 American Journal of Plant Sciences, 2012, 3, 1304-1310 http://dx.doi.org/10.4236/ajps.2012.39157 Published Online September 2012 (http://www.SciRP.org/journal/ajps) DNA Barcoding of Ricinus communis from Different Geographical Origin by Using Chloroplast matK and Internal Transcribed Spacers Mohamed Enan1,2, Nael Fawzi1,3, Mohammad Al-Deeb1, Khaled Amiri1 1Department of Biology, UAE University, Al-Ain, UAE; 2Agricultural Genetic Engineering Research Institute (AGERI), Agricul- tural Research Center (ARC), Giza, Egypt; 3Flora and Phyto-Taxonomy Research Department, Horticultural Research Institute, Ag- ricultural Research Center, Giza, Egypt. Email: mohamed.enan@uaeu.ac.ae Received June 27th, 2012; revised July 23rd, 2012; accepted August 1st, 2012 ABSTRACT Ricinus communis have attracted considerable attention because of its specific industrial and pharmacological activities. DNA barcodes can be used as reliable tools to facilitate the identification of medicinal plants for the safe use, quality control and forensic investigation. In this study, the differential identification of eight accessions of R. communis was investigated through DNA sequence analysis of two candidate DNA barcodes. The nucleotide sequence of internal transcribed spacers (ITS2) and chloroplast maturase gene (matK) have been determined to construct the phylogenetic tree. The phylogenetic relationships of accessions based on the nrITS2 region and partial matK region showed that all accessions in this study were related to three geographical origins. Based on sequence alignment and phylogenetic ana- lyses we concluded that the ITS2 sequences can distinguish R. communis accessions from different geographical distri- butions. Keywords: DNA Barcoding; Internal Transcribed Spacer; Maturase K; Ricinus communis 1. Introduction The castor oil plant, Ricinus communis also known as Palma(e) christi or wonder tree. It is a perennial scrub of the family Euphorbiaceae. The plant can vary greatly in its growth habit and morphology. The variability has been increased by breeders who have selected a range of cultivars for use as ornamental plants, and for commer- cial production of castor oil [1]. The castor oil is a wonderful universal remedy for a large number of health concerns. The oil has been used as for warts, cold tumors, indurations of the abdominal organs, lacteal tumors and indurations of the mammary gland [2]. Seeds have high oil content, with multiple industrial applications such as paints, lubricants, cosmetics, polymers and biofuels [3]. DNA barcoding is a method of describing and identify- ing species by analyzing sequence information from one or a few short standardized loci amplified with universal primers. To standardize the international use of DNA barcodes, the scientific community has made consider- able efforts searching for suitable DNA regions to bar- code every species [4-8]. DNA barcoding provides a ra- pid identification tool, utilizing only minute amount of tissue from any stage of development of a plant or animal. DNA barcodes can be used to identify specimens corre- ctly, to expand the discovery of new species, in tackling illegal trade of endangered species of both plant and ani- mals and in forensic investigation to help detect poison- ous materials in life-threatening cases [9,10]. DNA bar- coding has been proposed as a novel and powerful taxo- nomic tool [6,11], the mitochondrial cytochrome oxidase subunit I (CO1) is a widely used barcode in a range of animal groups [12-15] this locus is unsuitable for use in plants due to its low mutation rate [4,14,15]. A variety of loci have been suggested as DNA barcodes for plants, including coding genes in plastid genome and the multi- copy nuclear Internal Transcribed Spacer (ITS) are two of the leading candidates [4]. Thus, this issue is add- ressed in the present study by comparing the feasibility of using each of these proposed DNA barcodes (matK, ITS1, ITS2) to identify gen etic variations of R. communis accessions from different geographical distributions. 