Advances in Microbiology
Vol.2 No.4(2012), Article ID:25951,6 pages DOI:10.4236/aim.2012.24074
Antimicrobial Activities of Seed Extracts of Mango (Mangifera indica L.)
1Department of Pharmaceutics, College of Pharmacy, King Khalid University, Abha, KSA
2Department of Clinical Microbiology and Parasitology, College of Medicine, King Khalid University, Abha, KSA
3Department of Pharmaceutics, College of Pharmacy, Omdurman Islamic University, Omdurman, Sudan
Email: *mehamid2@yahoo.com
Received September 12, 2012; revised October 23, 2012; accepted October 29, 2012
Keywords: Antimicrobial; Mango Seed; Mangifera indica L.; Ethnopharmacology
ABSTRACT
Mangifera indica L. is a species of mango in the Anacardiaceae family. It is found in the wild in tropical regions and cultivated varieties have been introduced to other warm regions of the world. This present study aimed to investigate the in vitro antimicrobial activities of methanol and ethanol extracts of mango seed against 25 representatives gram positive, gram negative, acid fast bacteria and fungi. Mango fruit seed were extracted by Soxhlet using methanol and ethanol as solvents. The extracts were tested against the microorganisms using disc diffusion method at different concentrations: 5 mg/mL, 3.75 mg/mL, 3.125 mg/mL, 2.5 mg/mL, 1.875 mg/mL and 1.25 mg/mL). In vitro antibacterial activities of methanol and ethanol extracts of mango bulb showed inhibitions to tested organisms with variable inhibition zones. Except one organism (Rhodococcus equi), no resistance among the tested strains was shown. The mean zone of inhibition produced ranged between 5 mm and 18 mm with 18 mm/Mycobacterium smegmatis showed the highest zone of inhibition. In most test strains comparable zones of inhibitions were noted for both methanol and ethanol extract. Candida albicans and Aspergilllus niger were both inhibited by the extracts. The methanol and ethanol extracts of mango seed showed good inhibitory effects against almost all tested strains. The inhibition zones produced by mango extract were less than those produced by standard positive control drug. This could be due to low diffusion rate of mango extract in agarose medium, a thing needed to be further investigated. The products are potential new antimicrobial therapy in the ethnopharmacology domain.
1. Introduction
Due to report of increasing developments of drug resistance in human pathogen as well as undesirable side effects of certain antimicrobial agents, it is necessary to search for new agents that are better, cheaper and without side effect for treating infectious diseases especially in developing countries. A wide variety of plant/natural products are used in the treatment of infections. Phytoconstituents have been found to inhibit bacteria, fungi, viruses and pests [1].
There have been several reports on the pharmacological effects and suitability of medicinal plants as phytotherapies for diseases. Mango stem-bark aqueous extract has been reported to possess anti-inflammatory, analgesic and immunoprotective effects [2,3]. A number of biological activities of mangiferin have been suggested, including anti-diabetic and anti-inflammatory abilities [4].
Mango was also claimed to have anti malaria effect [5] and was found to display in vitro activity against Plasmodium falciparum [6].
Methanol extract of M. indica seeds was done against 61 bacterial strains. The extract showed potent antibacterial activity against all strains tested [7]. The leaves of M. indica have been reported to possess antibacterial activity against E. coli and other bacteria in the family Enterobacteriaceae and the bioactive component mangiferin which was isolated from M. indica was reported to possess remarkable anti-influenza activity [8,9]. Ojewole [3] found anti-inflammatory, analgesic and hypoglycemic effects on the aqueous extract of the stem-bark of M. indica. Doughari and Manzara [9] found that both acetone and methanol extracts inhibited the growth of gram positive bacteria, with acetone extract exerting more activities on all the gram positive bacteria with zone of inhibition between 15 - 16 mm, and a gram negative bacterium S. typhi (14 mm) at 250 mg/mL. Stem park of M. indica showed significant antibacterial and antifungal activities against Streptococcus pneumonia, Enterobacter aerogenes, Klebsiella pneumonia and Candida albicans with MIC of 0.08 mg/mL [10].
The presence of phytoconstituents in the leaf extracts may be responsible for the antibacterial activity of the plant [1]. No research has been carried out on antimicrobial activity of seeds extract of mango (M. indica). In this study, the in vitro antimicrobial activity of methanol and ethanol extracts of the seeds of of mango (Mangifera indica L.) was investigated.
