 American Journal of Plant Sciences, 2012, 3, 1181-1186 http://dx.doi.org/10.4236/ajps.2012.39143 Published Online September 2012 (http://www.SciRP.org/journal/ajps) 1181 An Improved System for Shoot Regeneration from Stem Explants of Lombardy Poplar (Populus nigra L. var. italica Koehne) Kamal Kanti Biswas1, Takeshi Mohri2, Satoshi Kogawara2, Yoshihiro Hase1, Issay Narumi1, Yutaka Oono1 1Medical and Biotechnological Application Division, Japan Atomic Energy Agency (JAEA), Takasaki, Japan; 2Forestry and Forest Products Research Institute (FFPRI), Tsukuba, Japan. Email: biswas.kamal@jaea.go.jp Received July 5th, 2012; revised July 30th, 2012; accepted August 10th, 2012 ABSTRACT We developed a system for the regeneration of Lombardy poplar (Populus nigra L. var. italica ) shoo ts from internodal stem explants. Using this system, shoots regenerated from 87% of the stem explants placed on Murashige and Skoog (MS) medium supplemented with 0.1 mg/L indole-3-acetic acid and 0.5 mg/L benzylaminopurine without undergoing callus formation. About 80% of the in vitro regenerated shoots developed roots on MS medium supplemented with 0.5 mg/L indole-3-butyric acid and 0.02 mg/L 1-naphthylacetic acid. Well-rooted seven-to eight-week-old regenerated plants could be transferred to soil for further growth and the survival rate of such plants after three weeks was 88%. The protocol presented here is simple and economical because it does not rely on pre-incubation in callus induction medium or repeated subculture in shoot induction medium containing trans-zeatin, an expensive substance. The in vitro regen- eration system presented here could be used for evaluation of radiation sensitivity for Lombardy poplar tissues. Keywords: Lombardy Poplar; Shoot Regeneration; Stem Explants; Auxin and Benzylaminopurine; Radiation Sensitivity 1. Introduction Poplars are of growing scientific importance due to their small genome size, short rotation cycle, ability to under- go in vitro regeneration, and ease of transformation and vegetative propagation. These important traits, coupled with the existence of a library of full-length enriched expressed sequence tags [1] and availability of the ge- nome sequence of Populus tricocarpa, have established poplar as a model system in molecular genetics [2-5] and tree physiology [6-8] studies. Furthermore, these rapidly growing plants, which have the potential to enhance wood supplies for the plywood, hardboard, pulp, and paper industries, represent a commercially important re- source [9]. In vitro plant regeneration of Lombardy poplar [11] or its close hybrids [10,12-14] was achieved from leaf or stem explants. These methods consists of two steps of tissue culture stages; first, stem or leaf explants were in- cubated on the medium containing high concentration of plant hormone auxin (in most case, it is called callus in- ducing medium, CIM) for several days then transferred to shoot induction medium (SIM) that dominantly con- tains plant hormone cytokinin. The system is also avail- able for Agrobacterium-mediated gene transformation [10,11,15]. In an early study of Lombardy poplar regen- eration by Mohri et al. [15], 0.5 mg/L 2,4-dichlorophe- noxyacetic acid (2,4-D) was added to CIM for calli in- duction. Then, four weeks old calli developed in CIM were repeatedly subcultured in SIM containing 2 mg/L trans-zeatin and 0.2 mg/L benzylaminopurine (BA), fol- lowed by re-transfer to root induction medium (RIM). In later by Nishiguchi et al. [11], much simple regeneration method had been developed for Lombardy poplar rege- neration, without callus formation. But, still they relied on pre incubation of explants in CIM (0.5 mg/L 2,4-D and 1 mg/L BA) for 6 days. There after, pre incubated explants in CIM were subcultured in SIM (0.5 mg/L zeatin and 0.1 mg/L BA) for several times, followed by re-transfer to RIM. However, in our experience, we were unable to perform efficient shoot regeneration with these protocols. Thus, we tried to re-optimize shoot regenera- tion protocol for Lombardy poplar by using different types of auxins and cytokinins at various concentrations. Here, we report a simple shoot regeneration protocol from stem explants of Lombardy poplar that is charac- Copyright © 2012 SciRes. AJPS 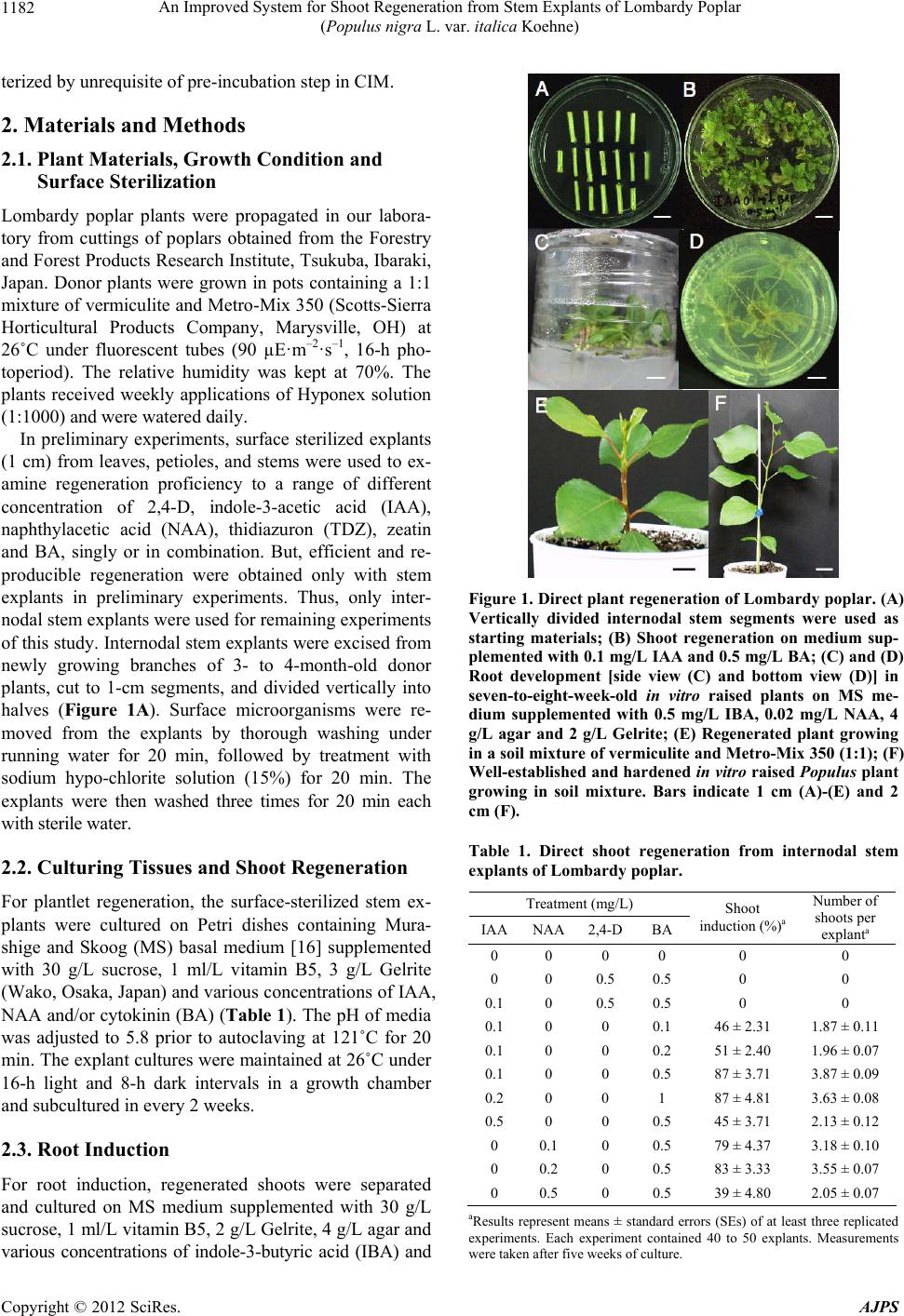 An Improved System for Shoot Regeneration from Stem Explants of Lombardy Poplar (Populus nigra L. var. italica Koehne) 1182 terized by unre qui si te of p re-incubation ste p i n CIM. 2. Materials and Methods 2.1. Plant Materials, Growth Condition and Surface Sterilization Lombardy poplar plants were propagated in our labora- tory from cuttings of poplars obtained from the Forestry and Forest Products Research Institu te, Tsukuba, Ibaraki, Japan. Donor plants were grown in pots containing a 1:1 mixture of v ermiculite and Metro-Mix 3 50 (Scotts-Sierra Horticultural Products Company, Marysville, OH) at 26˚C under fluorescent tubes (90 µE·m–2·s–1, 16-h pho- toperiod). The relative humidity was kept at 70%. The plants received weekly applications of Hyponex solution (1:1000) and were watered daily. In preliminary experiments, surface sterilized explants (1 cm) from leaves, petioles, and stems were used to ex- amine regeneration proficiency to a range of different concentration of 2,4-D, indole-3-acetic acid (IAA), naphthylacetic acid (NAA), thidiazuron (TDZ), zeatin and BA, singly or in combination. But, efficient and re- producible regeneration were obtained only with stem explants in preliminary experiments. Thus, only inter- nodal stem explants were used for remaining experiments of this study. Intern odal stem explants were excised from newly growing branches of 3- to 4-month-old donor plants, cut to 1-cm segments, and divided vertically into halves (Figure 1A). Surface microorganisms were re- moved from the explants by thorough washing under running water for 20 min, followed by treatment with sodium hypo-chlorite solution (15%) for 20 min. The explants were then washed three times for 20 min each with sterile water. 