2. Materials and Methods 2.1. Plant Materials Eight accessions from R. communis were examined. The *Corresponding a uthor. Copyright © 2012 SciRes. AJPS 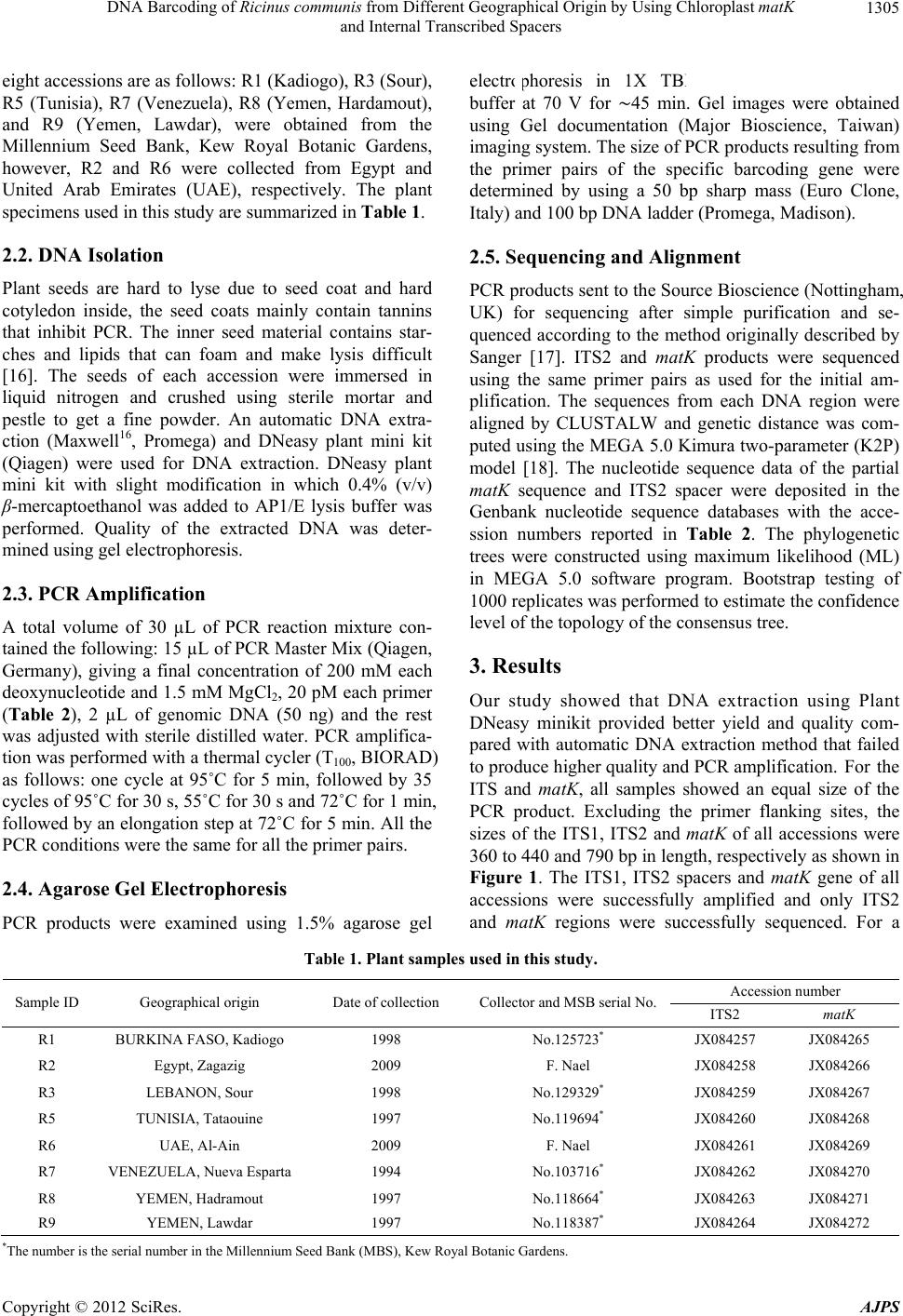 DNA Barcoding of Ricinus communis from Different Geographical Origin by Using Chloroplast matK and Internal Transcribed Spacers 1305 eight accessions are as follows: R1 (Kadiogo), R3 (Sour), R5 (Tunisia), R7 (Venezuela), R8 (Yemen, Hardamout), and R9 (Yemen, Lawdar), were obtained from the Millennium Seed Bank, Kew Royal Botanic Gardens, however, R2 and R6 were collected from Egypt and United Arab Emirates (UAE), respectively. The plant specimens used in this study are summarized in Table 1. 2.2. DNA Isolation Plant seeds are hard to lyse due to seed coat and hard cotyledon inside, the seed coats mainly contain tannins that inhibit PCR. The inner seed material contains star- ches and lipids that can foam and make lysis difficult [16]. The seeds of each accession were immersed in liquid nitrogen and crushed using sterile mortar and pestle to get a fine powder. An automatic DNA extra- ction (Maxwell16, Promega) and DNeasy plant mini kit (Qiagen) were used for DNA extraction. DNeasy plant mini kit with slight modification in which 0.4% (v/v) β-mercaptoethanol was added to AP1/E lysis buffer was performed. Quality of the extracted DNA was deter- mined using gel electrophoresis. 2.3. PCR Amplification A total volume of 30 µL of PCR reaction mixture con- tained the following : 15 µL of PCR Master Mix (Qiagen, Germany), giving a final concentration of 200 mM each deoxynucleotide and 1.5 mM MgCl2, 20 pM each primer (Table 2), 2 µL of genomic DNA (50 ng) and the rest was adjusted with sterile distilled water. PCR amplifica- tion was performed with a thermal cycler (T100 , BIORAD) as follows: one cycle at 95˚C for 5 min, followed by 35 cycles of 95˚C for 30 s, 55˚C for 30 s and 72˚C for 1 min, followed by an elongation step at 72˚C for 5 min. All the PCR conditions were the same for all the primer pairs. 2.4. Agarose Gel Electrophoresis PCR products were examined using 1.5% agarose gel electrophoresis in 1X TBE (Tris-Boric acid-EDTA) buffer at 70 V for ∼45 min. Gel images were obtained using Gel documentation (Major Bioscience, Taiwan) imaging system. The size of PCR products resulting from the primer pairs of the specific barcoding gene were determined by using a 50 bp sharp mass (Euro Clone, Italy) and 100 bp DNA ladder (Promega, Madison). 2.5. Sequencing and Alignment PCR products sent to the Source Bioscience (Notting ham, UK) for sequencing after simple purification and se- quenced according to the method originally described by Sanger [17]. ITS2 and matK products were sequenced using the same primer pairs as used for the initial am- plification. The sequences from each DNA region were aligned by CLUSTALW and genetic distance was com- puted using the MEGA 5.0 Kimura two-parameter (K2P) model [18]. The nucleotide sequence data of the partial matK sequence and ITS2 spacer were deposited in the Genbank nucleotide sequence databases with the acce- ssion numbers reported in Table 2. The phylogenetic trees were constructed using maximum likelihood (ML) in MEGA 5.0 software program. Bootstrap testing of 1000 replicates was performed