2. Materials and Methods
2.1. Plant Collection and Identification
Fresh seeds of Mangifera indica L. were collect from the wild from around Khartoum vegetable, Sudan and were identified and authenticated in the Department of Biological Sciences; King Khalid University. The Sudanese Mangifera indica L. was identified as mango. The collected seeds were kept at plastic bags at room temperature till use.
2.2. Extraction of Mango Seeds
1000 grams of air dried and coarsely powdered of clean seeds of Mangifera indica seed were extracted in Soxhlet apparatus to obtain methanolic and ethanolic extracts. The extracts were filtered, and the filtrates were vaporized to dryness, and weighed in order to determine the % yield of the extracts, following the formula: % yield = (weight of extract/weight of ground plant material) × 100. The stock solutions of the crude methanolic and ethanolic extracts were prepared by dilution the dried extracts with 50% methanol and 50% ethanol to obtain the desired final concentrations of: 5 mg/mL, 3.75 mg/mL, 3.125 mg/mL, 2.5 mg/mL, 1.875 mg/mL and 1.25 mg/mL. These concentrations were used to impregnate filter paper disks (5.5 mm diameters). Disk impregnated into 50% methanol and 50% ethanol was used as control, while standard antimicrobial discs: amikacin, chlorophenicol, oxacillin, amoxicillin, nystatin and metronidazole (Difco) were used as positive control.
2.3. Preparation of Extracts
The extraction was carried out using methanol and ethanol (separately). 400 gm of dry seeds of Mangifera indica fruit were extracted with 80% methanol using Soxeight hour till the color of the solvent returned colorless.
Solvent was evaporated under reduced pressure using Rotary evaporator apparatus (BUCHI Rotavapor R-200/ 20). Extract was finally allowed to dry at air at room temperature till complete dryness. Extraction using ethanol followed the above procedures.
2.4. Determination of Phytochemical Constituents
Preliminary phytochemical analysis was undertaken using standard qualitative methods [11]. The different crude extracts obtained were qualitatively tested for the presence of various phytochemical constituents using standard protocols [12]. The extractive values of mango were calculated as per standard methods [12]. The concentrated crude extracts obtained through were weighed and dissolved in the respective solvent used for the extraction (1 g/100 mL, w/v).
2.5. Assay for Antimicrobial Activity
The original extract of Mangifera indica was subject to serial dilution was made as follows: 5 mg/mL, 3.75 mg/mL, 3.125 mg/mL, 2.5 mg/mL, 1.875 mg/mL and 1.25 mg/mL. Filter discs (5.5/mm) were made and impregnated into each of the above dilutions. The discs were dried at 37˚C for one hour. The dried discs were therefore having the following concentration: 5 mg/mL, 3.75 mg/mL, 2.5 mg/mL, 1.75 mg/mL, and 1 mg/mL. The discs were dried at 37˚C for one hour. The dried discs were therefore having the following concentration: 5 mg/mL, 3.75 mg/mL, 2.5 mg/mL, 1.75 mg/mL, and 1 mg/mL.
2.6. Bacterial and Fungal Strains
Bacterial strains (n = 22) and fungal strains (n = 3) (Table 1) recovered from frozen stocks at King Khalid University or from clinical materials isolated from patients at Aseer Central Hospital were used in the study. One to three loopful of 24 h old cultures from each test strains were used to prepare 0.5 McFarland standard suspendsions. Slow growing culture, namely Mycobacterium spp, Nocardia spp., Gordonia spp. and the three fungal strains were incubated to 3 to 5 days.
Mueller Hinton’s agar (DIFCO) plates were used the in vitro antimicrobial testing as recommended by clinical and Laboratory Standards Institute [13]. Then impregnated dried discs plus positive and negative control discs hlet extractor apparatus. Extraction was carried out for
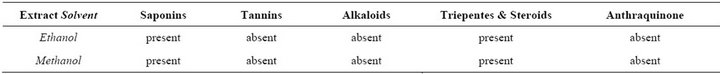
Table 1. Phytochemical constituents of seed extract of Mangifera indica L.
were placed on the inoculated agar. The inoculated plates were incubated at 37˚C and examined after 24, 48 and 72 h for inhibition zones under and around 1. Z was measured in mm by a ruler for each disc exhibiting inhibition zones under and around discs.
3. Results
3.1. Phytochemical Constituents
Preliminary phytochemical analysis revealed that M. indica possessed the phytoconstituents tannins, alkaloids, flavonoids, Anthraquinone, glycosides and Saponins (Table 1).