2.2. Culturing Tissues and Shoot Regeneration For plantlet regeneration, the surface-sterilized stem ex- plants were cultured on Petri dishes containing Mura- shige and Skoog (MS) basal medium [16] supplemented with 30 g/L sucrose, 1 ml/L vitamin B5, 3 g/L Gelrite (Wako, Osaka, Japan) and various concentrations of IAA, NAA and/or cytokinin (BA) (Table 1). The pH of media was adjusted to 5.8 prior to autoclaving at 121˚C for 20 min. The explant cu ltures were maintained at 26˚C under 16-h light and 8-h dark intervals in a growth chamber and subcultured in every 2 weeks. 2.3. Root Induction For root induction, regenerated shoots were separated and cultured on MS medium supplemented with 30 g/L sucrose, 1 ml/L vitamin B5, 2 g/L Gelrite, 4 g/L agar and various concentrations of indole-3-butyric acid (IBA) and Figure 1. Direct plant regeneration of Lombardy poplar. (A) Vertically divided internodal stem segments were used as starting materials; (B) Shoot regeneration on medium sup- plemented with 0.1 mg/L IAA and 0.5 mg/L BA; (C) and (D) Root development [side view (C) and bottom view (D)] in seven-to-eight-week-old in vitro raised plants on MS me- dium supplemented with 0.5 mg/L IBA, 0.02 mg/L NAA, 4 g/L agar and 2 g/L Gelrite; (E) Regenerated plant growing in a soil mixture of vermiculite and Metro-Mix 350 (1:1); (F) Well-established and hardened in vitro raised Populus plant growing in soil mixture. Bars indicate 1 cm (A)-(E) and 2 cm (F). Table 1. Direct shoot regeneration from internodal stem explants of Lombardy poplar. Treatment (mg/L) IAANAA2,4-DBA Shoot induction (%)a Number of shoots per explanta 0 0 0 0 0 0 0 0 0.5 0.5 0 0 0.1 0 0.5 0.5 0 0 0.1 0 0 0.1 46 ± 2.31 1.87 ± 0.11 0.1 0 0 0.2 51 ± 2.40 1.96 ± 0.07 0.1 0 0 0.5 87 ± 3.71 3.87 ± 0.09 0.2 0 0 1 87 ± 4.81 3.63 ± 0.08 0.5 0 0 0.5 45 ± 3.71 2.13 ± 0.12 0 0.1 0 0.5 79 ± 4.37 3.18 ± 0.10 0 0.2 0 0.5 83 ± 3.33 3.55 ± 0.07 0 0.5 0 0.5 39 ± 4.80 2.05 ± 0.07 aResults r epresent means ± standard errors (SEs) of at least three replicated experiments. Each experiment contained 40 to 50 explants. Measurements were taken after five weeks of culture. Copyright © 2012 SciRes. AJPS  An Improved System for Shoot Regeneration from Stem Explants of Lombardy Poplar (Populus nigra L. var. italica Koehne) 1183 NAA. Again, the pH of the medium was adjusted to 5.8. 2.4. Irradiation of Tissues For investigation of radiation dose response relationship on viability test and growth proficiency of regenerated shoots, stem explants were excised to 1 cm, surface ster- ilized, divided vertically into halves and cultured on me- dium with 0.1 mg/L IAA and 0.5 mg/L BA in Petri dishes. One week after from culture, Petri dishes con- taining stem segments were subjected for irrad iation with a range of doses (0 Gy to 30 Gy). The explants were ex- posed to 320 MeV carbon ions accelerated by the azi- muthally varying field cyclotron [22,23] or gamma rays. Gamma rays obtained from 60Co source maintaining at Japan Atomic Energy Agency (Takasaki, Japan). Irradia- tion time for gamma rays was fixed to 30 min. Shoots above 1 cm in length at five weeks after irradiation were counted as regenerated shoots. Regeneration ratio was calculated with dividing the number of stem explants that produce regenerated shoots by total number of stem ex- plants irradiated. The regeneration ratio of non-irradiated control stems was plotted as 100%. 