to estimate the confidence level of the topology of the cons ensus tree. 3. Results Our study showed that DNA extraction using Plant DNeasy minikit provided better yield and quality com- pared with automatic DNA extraction method that failed to produce higher quality and PCR amplification . For the ITS and matK, all samples showed an equal size of the PCR product. Excluding the primer flanking sites, the sizes of the ITS1, ITS2 and matK of all accessions were 360 to 440 and 790 bp in length, resp ectiv ely as shown in Figure 1. The ITS1, ITS2 spacers and matK gene of all accessions were successfully amplified and only ITS2 and matK regions were successfully sequenced. For a Table 1. Plant samples used in this study. Accession number Sample ID Geographical origin Date of collection Collector and MSB serial No.ITS2 matK R1 BURKINA FAS O, Ka d io g o 1998 No.125723* JX084257 JX084265 R2 Egypt, Zagazig 2009 F. Nael JX084258 JX084266 R3 LEBANON, Sour 1998 No.129329* JX084259 JX084267 R5 TUNISIA, Tataouine 1997 No.119694* JX084260 JX084268 R6 UAE, Al-Ain 2009 F. Nael JX084261 JX084269 R7 VENEZUELA, Nueva Espart a 1994 No.103716* JX084262 JX084270 R8 YEMEN, Hadramout 1997 No.118664* JX084263 JX084271 R9 YEMEN, Lawdar 1997 No.118387* JX084264 JX084272 *The number is the se rial number in the Millennium Seed Bank (MBS), Kew Royal Botanic Gardens. Copyright © 2012 SciRes. AJPS 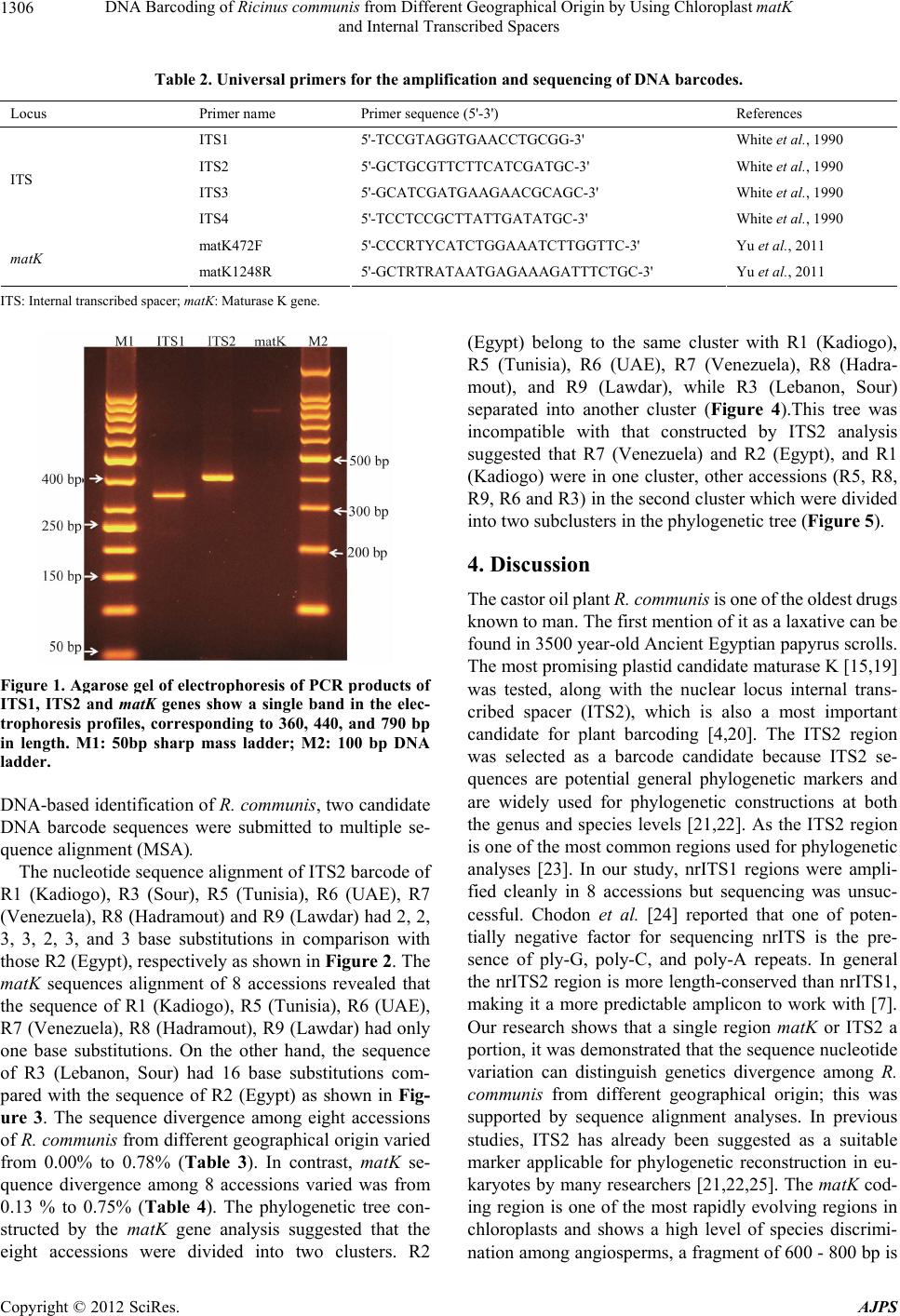 DNA Barcoding of Ricinus communis from Different Geographical Origin by Using Chloroplast matK and Internal Transcribed Spacers 1306 Table 2. Universal primers for the amplification and sequencing of DNA barcodes. Locus Primer name Primer s e q uence (5'-3') References ITS1 5'-TCCGTAGGTGAACCTGCGG-3' White et al., 1990 ITS2 5'-GCTGCGTTCTTCATCGATGC-3' White et al., 1990 ITS3 5'-GCATCGATGAAGAACGCAGC-3' White et al., 1990 ITS ITS4 5'-TCCTCCGCTTATTGATATGC-3' White et al., 1990 matK472F 5'-CCCRTYCATCTGGAAATCTTGGTTC-3' Yu et al., 2011 matK matK1248R 5'-GCTRTRATAATGAGAAAGATTTCTGC-3' Yu et al., 2011 ITS: Internal transcribed spacer; matK: Maturase K gene. Figure 1. Agarose gel of electrophoresis of PCR products of ITS1, ITS2 and matK genes show a single band in the elec- trophoresis profiles, corresponding to 360, 440, and 790 bp in length. M1: 50bp sharp mass ladder; M2: 100 bp DNA ladder. DNA-based identification of R. communis, two candidate DNA barcode sequences were submitted to multiple se- quence alignment (MSA). The nucleotide sequ ence alignment of ITS2 barcode of R1 (Kadiogo), R3 (Sour), R5 (Tunisia), R6 (UAE), R7 (Venezuela), R8 (Hadramout) and R9 (Lawdar) had 2, 2, 3, 3, 2, 3, and 3 base substitutions in comparison with those R2 (Egypt), respectively as shown in Figure 2. The matK sequences alignment of 8 accessions revealed that the sequence of R1 (Kadiogo), R5 (Tunisia), R6 (UAE), R7 (Venezuela), R8 (Hadramout), R9 (Lawdar) had only one base substitutions. On the other hand, the sequence of R3 (Lebanon, Sour) had 16 base substitutions com- pared with the sequence of R2 (Egypt) as shown in Fig- ure 3. The sequence divergence among eight accessions of R. communis from different geographical origin varied from 0.00% to 0.78% (Table 3). In contrast, matK se- quence divergence among 8 accessions varied was from 0.13 % to 0.75% (Table 4). The phylogenetic tree con- structed by the matK gene analysis suggested that the eight accessions were divided into two clusters. R2 (Egypt) belong to the same cluster with R1 (Kadiogo), R5 (Tunisia), R6 (UAE), R7 (Venezuela), R8 (Hadra- mout), and R9 (Lawdar), while R3 (Lebanon, Sour) separated into another cluster (Figure 4).This tree was incompatible with that constructed by ITS2 analysis suggested that R7 (Venezuela) and R2 (Egypt), and R1 (Kadiogo) were in one cluster, other accessions (R5, R8, R9, R6 and R3) in the second clus ter which were divided into two subclusters in the phylog enetic tree (Figure 5). 