3.2. Antimicrobial Activity
The antimicrobial activity of M. indica extract against the 25 organism is shown in Table 2. With the exception of Bacillus cereus and Rhodococcus equi all remaining 23 strains showed zones of inhibitions to one of the concentrations of ethanolic and methanolic extracts (Figure 1).
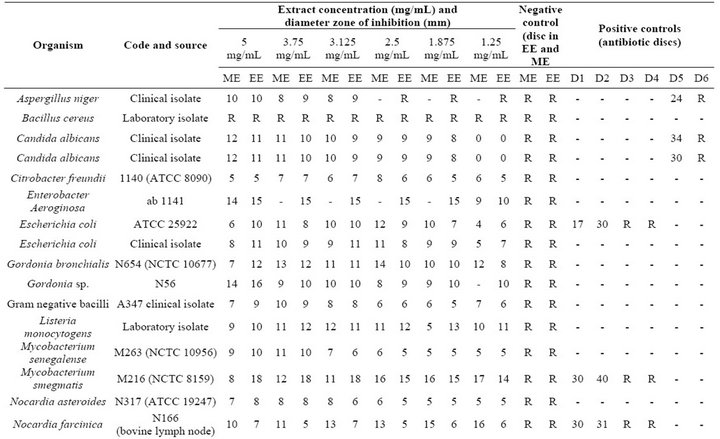
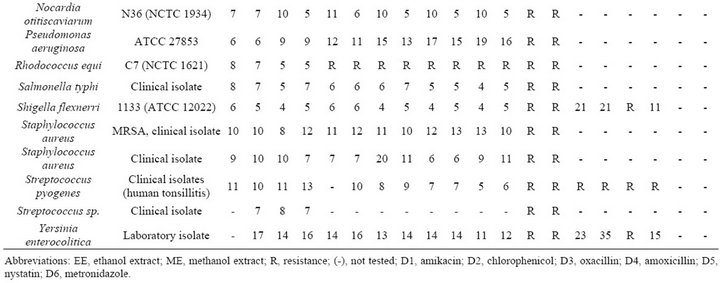
Table 2. Antimicrobial activity of seed extract of Mangifera indica L. against 25 bacterial and fungal strains.
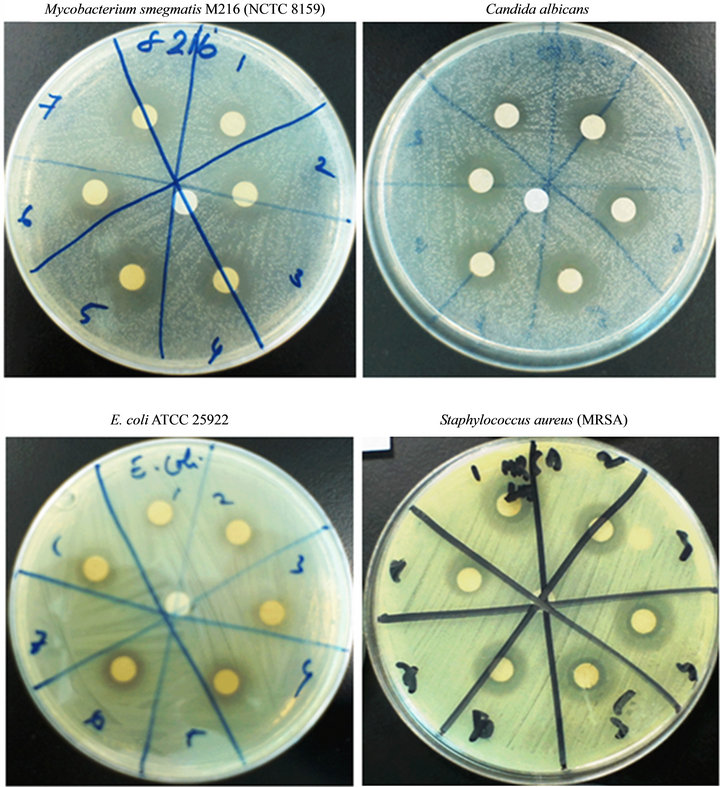
Figure 1. Zones of inhibitions revealed by various concentrations of the ethanolic extract of Mangifera indica L. against representatives of an acid fast (Mycobacterium smegmatis), a yeast (Candida albicans), a gram negative (E. coli) and a gram positive bacteria (Staphylococcus aureus).