3. Results and Discussion 3.1. Direct Shoot Regeneration In preliminary experiments, we cultured explants from leaves, petioles, and shoots to medium supplemented with different concentrations of 2,4-D, IAA, NAA, TDZ, zeatin and BA, singly or in combination (d ata not shown). Leaf and petiole explants did not develop any robust calli or shoots on any of the media mentioned above. On the other hand, stem explants developed calli on medium supplemented with 0.5 mg/L 2,4-D and 0.5 mg/L BA; however, no shoots emerged from these calli until five weeks of culture (Table 1). Interestingly, we found that direct shoot regeneration occurred when stem explants were cultured on medium supplemented with NAA and IAA in combination with BA (Table 1 and Figure 1(B)). Over 87% of the stem explants produced at least one shoot on medium supplemented with 0.1 mg/L IAA and 0.5 mg/L BA. The average number of shoots per explant was 3.87 ± 0.09 (Table 1). A further increase of IAA to 0.2 mg/L or 0.5 mg/L and BA to 1 mg/L failed to im- prove the regeneration efficiency. Thus, we used 0.1 mg/L IAA and 0.5 mg/L BA for the direct regeneration of Lombardy poplar in this study. Application of the synthetic auxin analog NAA yielded similar results as IAA. Over 83% of the explants induced shoots on me- dium supplemented with 0.2 mg/L NAA and 0.5 mg/L BA, and the average number of shoots per explant was 3.55 + 0.07 (Table 1). Once again, an increased NAA concentration (0.5 mg/L) failed to improve the regenera- tion efficiency (Table 1). In Eastern Co ttonwood (Popu- lus deltoides Bartram ex Marsh.), addition of cytokinin (1 mg/L zeatin) alone resulted in the most efficient shoot regeneration from stem explants in woody plant medium (WPM) [17]. However, we found that cytokinin alone could not stimulate the gro wth of calli or shoots in Lom- bardy poplar and an optimal combination of auxin and cytokinin (BA) was required for direct shoot regeneration. This indicates that the optimal hormone concentration for direct shoot regeneration in poplar is genotype specific. Alternatively, the difference in response might be for the reason of different basal medium, although there is no major difference between the composition of WPM and MS. Thus, for efficient regeneration, appropriate combi- nation and concentration of growth regulators need to be determined precisely. 3.2. Root Induction To optimize root induction conditions, the directly re- generated shoots were cultured on media con taining IBA and NAA. In agreement with a report [1 1], we fo und that a combination of 0.5 mg/L IBA and 0.02 mg/L NAA was the most effective dose for root development in Lom- bardy poplar (Figure 1(C) and 1(D)), although 0.5 mg/L IBA alone could induce roots in 50% of the regenerated shoots (Table 2). Doubling the concen tration of IBA and NAA in the medium did not improve the root induction efficiency (Table 2). The average number of roots per shoot in the presence of 0.5 mg/L IBA and 0.02 mg/L NAA was 5.2 ± 0.34 (Table 2). We separated the well-rooted plants that were approxi- mately seven weeks old from the culture medium, trans- ferred them to pots containing a 1:1 mixture of vermicu- lite and Metro-Mix 350 and incubated them at 26˚C un- der a 16/8 h light/dark regimen. Figure 1(E) shows a re- generated plant growing in soil mixture. Initially, the potted plants were covered with clear plastic bags to pro- tect them from any direct physical stress, and the plants Table 2. Root induction in the regenerated shoots. Treatment (mg/L) IBA NAA Root induction (%)a Number of roots per shoota 0 0 0 0 0.5 0 50.00 ± 4.33 3.6 ± 0.24 0 0.02 0 - 0.5 0.01 60.83 ± 4.41 3.67 ± 0.12 0.5 0.02 78.33 ± 3.00 5.2 ± 0.34 1 0.04 75.83 ± 3.63 5.1 ± 0.31 aResults represent means ± SEs of at least three replicated experiments. Each experiment contained 40 to 50 regenerated shoots. Measurements were taken after seven weeks of culture. Copyright © 2012 SciRes. AJPS 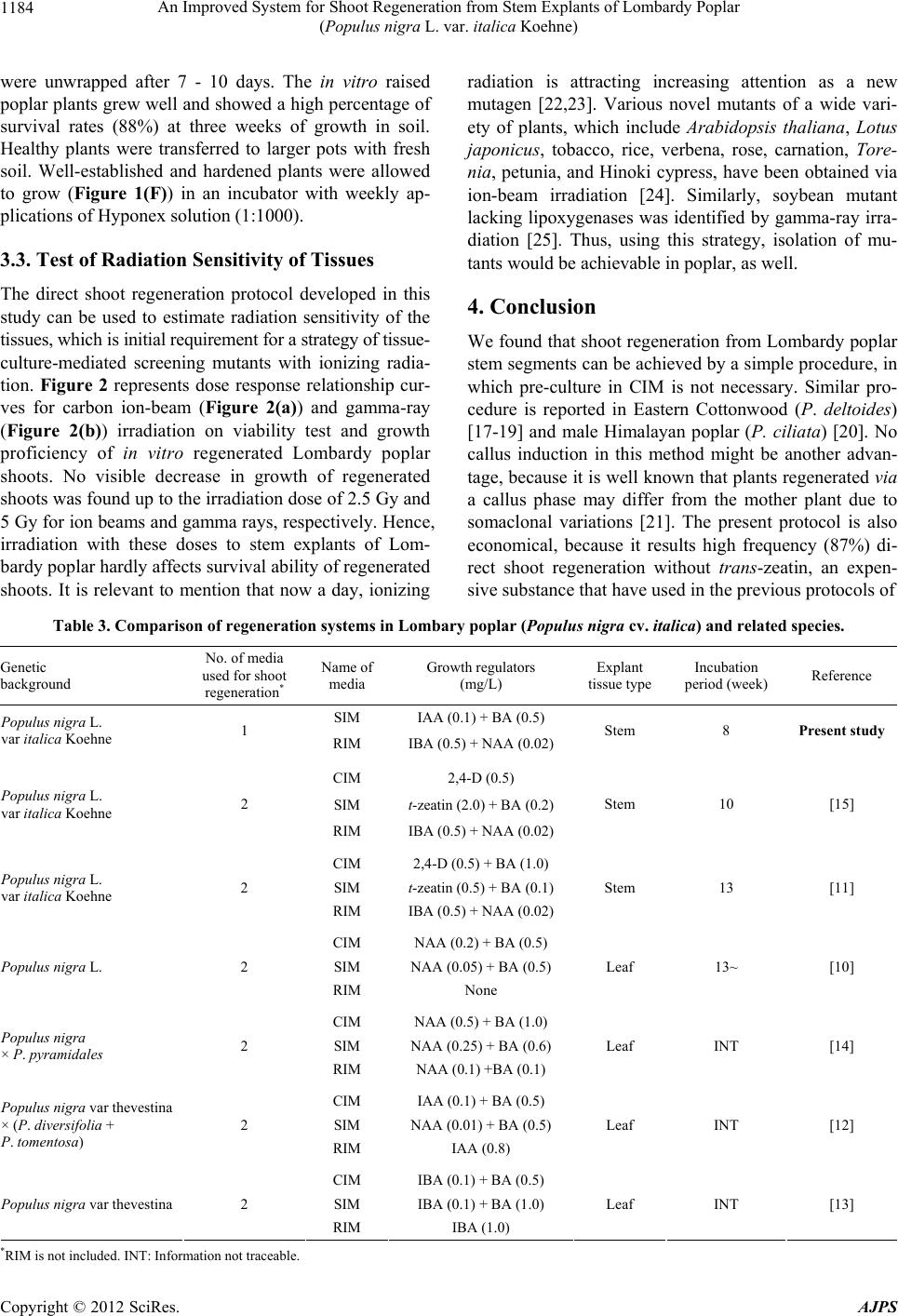 An Improved System for Shoot Regeneration from Stem Explants of Lombardy Poplar (Populus nigra L. var. italica Koehne) Copyright © 2012 SciRes. AJPS 1184 were unwrapped after 7 - 10 days. The in vitro raised poplar plants grew well and showed a high percentage of survival rates (88%) at three weeks of growth in soil. Healthy plants were transferred to larger pots with fresh soil. Well-established and hardened plants were allowed to grow (Figure 1(F)) in an incubator with weekly ap- plications of Hyponex solution (1:1000). 3.3. Test of Radiation Sensitivity of Tissues The direct shoot regeneration protocol developed in this study can be used to estimate radiation sensitivity of the tissues, which is initial requirement for a strategy of tissue- culture-mediated screening mutants with ionizing radia- tion. Figure 2 represents dose response relationship cur- ves for carbon ion-beam (Figure 2(a)) and gamma-ray (Figure 2(b)) irradiation on viability test and growth proficiency of in vitro regenerated Lombardy poplar shoots. No visible decrease in growth of regenerated shoots was found up to th e irradiation dose of 2.5 Gy and 5 Gy for ion beams and gamma rays, respectively. Hence, irradiation with these doses to stem explants of Lom- bardy poplar hardly affects survival ability of regen erated shoots. It is relevant to mention that now a day, ionizing radiation is attracting increasing attention as a new mutagen [22,23]. Various novel mutants of a wide vari- ety of plants, which include Arabidopsis thaliana, Lotus japonicus, tobacco, rice, verbena, rose, carnation, Tore- nia, petunia, and Hinoki cypress, have been obtained via ion-beam irradiation [24]. Similarly, soybean mutant lacking lipoxygenases was identified by gamma-ray irra- diation [25]. Thus, using this strategy, isolation of mu- tants would be achievable in poplar, as well. 4. Conclusion We found that shoot regeneration from Lombardy poplar stem segments can be achieved by a simple procedure, in