4. Discussion The castor oil plant R. communis is on e of the oldest drugs known to man. The first mention of it as a laxative can be found in 3500 year-old Ancient Egyptian papyrus scrolls. The most promising plastid candidate maturase K [15,19] was tested, along with the nuclear locus internal trans- cribed spacer (ITS2), which is also a most important candidate for plant barcoding [4,20]. The ITS2 region was selected as a barcode candidate because ITS2 se- quences are potential general phylogenetic markers and are widely used for phylogenetic constructions at both the genus and species levels [21,22]. As the ITS2 region is one of the most common regions used for phylogenetic analyses [23]. In our study, nrITS1 regions were ampli- fied cleanly in 8 accessions but sequencing was unsuc- cessful. Chodon et al. [24] reported that one of poten- tially negative factor for sequencing nrITS is the pre- sence of ply-G, poly-C, and poly-A repeats. In general the nrITS2 region is more length-conserved than nrITS1, making it a more predictable amplicon to work with [7]. Our research shows that a single region matK or ITS2 a portion, it was demonstrated that the seq uence nucleotide variation can distinguish genetics divergence among R. communis from different geographical origin; this was supported by sequence alignment analyses. In previous studies, ITS2 has already been suggested as a suitable marker applicable for phylogenetic reconstruction in eu- karyotes by many researchers [21,22,25]. The matK cod- ing region is one of the most rapidly evolving regions in chloroplasts and shows a high level of species discrimi- nation among angiosperms, a fragment of 600 - 800 bp is Copyright © 2012 SciRes. AJPS 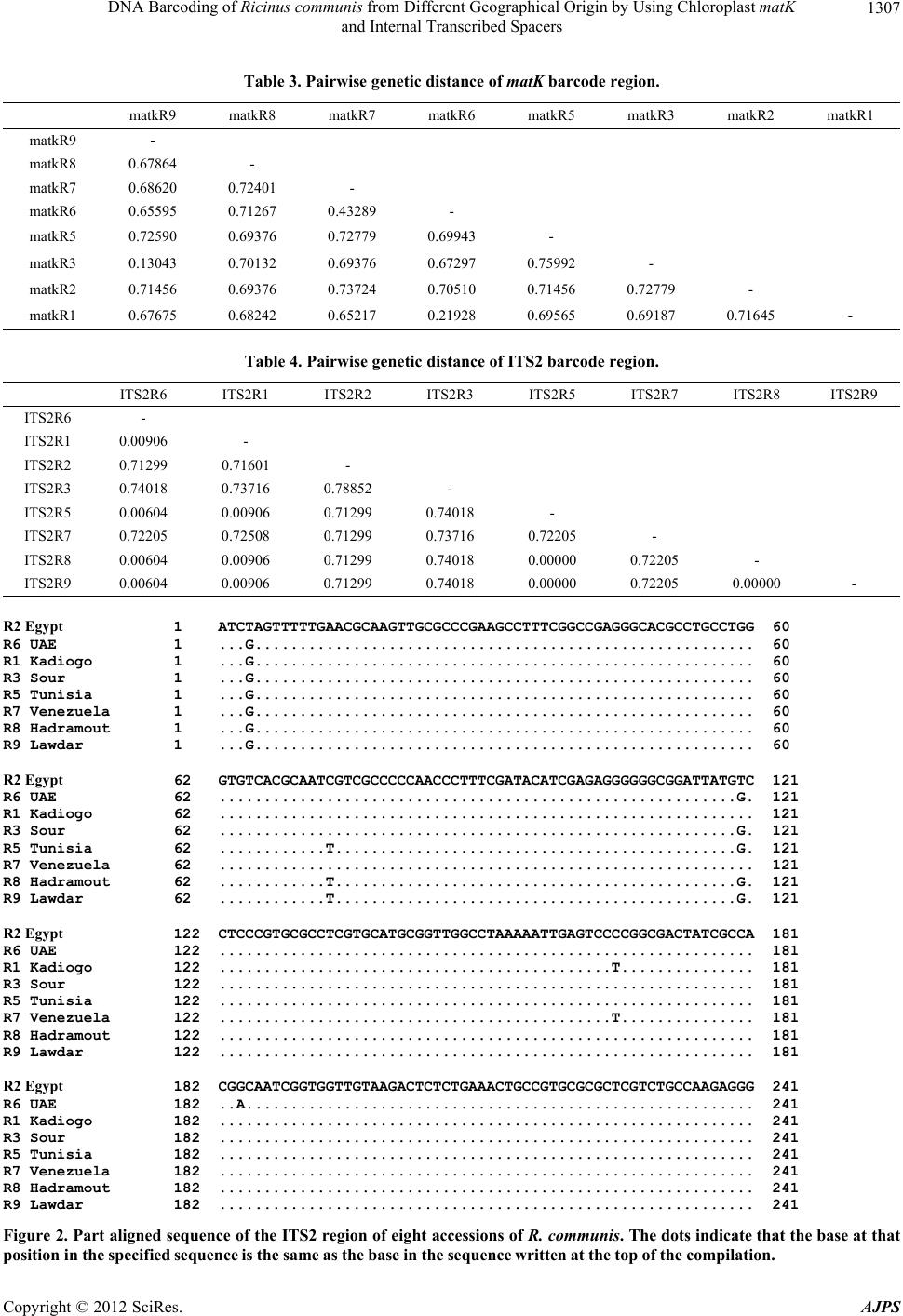 DNA Barcoding of Ricinus communis from Different Geographical Origin by Using Chloroplast matK and Internal Transcribed Spacers 1307 Table 3. Pairwise genetic distance of matK barcode region. matkR1 matkR2 matkR3 matkR5 matkR6 matkR7 matkR8 matkR9 - matkR9 - 0.67864 matkR8 - 0.72401 0.68620 matkR7 - 0.43289 0.71267 0.65595 matkR6 - 0.69943 0.72779 0.69376 0.72590 matkR5 - 0.75992 0.67297 0.69376 0.70132 0.13043 matkR3 - 0.72779 0.71456 0.70510 0.73724 0.69376 0.71456 matkR2 - 0.71645 0.69187 0.69565 0.21928 0.65217 0.68242 0.67675 matkR1 Table 4. Pairwise genetic distance of ITS2 barcode region. ITS2R9 ITS2R8 ITS2R7 ITS2R5 ITS2R3 ITS2R2 ITS2R1 ITS2R6 - ITS2R6 - 0.00906 ITS2R1 - 0.71601 0.71299 ITS2R2 - 0.78852 0.73716 0.74018 ITS2R3 - 0.74018 0.71299 0.00906 0.00604 ITS2R5 - 0.72205 0.73716 0.71299 0.72508 0.72205 ITS2R7 - 0.72205 0.00000 0.74018 0.71299 0.00906 0.00604 ITS2R8 - 0.00000 0.72205 0.00000 0.74018 0.71299 0.00906 0.00604 ITS2R9 R2 Egypt 1 ATCTAGTTTTTGAACGCAAGTTGCGCCCGAAGCCTTTCGGCCGAGGGCACGCCTGCCTGG 60 R6 UAE 1 ...G........................................................ 60 R1 Kadiogo 1 ...G........................................................ 60 R3 Sour 1 ...G........................................................ 60 R5 Tunisia 1 ...G........................................................ 60 R7 Venezuela 1 ...G........................................................ 60 R8 Hadramout 1 ...G........................................................ 60 R9 Lawdar 1 ...G........................................................ 60 R2 Egypt 62 GTGTCACGCAATCGTCGCCCCCAACCCTTTCGATACATCGAGAGGGGGGCGGATTATGTC 121 R6 UAE 62 ..........................................................G. 121 R1 Kadiogo 62 ............................................................ 