4. Discussion
Physiotherapy has been shown to have indisputable effectiveness against diseases worldwide. Many sections in the rural African population rely on medicinal plants and folklore healing methods for primary health care [1,8]. While 25% to 50% of current pharmaceuticals are derived from plants, none are used as antimicrobials. Traditional healers have long used plants to prevent or cure infectious conditions [14]. Medicinal plants constitute an effective source of both traditional and modern medicines, but assessment of antimicrobial potential of these sources is essential. The present study found a very promising and readily available source (mango, M. indica) for treating infections caused by bacteria and fungi. This is particularly significant because drug resistance to human pathogens has been increasing not only in the developing countries but throughout the world due to indiscriminate use of antibiotics [15]. The drug resistance bacterial and fungal pathogens have further complicated the treatment of infectious diseases in immunocompromised AIDS and cancer patients [16]. In the present scenario due to emergence of multiple drug resistance to human pathogenic bacteria and fungi, especially the antibiotic penicillins, cephalosporins and chloromphenicol types involve the enzymic inactivation of the antibiotic by hydrolysis or by the formation of an active derivative. This has opened a new vista for the search of new antimicrobial substance.
The results of the present study revealed wide ranges of inhibition zones from as low as 5 mm up to 20 mm. Both methanol and ethanol extract of the mango seed showed zones of inhibitions in most strains tested. The antimicrobial activity of M. indica extract against the 25 organisms was found to have effect but only Bacillus cereus and Rhodococcus equi, which seems to have resistance against these extracts. These levels of activities 92%) is promising. More application on these products will ensure its wide applicability and their bacteriostatic or bactericidal, fungostatic or fungicidal actions. It is clear that further research will be needed to cover a wide range of bacteria including multi drug (MDR) resistance ones. In this study Staphylococcus aureus (MRSA) was inhibited by these extracts, this could open way for testing MDR organisms and to determine the MIC concentration of these extracts.
In a previous research, Vaghasiya [7] found that methanol extract of M. indica seeds has potent antibacterial activity with concentration ranging from 0.6 mg/mL to 1.2 mg/mL. In our present study methanol extract was tested at higher concentrations (1.25 mg/mL onward). But the inhibition to many organism at 1.25 mg/mL is comparable to those shown by Vaghasiya [7]. In addition in the present study we tested the methanolic extract of M. indica and it showed potent antimicrobial activities. Ethanol extract is more likely to be selected for further pharmaceutical experimentation for human and animal use. This is because methanol is risky due to its high toxicity and not applicable for usage. It is noticed in this study, as expected, that inhibition of microorganisms is directly proportional with the concentration of the extract. The use of Mueller Hinton’s agar along with a unified inoculums size (0.5 McFarladn) follows the standard methods [13]. But is it expected that antimicrobial activeity and inhibition zone diameter might have been affected by factor related to its diffusion in agars such as the Mueller Hinton’s.
The present study is the first to explore mango seed as antimicrobial. It agrees with previous studies which used leaves of mango rather than seeds that M. indica L. has antibacterial activity [8,9]. In the present study both ethanolic and methanolic were investigated. Both solvents are low molecular weight alcohols, polar compound and shows very little difference in their extractive abilities. But ethanol extraction is more convenient and non toxic for the biological purpose. Ethanol extraction shows little increase in the yield percentage, zone of inhibition of ethanol extract shows little increase than methanol extract when treated with different micro organisms and Methanol extraction which we have done for comparative studies for our knowledge Doughari and Manzara [9] found that both acetone and methanol extracts inhibited the growth of gram positive bacteria, with acetone extract exerting more activities on all the gram positive bacteria with zone of inhibition between 15 - 16 mm, and a gram negative bacterium Salmonella typhi (14 mm) at 250 mg/mL. Singh [10] extracted stem park of M. indica and excellent results have been generated with significant antibacterial and antifungal activities (MIC = 0.08 mg/mL) against Streptococcus pneumonia, Enterobacter aerogenes, Klebsiella pneumonia and Candida albicans. In the present study encouraging results have been produced with both ethanolic and methanolic extracts but interestingly with the seeds of mango.
From the present screening, it could be concluded that the seed of M. indica is more potent antimicrobial agent than leaves and could be compared to the known antibiotics. Further, the detailed phytochemical research is required to identify the active principal responsible for aforementioned activities and testing wider range of organisms would be encouraged.
REFERENCES
- M. C. Marjorie, “Plant Products as anTimicrobial Agents,” Clinical and Microbiology Reviews, Vol. 12, 1999, pp. 564-582.