which pre-culture in CIM is not necessary. Similar pro- cedure is reported in Eastern Cottonwood (P. de ltoides) [17-19] and male Himalayan poplar (P. ciliata) [20]. No callus induction in this method might be another advan- tage, because it is well known that plants regenerated via a callus phase may differ from the mother plant due to somaclonal variations [21]. The present protocol is also economical, because it results high frequency (87%) di- rect shoot regeneration without trans-zeatin, an expen- sive subst ance that have used i n t he previous protocols of Table 3. Comparison of regeneration systems in Lombary poplar (Populus nigra cv. italica) and related species. Genetic background No. of media used for shoot regeneration* Name of media Growth regulators (mg/L) Explant tissue typeIncubation period (week) Reference SIM IAA (0.1) + BA (0.5) Populus nigra L. var italica Koehne 1 RIM IBA (0.5) + NAA (0.02) Stem 8 Present study CIM 2,4-D (0.5) SIM t-zeatin (2.0) + BA (0.2) Populus nigra L. var italica Koehne 2 RIM IBA (0.5) + NAA (0.02) Stem 10 [15] CIM 2,4-D (0.5) + BA (1.0) SIM t-zeatin (0.5) + BA (0.1) Populus nigra L. var italica Koehne 2 RIM IBA (0.5) + NAA (0.02) Stem 13 [11] CIM NAA (0.2) + BA (0.5) SIM NAA (0.05) + BA (0.5) Populus nigra L. 2 RIM None Leaf 13~ [10] CIM NAA (0.5) + BA (1.0) SIM NAA (0.25) + BA (0.6) Populus nigra × P. pyramidales 2 RIM NAA (0.1) +BA (0.1) Leaf INT [14] CIM IAA (0.1) + BA (0.5) SIM NAA (0.01) + BA (0.5) Populus nigra var thevestina × (P. diversifolia + P. tomentosa) 2 RIM IAA (0.8) Leaf INT [12] CIM IBA (0.1) + BA (0.5) SIM IBA (0.1) + BA (1.0) Populus nigra var thevestina 2 RIM IBA (1.0) Leaf INT [13] *RIM is not included. INT: Information not traceable.  An Improved System for Shoot Regeneration from Stem Explants of Lombardy Poplar (Populus nigra L. var. italica Koehne) 1185 0 25 50 75 100 0510 15 20 25 30 35 Irradiation dose (Gy) (a) Regeneration ratio (% of control) 0 25 50 75 100 0510 15 20 25 30 35 Irradiation dose (Gy) (b) Regeneration ratio (% of control) Figure 2. Dose response relationship curves for carbon ion- beam (a) and gamma-ray (b) irradiation on regeneration ratio of in vitro raised Lombardy poplar. Shoots above 1 cm in length at five weeks after irradiation were counted as regenerated shoots. Regeneration ratio was calculated with dividing the number of stem explants that produce regen- erated shoots by total number of stem explants irradiated. The regeneration ratio of non-irradiated control stems was plotted as 100%. Lombardy poplar regeneration (Table 3) [11,15,17]. Our method is able to produce and perpetuate a large number of disease-free Lombardy poplar plants, and will thus benefit physiological and genetic studies of hardwood plants by providing a constant supply of competent and efficient plant materials. 5. Acknowledgements The authors wish to express their gratitude to Dr. Kenji Shinohara, FFPRI, Tsukuba for providing Populus nigra plants. REFERENCES [1] T. Igasaki, Y. Watanabe, M. Nishiguchi and N. Kotoda, “The Flowering Locus T/Terminal Flower1 Family in Lombardy Poplar,” Plant Cell Physiology, Vol. 49, No. 3, 2008, pp. 291-300. doi:10.1093/pcp/pcn010 [2] K.