121 R3 Sour 62 ..........................................................G. 121 R5 Tunisia 62 ............T.............................................G. 121 R7 Venezuela 62 ............................................................ 121 R8 Hadramout 62 ............T.............................................G. 121 R9 Lawdar 62 ............T.............................................G. 121 R2 Egypt 122 CTCCCGTGCGCCTCGTGCATGCGGTTGGCCTAAAAATTGAGTCCCCGGCGACTATCGCCA 181 R6 UAE 122 ............................................................ 181 R1 Kadiogo 122 ............................................T............... 181 R3 Sour 122 ............................................................ 181 R5 Tunisia 122 ............................................................ 181 R7 Venezuela 122 ............................................T............... 181 R8 Hadramout 122 ............................................................ 181 R9 Lawdar 122 ............................................................ 181 R2 Egypt 182 CGGCAATCGGTGGTTGTAAGACTCTCTGAAACTGCCGTGCGCGCTCGTCTGCCAAGAGGG 241 R6 UAE 182 ..A......................................................... 241 R1 Kadiogo 182 ............................................................ 241 R3 Sour 182 ............................................................ 241 R5 Tunisia 182 ............................................................ 241 R7 Venezuela 182 ............................................................ 241 R8 Hadramout 182 ............................................................ 241 R9 Lawdar 182 ............................................................ 241 Figure 2. Part aligned sequence of the ITS2 region of eight accessions of R. communis. The dots indicate that the base at that position in the specifi ed s eq uence is the same as th e b ase in the seq uen ce written at the top of t he compilation. Copyright © 2012 SciRes. AJPS  DNA Barcoding of Ricinus communis from Different Geographical Origin by Using Chloroplast matK and Internal Transcribed Spacers 1308 matKR2 Egypt 1 GACTCTTTCTTCATGAGTATTGGAATTGGAACAGTTTTATTATTCCGAAAGAAATCAATT 59 matKR6 UAE 1 ............................................................ 59 matKR1 Kadiogo 1 ............................................................ 59 matKR3 Sour 1 ..........G...............G..CC..T....T..............G...... 59 matKR5 Tunisia 1 ............................................................ 59 matKR7 Venezuela 1 ............................................................ 59 matKR8 Hadramout 1 ............................................................ 59 matKR9 Lawdar 1 ............................................................ 59 matKR2 Egypt 60 TCTATTTTTACAAAAAGTAATCCAAGATTTTTCGTGTTCCTATATAATTCTCATGTATAT 119 matKR6 UAE 60 ............................................................ 119 matKR1 Kadiogo 60 ............................................................ 119 matKR3 Sour 60 ..................................G..................A.A.... 119 matKR5 Tunisia 60 ............................................................ 119 matKR7 Venezuela 60 ............................................................ 119 matKR8 Hadramout 60 ............................................................ 119 matKR9 Lawdar 60 ............................................................ 119 matKR2 Egypt 120 GAATATGAATCCCTCTTCTTTTTTCTCCGTAACCAATCCTTTCATTTACGATCAACATTT 179 matKR6 UAE 120 ............................................................ 