- G. Garrido, M. Blanco-Molina, R. Sancho, A. Macho, R. Delgado and E. Munoz, “An Aqueous Stem Bark Extract of Mangifera indica (Vimang) Inhibits T Cell Proliferation and TNF-Induced Activation of Nuclear Transcription Factor NF-kappa B,” Phytotherapy Research, Vol. 19, No. 3, 2005, pp. 211-215. doi:10.1002/ptr.1656
- J. A. Ojewole, “Antiinflammatory, Analgesic and Hypoglycemic Effects of Mangifera indica Linn. (Anacardiaceae) Stem-Bark Aqueous Extract,” Methods and Findings in Experimental and Clinical Pharmacology, Vol. 27, No. 8, 2005, pp. 547-554. doi:10.1358/mf.2005.27.8.928308
- A. Vyas, K. Syeda, A. Ahmad, S. Padhye and F. H. Sarkar, “Perspectives on Medicinal Properties of Mangiferin,” Mini-Reviews in Medicinal Chemistry, Vol. 12, No. 5, 2012, pp. 412-425. doi:10.2174/138955712800493870
- N. Tsabang, P. V. Fokou, L. R. Tchokouaha, B. Noguem, I. Bakarnga-Via, M. S. Nguepi, B. A. Nkongmeneck and F. F. Boyom, “Ethnopharmacological Survey of Annonaceae Medicinal Plants Used to Treat Malaria in Four Areas of Cameroon,” Journal of Ethnopharmacology, Vol. 139, No. 1, 2012, pp. 171-180. doi:10.1016/j.jep.2011.10.035
- P. Rasoanaivo, D. Ramanitrahasimbola, H. Rafatro, D. Rakotondramanana, B. Robijaona, A. Rakotozafy, S. Ratsimamanga-Urverg, M. Labaïed, P. Grellier, L. Allorge, L. Mambu and F. Frappier, “Screening Extracts of Madagascan Plants in Search of Antiplasmodial Compounds,” Phytotherapy Research, Vol. 18, No. 9, 2004, pp. 742- 747. doi:10.1002/ptr.1533
- Y. Vaghasiya, H. Patel and S. Chanda, “Antibacterial Activity of Mangifera indica L. Seeds against Some Human Pathogenic Bacterial Strains,” African Journal of Biotechnology, Vol. 10, 2011, pp. 15788-15794. doi:10.5897/AJB10.632
- B. Neon, “Medicinal Plants in Nigeria, Private Edition,” Nigeria College of Arts, Science and Technology, Ibadan, 1984, pp. 1-84.
- J. H. Doughari and S. Manzara, “In Vitro Antibacterial Activity of Crude Leaf Extracts of Mangifera indica Linn,” African Journal of Microbiology Research, Vol. 2, 2008, pp. 67-72.
- M. Singh, S. Khatoon, S. Singh, V. Kumar, A. K. Rawat and S. Mehrotra, “Antimicrobial Screening of Ethnobotanically Important Stem Bark of Medicinal Plants,” Pharmacognosy Research, Vol. 2, 2010, pp. 254-257. doi:10.4103/0974-8490.69127
- S. C. Chhabra, F. C. Uiso and E. N. Mshiu, “Phytochemical Screening of Tanzanian Medicinal Plants,” Journal of Ethnopharmacology, Vol. 11, 1984, pp. 157-179.
- A. Sofowora, “Medicinal Plants and Traditional Medicines in Africa,” John Wiley and Sons Ltd., New York, 1982.
- Clinical and Laboratory Standards Institute (CLSI), “Performance Standards for Antimicrobial Disk Susceptibility Tests,” 21st Informational Supplement, CLSI Document M100-S21, Vol. 31, No. 1, Wayne, Clinical and Laboratory Standards Institute, 2011.
- M. M. Cowan, “Plant Products as Antimicrobial Agents,” Clinical and Microbiology Reviews, Vol. 12, 1999, pp. 564-582.
- P. S. Barie, “Multidrug-Resistant Organisms and Antibiotic Management,” Surgical Clinics of North America, Vol. 92, No. 2, 2012, pp. 345-391. doi:10.1016/j.suc.2012.01.015
- P. R. Byam, R. B. Pierre, C. D. Christie, W. A. Andiman and M. Pettigrew, “Kingston Paediatric and Perinatal HIV/AIDS (KPAIDS) Study Group, Antibiotic Resistance among Pathogens Causing Disease in Jamaican Children with HIV/AIDS,” West Indian Medical Journal, Vol. 59, 2010, pp. 386-392.
NOTES
*Corresponding author.