-H. Han, C. Ma and S. H. Strauss, “An Agrobacterium tumefaciens Transformation Protocol Effective on a Vari- ety of Cottonwood Hybrids (Genus Populus),” Plant Cell Report, Vol. 19, No. 3, 2000, pp. 315-320. doi:10.1007/s002990050019 [3] W. H. Dai, Z. M. Cheng and W. Sargent, “Plant Regen- eration and Agrobacterium-Mediated Transformation of Two Elite Aspen Hybrid Clones from in Vitro Leaf Tis- sues,” In Vitro Cellular and Developmental Biology-Plant, Vol. 39, No. 6, 2003, pp. 6-11. doi:10.1079/IVP2002355 [4] N. R. Street, O. Skogström, A. Sjödin, J. Tucker, R. A. Maricela, P, Nilsson, S. Jansson and G. Taylor, “The Ge- netics and Genomics of Drought Response in Populus,” The Plant Journal, Vol. 48, No. 3, 2006, pp. 321-341. doi:10.1111/j.1365-313X.2006.02864.x [5] S. Raj, K. Bräutigam, E. T. Hamanishi, O. Wilkins, B. R. Thomas, W. Schroeder, S. D. Mansfield, A. L. Plant and M. M. Campbell , “Clone History Shapes Populus Drought Responses,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 108, No. 30, 2011, pp. 12521-12526. doi:10.1073/pnas.1103341108 [6] S. H. Son and R. B. Hall, “Multiple Shoot Regeneration from Root Organ Cultures of Populus alba × P. gran- didentata,” Plant Cell Tissue and Organ Culture, Vol. 20, No. 1, 1990, pp. 53-57. doi:10.1007/BF00034757 [7] T. Ruttink, M. Arend, K. Morreel, V. Strome, S. Rom- bauts, J. Fromm, R. P. Bhalerao, W. Boerjan and A. Rohde, “A Molecular Timetable for Apical Bud Forma- tion and Dormancy Induction in Poplar,” The Plant Cell, Vol. 19, No. 8, 2007, pp. 2370-2390. doi:10.1105/tpc.107.052811 [8] A. Caruso, F. Chefdor, S. Carpin, C. Depierreux, F. M. Delmotte, G. Kahlem and D. Morabito, “Physiological Characterization and Identification of Genes Differen- tially Expressed in Response to Drought Induced by PEG 6000 in Populus canadensis Leaves,” Journal of Plant Physiology, Vol. 165, No. 9, 2008, pp. 932-941. doi:10.1016/j.jplph.2007.04.006 [9] A. Matthias and J. Fromn, “Seasonal Change in the Drought Response of Wood Cell Development in Pop- lar,” Tree Physiology, Vol. 27, No. 7, 2007, pp. 985-992. doi:10.1093/treephys/27.7.985 [10] M. Confalonieri, A. Balestrazzi and S. Bisoffi, “Genetic Transformation of Populus nigra by Agrobacterium tu- mefaciens,” Plant Cell Reports, Vol. 13, No. 5, 1994, pp. 256-261. doi:10.1007/BF00233315 [11] M. Nishiguchi, K. Yoshida, T. Mohri, T. Igasaki and K. Shinohara, “An Improved Transformation System for Lombardy poplar (Populus nigra var. italica),” Journal of Forest Research, Vol. 11, No. 3, 2006, pp. 175-180. doi:10.1007/s10310-006-0203-1 [12] L. Yi and D. Li, “A Study on Tissue Culture Technique of Populus nigra var. thevestina × (P. diversifolia + P. tomentosa),” Journal of Gansu Agricultural University, Vol. 37, 2002, pp. 180-184. [13] Y. Tao, F. Li and Y. Li, “Establishment of Tissue Culture Regeneration System of Populus nigra var. thevestina,” Journal of Northwest A&F University, Vol. 37, 2008, pp. 203-207. [14] L. Zhao, L. Xu, S. Shi and C. Wu, “Establishment