179 matKR1 Kadiogo 120 ............................................................ 179 matKR3 Sour 120 ...............G....G....C.................................. 179 matKR5 Tunisia 120 ............................................................ 179 matKR7 Venezuela 120 ............................................................ 179 matKR8 Hadramout 120 ............................................................ 179 matKR9 Lawdar 120 ............................................................ 179 matKR2 Egypt 360 AAATATTACCTTGTCCATTTATGTCAATGTCATTTTTATGTGTGGTTTCAACCGGAAAAG 419 matKR6 UAE 359 ............................................................ 418 matKR1 Kadiogo 360 ............................................................ 419 matKR3 Sour 360 .................................................C.......... 419 matKR5 Tunisia 360 ............................................................ 419 matKR7 Venezuela 360 ............................................................ 419 matKR8 Hadramout 360 ............................................................ 419 matKR9 Lawdar 360 ............................................................ 419 matKR2 Egypt 420 ATCTATATAAATTCATTATCTAAGCATTCTCTCAACTTTTTGGGCTATCTTTCAAATGTA 479 matKR6 UAE 420 ............................................................ 479 matKR1 Kadiogo 420 ............................................................ 479 matKR3 Sour 420 ....................................................G....... 479 matKR5 Tunisia 420 ............................................................ 479 matKR7 Venezuela 420 ............................................................ 479 matKR8 Hadramout 420 ............................................................ 479 matKR9 Lawdar 420 ............................................................ 479 matKR2 Egypt 480 CAATTTAATCCTTCGTTGGTACGGAGTCAAATGAAAGAAAAT 521 matKR6 UAE 480 ..................................T....... 521 matKR1 Kadiogo 480 ..................................T....... 521 matKR3 Sour 480 ..................................T....... 521 matKR5 Tunisia 480 ..................................T....... 521 matKR7 Venezuela 480 ..................................T....... 521 matKR8 Hadramout 480 ..................................T....... 521 matKR9 Lawdar 480 ..................................T....... 521 Figure 3. Part aligned se quenc e of the matK barcode region of eight accessions of R. communis. The dots indicate that the bas e at that position in the s pecified sequence is the sam e as th e bas e in the s equ ence wr itten at the top of the compilation. matKR8 matKR7 matKR6 matKR5 matKR1 matKR9 matKR2 matKR3 Figure 4. Maximum likelihood tree constructed by partial sequence of matK gene from eight R. communis accession. usually sufficient [15,26]. The matK region varied suffi- ciently to distinguish “Sanqi” (Panax notoginseng; Ar- aliaceae) from different geographical origins [27], but it failed to differentiate among Sanqi cultivars [28]. The partial matK sequence of 7 accessions (R1, R5, R6, R7, R8, and R9) shows only one nucleotide substations at Copyright © 2012 SciRes. AJPS  DNA Barcoding of Ricinus communis from Different Geographical Origin by Using Chloroplast matK and Internal Transcribed Spacers 1309 ITS2R5 69 ITS2R8 26 ITS2R9 ITS2R3 ITS2R6 ITS2R2 ITS2R1 55 ITS2R7 73 Figure 5. Maximum likelihood tree constructed ITS2 sequence from eight R. communis accessions. one position compared with accession R2 from Egypt. However, R3 accession from Lebanon had 16 base sub- stitutions. In this study access ions from Yemen, Kadiogo, Venezuela, United Arab Emirates and Tunisia had the same matK sequence which might be ascribed to the same ancestor and different Environment. In the present study, nrITS2 sequence was found to correlate with geo- graphical distributions of the samples which matK gene sequence was conserved than the ITS2. 