 146-147.
- Woodward, A.W. and Bartel, B. (2005) Auxin: Regulation, Action, and Interaction. Annals of Botany, 95, 707-735.
- Brunn, S.A., Muday, G.K. and Haworth, P. (1992) Auxin Transport and the Interaction of Phytotropins Probing the Properties of a Phytotropin Binding Protein. Plant Physiology, 98, 101-107. http://dx.doi.org/10.1104/pp.98.1.101
- Baziramakenga, R., Leroux, G. and Simard, R. (1995) Effects of Benzoic and Cinnamic Acids on Membrane Permeability of Soybean Roots. Journal of Chemical Ecology, 21, 1271-1285. http://dx.doi.org/10.1007/BF02027561
- Karuppanapandian, T., Moon, J.-C., Kim, C., Manoharan, K. and Kim, W. (2011) Reactive Oxygen Species in Plants: Their Generation, Signal Transduction, and Scavenging Mechanisms. Australian Journal of Crop Science, 5, 709-725.
- Fitter, A. (2003) Making Allelopathy Respectable. Science, 301, 1337-1338. http://dx.doi.org/10.1126/science.1089291
- Paul, N. and Begum, N. (2007) Influence of Root and Leaf Extracts of Argemone mexicana on Germination and Seedling Growth of Blackgram, Rapeseed and Wheat. Bangladesh Journal of Scientific and Industrial Research, 42, 229- 234. http://dx.doi.org/10.3329/bjsir.v42i2.477
- Alagesaboopathi, C. (2013) Allelopathic Effect of Different Concentration of Water Extract of Argemone mexicana L. on Seed Germination and Seedling Growth of Sorghum bicolor (L.) Moench. Journal of Pharmacy and Biological Sciences, 5, 52-55. http://dx.doi.org/10.9790/3008-0515255
- Jilani, G., Mahmood, S., Chaudhry, A.N., Hassan, I. and Akram, M. (2008) Allelochemicals: Sources, Toxicity and Microbial Transformation in Soil—A Review. Annals of Microbiology, 58, 351-357. http://dx.doi.org/10.1007/BF03175528
- Einhellig, F.A. and Rasmussen, J.A. (1979) Effects of Three Phenolic Acids on Chlorophyll Content and Growth of Soybean and Grain Sorghum Seedlings. Journal of Chemical Ecology, 5, 815-824. http://dx.doi.org/10.1007/BF00986566
- Rimando, A.M., Dayan, F.E., Czarnota, M.A., Weston, L.A. and Duke, S.O. (1998) A New Photosystem II Electron Transfer Inhibitor from Sorghum bicolor. Journal of Natural Products, 61, 927-930. http://dx.doi.org/10.1021/np9800708
- Niinemets, Ü. and Tenhunen, J. (1997) A Model Separating Leaf Structural and Physiological Effects on Carbon Gain along Light Gradients for the Shade—Tolerant Species Acer saccharum. Plant, Cell & Environment, 20, 845-866. http://dx.doi.org/10.1046/j.1365-3040.1997.d01-133.x
- Gitelson, A.A. and Merzlyak, M.N. (2003) Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. Journal of Plant Physiology, 160, 271-282. http://dx.doi.org/10.1078/0176-1617-00887
- Curran, P.J., Dungan, J.L. and Gholz, H.L. (1990) Exploring the Relationship between Reflectance Red Edge and Chlorophyll Content in Slash Pine. Tree Physiology, 7, 33-48.
- Filella, I., Serrano, L., Serra, J. and Penuelas, J. (1995) Evaluating Wheat Nitrogen Status with Canopy Reflectance Indices and Discriminant Analysis. Crop Science, 35, 1400-1405. http://dx.doi.org/10.2135/cropsci1995.0011183X003500050023x
- Wu, C., Niu, Z., Tang, Q. and Huang, W. (2008) Estimating Chlorophyll Content from Hyperspectral Vegetation Indices: Modeling and Validation. Agricultural and Forest Meteorology, 148, 1230-1241. http://dx.doi.org/10.1016/j.agrformet.2008.03.005
- Moran, J.A., Mitchell, A.K., Goodmanson, G. and Stockburger, K.A. (2000) Differentiation among Effects of Nitrogen Fertilization Treatments on Conifer Seedlings by Foliar Reflectance: A Comparison of Methods. Tree Physiology, 20, 1113-1120. http://dx.doi.org/10.1093/treephys/20.16.1113
- Yang, C.-M., Lee, C.-N. and Chou, C.-H. (2002) Effects of Three Allelopathic Phenolics on Chlorophyll Accumulation of Rice (Oryza sativa) Seedlings: I. Inhibition of Supply-Orientation. Botanical Bulletin of Academia Sinica, 43, 299- 304.
- Sarkar, E., Chatterjee, S.N. and Chakraborty, P. (2012) Allelopathic Effect of Cassia tora on Seed Germination and Growth of Mustard. Turkish Journal of Botany, 36, 488-494.
- Stupnicka-Rodzynkiewicz, E., Dabkowska, T., Stoklosa, A., Hura, T., Dubert, F. and Lepiarczyk, A. (2006) The Effect of Selected Phenolic Compounds on the Initial Growth of Four Weed Species. Zeitschrift Fur Pflanzenkrankheiten und Pflanzenschutz-Sonderheft, 20, 479-486.
- Yang, C.-M., Chang, F., Lin, S.-J. and Chou, C.-H. (2004) Effects of Three Allelopathic Phenolics on Chlorophyll Accumulation of Rice (Oryza sativa) Seedlings: II. Stimulation of Consumption-Orientation. Botanical Bulletin of Academia Sinica, 45, 119-125.
- Makoi, J.H. and Ndakidemi, P.A. (2012) Allelopathy as Protectant, Defence and Growth Stimulants in Legume Cereal Mixed Culture Systems. New Zealand Journal of Crop and Horticultural Science, 40, 161-186. http://dx.doi.org/10.1080/01140671.2011.630737
NOTES

*Corresponding author.