of Copyright © 2012 SciRes. AJPS  An Improved System for Shoot Regeneration from Stem Explants of Lombardy Poplar (Populus nigra L. var. italica Koehne) 1186 Tissue Culture Regeneration System for Populus nigra × P. pyramidalis,” Protection Forest Science and Technol- ogy, Vol. 2008, No. 6, 2008, pp. 22-24. [15] T. Mohri, N. Yamamoto and K. Shinohara, “Agrobacte- rium-Mediated Transformation of Lombardy poplar (Populus nigra L. var. italica Koehne) Using Stem Seg- ments,” Journal of Forest Research, Vol. 1, No. 1, 1996, pp. 13-16. doi:10.1007/BF02348333 [16] T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cul- ture,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x [17] A. Cavusoglu, Z. I. Altas, K. Bajrovic, N. Gozukirmizi, A. Zehir, “Direct and Indirect Plant Regeneration from Various Explants of Eastern Cottonwood Clones (Popu- lus deltoides Bartram ex Marsh.) with Tissue Culture,” African Journal of Biotechnology, Vol. 10, No. 16, 2011, pp. 3216-3221. [18] R. Yadav, P. Arora, D. Kumar, D. Katyal, N. Dilbaghi and A. Chaudhury, “High Frequency Direct Plant Regen- eration from Leaf, Internode, and Root Segments of Easter Cottonwood (Populus deltoids),” Plant Biotech- nology Reports, Vol. 3, No. 3, 2009, pp. 175-182. doi:10.1007/s11816-009-0088-5 [19] F. E. Sherif and S. Khattab, “Direct Shoot Regeneration from Leaf, Root and Stem Internode Segments of Male Poplar Trees and the Molecular Analysis of Variant Re- generated Plants,” Journal of American Science, Vol. 7, No. 8, 2011, pp. 200-206. [20] A. K. Thakur and D. K. Srivastava, “High-Efficiency Plant Regeneration from Leaf Explants of Male Himala- yan Poplar (Populus ciliata Wall.),” In Vitro Cell Devel- opmental Bio logy-Plant, Vol. 42, 2006, pp. 144-147. [21] T. Ramanathan, K. Satyavani and S. Gurudeeban, “In Vitro Plant Regeneration from Leaf Primordial of Gum- Bearing Tree Aegle marmelos,” E-International Scientific Research Journal, Vol. 3, No. 1, 2011, pp. 47-50. [22] A. Tanaka, N. Shikazono and Y. Hase, “Studies on Bio- logical Effects of Ion Beams on Lethality, Molecular Na- ture of Mutation Rate, and Spectrum of Mutation Pheno- type for Mutation Breeding in Higher Plants,” Journal of Radiation Research, Vol. 51, No. 3, 2010, pp. 223-233. doi:10.1269/jrr.09143 [23] S. Arase, Y. Hase, J. Abe, M. Kasai, T. Yamada, K. Ki- tamura, I. Narumi, A. Tanaka and A. Kanazawa, “Opti- mization of Ion-Beam Irradiation for Mutagenesis in Soybean: Effects on Plant Growth and Production of Visibly Altered Mutants,” Plant Biotechnology, Vol. 28, 2011, pp. 323-329. doi:10.5511/plantbiotechnology.11.0111a [24] A. Tanaka and Y. Hase, “Establishment of Ion Beam Technology for Breeding,” In: Q. Y. Shu, Ed., Induced Plant Mutations in the Genomics Era, Food and Agricul- ture Organization of the United Nations, 2009, pp. 243- 246. [25] M. Hajika, K. Igita and K. Kitamura, “A Line Lacking All the See d Li poxy ge nase Isozymes in Soy bean [Glycine max (L.) Merr.] Induced by Gammaray Irradiation,” Japa- nese Journal of Breeding, Vol. 41, 1991, pp. 507-509. Copyright © 2012 SciRes. AJPS
|