5. Conclusion Based on our own findings, we propose that ITS2 be used as the desired barcode to study geographical dis- tributions of Euphorbiaceae species. REFERENCES [1] B. Christopher, “The Royal Horticultural Society A-Z Encyclopedia of Garden Plants,” Dorling Kindersley, London, 1996, pp. 884-885. [2] A. Scarpa and A. Guerci, “Various Uses of the Castor Oil Plant (Ricinus communis L.): A Review,” Journal of Ethnopharmacology, Vol. 5, No. 2, 1982, pp. 117-137. doi:10.1016/0378-8741(82)90038-1 [3] M. F. Jose and V. Leonardo, “Castor,” In: S. K. Gupta, Ed., Technological Innovations in Major World Oil Crops, Volume 1: Breeding, Springer, 2012, pp. 237-265. [4] W. J. Kress, K. J. Wurdack, E. A. Zimmer, L. A. Weigt and D. H. Janzen, “Use of DNA Barcodes to Identify Flowering Plants,” Proceedings of the National Academy of Sciences of the USA, Vol. 102, 2005, pp. 8369-8374. doi:10.1073/pnas.0503123102 [5] W. J. Kress and D. L. Erickson, “A Two-Locus Global DNA Barcode for Land Plants: The Coding rbcL Gene Complements the Non-Coding trnH-psbA Spacer Re- gion,” PloS One, Vol. 2, 2007, p. e508. doi:10.1371/journal.pone.0000508 [6] CBOL Plant Working Group, “A DNA Barcode for Land Plants,” Proceedings of the National Academy of Sciences of the USA, Vol. 106, 2009, pp. 12794-12797. doi:10.1073/pnas.0905845106 [7] S. Chen, H. Yao, J. Han, C. Liu, J. Song, L. Shi, Y. Zhu, X. Ma, T. Gao, X. Pang, K. Luo, Y. Li, X. Li, X. Jia, Y. Lin and C. Leon, “Validation of ITS2 Region as a Novel DNA Barcode for Identifying Medicinal Plant Species,” PLoS One, Vol. 5, 2010, p. e8613. doi:10.1371/journal.pone.0008613 [8] H. Yao, J. Song, C. Liu, K. Luo, J. Han, Y. Li, X. Pang, H. Xu, Y. Zhu, P. Xiao and S. Chen, “Use of ITS2 Re- gion as the Universal DNA Barcode for Plants and Ani- mals,” PLoS One, Vol. 5, No. 10, 2010, p. e13102. doi:10.1371/journal.pone.0013102 [9] E. M. Soininen, A. Valentini, E. Coissac, C. Miquel, L. Gielly, C. Brochmann, A. K. Brysting, J. H Sonstebo, R. A. Ims , N. G. Yoc co z an d P. Taberlet, “Analyzing Diet of Small Herbivores: The Efficiency of DNA Barcoding Coupled with High-Throughput Pyrosequencing for De- ciphering the Composition of Complex Plant Mixtures,” Frontier in Zoology, Vol. 6, 2009, p. 16. doi:10.1186/1742-9994-6-16 [10] M. Stech, E. Kolvoort, M. J. J. E Loonen, K. Verieling and J. D. Kruijer, “Bryophyte DNA Sequences from Fae- ces of an Arctic Herbivore, Barnacle Goose (Branta leu- copsis),” Molecular Ecology Resources, Vol. 11, No. 2, 2011, pp. 404-408. doi:10.1111/j.1755-0998.2010.02938.x [11] K. M. Evans, A. H. Wortley and D. G. Mann, “An As- sessment of Potential Diatom ‘Barcode’ Genes (cox1, rbcL, 18S and ITS rDNA) and Their Effectiveness in De- termining Relationships in Sellaphora (Bacillariophyta),” Protist, Vol. 158, No. 3, 2007, pp. 349-364. doi:10.1016/j.protis.2007.04.001 [12] P. D. Hebert, S. Ratnasingham and J. R. deWaard, “Bar- coding Animal Life: Cytochrome c Oxidase Subunit 1 Divergences among Closely Related Species,” Proceed- ings of the Royal Society B: Biological Sciences, Vol. 270, 2003, pp. 95-99. [13] M. Hajibabaei, G. A. C. Singer, P. D. N. Hebert and D. A. Hickey, “DNA Barcoding: How It Complements Tax- onomy, Molecular Phylogenetics and Population Genet- ics,” Trends in Genetics, Vol. 23, No. 4, 2007, pp. 167- 172. doi:10.1016/j.tig.2007.02.001 [14] R. S. Cowan, M. W. Chase, W. J. Kress and V. Savolai- nen, “300,000 Species to Identify: Problems, Progress, and Prospects in DNA Barcoding of Land Plants,” Taxon, Vol. 55, No. 3, 2006, pp. 611-616. doi:10.2307/25065638 [15] A. J. Fazekas, K. S. Burgess, P. R. Kesanakurti, S. W. Graham and S. G. Newmaster, “Multiple Multilocus Copyright © 2012 SciRes. AJPS  DNA Barcoding of Ricinus communis from Different Geographical Origin by Using Chloroplast matK and Internal Transcribed Spacers 1310 DNA Barcodes from the Plastid Genome Discriminate Plant Species Equally Well,” PLoS One, Vol. 3, No. 7, 2008, p. e2802. doi:10.1371/journal.pone.0002802 [16] D. A. Eisenbarth and A. R. Weig, “Dynamics of Aquapo- rins and Water Relations during Hypocotyl Elongation in Ricinus communis L. Seedlings,” Journal of Experimental Botany, Vol. 56, No. 417, 2005, pp. 1831-1842. doi:10.1093/jxb/eri173 [17] F. Sanger, S. Nicklen and A. R. Coulson, “DNA Se- quencing with Chain-Terminating Inhibitors,” Proceed- ings of the National Academy of Sciences of the USA, Vol. 74, 1977, pp. 5463-5467. doi:10.1073/pnas.74.12.5463 [18] K. Tamura, D. Peterson, N. Peterson, G. Stecher, M. Nei and S. Kumar, “MEGA5: Molecular Evolutionary Genet- ics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods,” Molecular Biology and Evolution, Vol. 28, No. 10, 2011, pp. 2731- 2739. doi:10.1093/molbev/msr121 [19] P. M. Hollingsworth, S. W. Graham and D. P. Little, “Choosing and Using a Plant DNA Barcode,” PLoS One, Vol. 6, No. 5, 2011, p. e19254. doi:10.1371/journal.pone.0019254 [20] M. W. Chase, N. Salamin, M. Wilkinson, J. M. Dunwell, R. P. Kesanakurthi, N. Haidar and V. Savolainen, “Land Plants and DNA Barcodes: Short-Term and Long-Term Goals,” Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 360, 2005, pp. 1889-1895. doi:10.1098/rstb.2005.1720 [21] J. Schultz, S. Maisel, D. Gerlach, T. Müller and M. Wolf, “A Common Core of Secondary Structure of the Internal Transcribed Spacer 2 (ITS2) throughout the Eukaryota,” RNA, Vol. 11, 2005, pp. 361-364. doi:10.1261/rna.7204505 [22] M. Miao, A. Warren, W. B. Song, S. Wang and H. M. Shang, “Analysis of the Internal Transcribed Spacer 2 (ITS2) Region of Scuticociliates and Related Accessions (Ciliophora, Oligohymenophorea) to Infer Their Evolu- tion and Phylogeny,” Protist, Vol. 159, No. 4, 2008, pp. 519-533. doi:10.1016/j.protis.2008.05.002 [23] P. B. Pelser, B. Nordenstam, J. W. Kadereit and L. E. Watson, “An ITS Phylogeny of Tribe Senecioneae (As- teraceae) and a New Delimitation of Senecio L.,” Taxon, Vol. 5694, 2007, pp. 1062-1077. [24] S. Chodon, D. P. Little, D. W. Stevenson and C. D. Specht, “DNA Barcoding in the Cycadales: Testing the Potential of Proposed Barcoding Markers for Species Identification of Cycads,” PLoS One, Vol. 11, 2007, p. e1154. [25] A. W. Coleman, “Pan-Eukaryote ITS2 Homologies Re- vealed by RNA Secondary Structure,” Nucleic Acid Re- search, Vol. 35, No. 10, 2007, pp. 3322-3329. doi:10.1093/nar/gkm233 [26] F. W. Li, L. Y. Kuo, C. J. Rothfels, A. Ebihara, W. L. Chiou, M. D. Windham and K. M. Pryer, “rbcL and matK Earn Two Thumbs Up as the Core DNA Barcode for Ferns,” PLoS One, Vol. 6, No. 10, 2011, p. e26597. doi:10.1371/journal.pone.0026597 [27] H. Fushimi, K. Komatsu, T. Namba and M. Isobe, “Ge- netic Heterogeneity of Ribosomal RNA Gene and matK Gene in Panax Notoginseng,” Planta Medica, Vol. 66, N o . 7, 2000, pp. 659-661. doi:10.1055/s-2000-8636 [28] Y. Zhang, J. C. Zhang, M. H. Huang, M. S. Yang and H. Cao, “Detection of Genetic Homogeneity of Panax Noto- ginseng Cultivars by Sequencing Nuclear 18S rRNA and Plastid matK Genes,” Planta Medica, Vol. 72, 2006, pp. 860-862. doi:10.1055/s-2006-946685 Copyright © 2012 SciRes. AJPS
|