G. PASUPATHI, P. PHILOMINATHAN
Copyright © 2012 SciRes. JMMCE
907
[4] M. Karppinnen, J. O. Lundgren and R. Liminga, “Struc-
ture of Pyroelectric Lithium Potassium Sulphate, LiKSO4,”
Acta Crystallographica Section C, Vol. 39, 1983, pp. 34-
38. doi:10.1107/S0108270183003509
[5] M. A. Pimenta, S. L. V. Vierira, F. O. V. Letelier, N. L.
Speziali and M. S. Dantas, “Ionic Conductivity in
LiK0.9Na0.1SO4 Single Crystals,” Solid State Communica-
tions, Vol. 82, No. 10, 1992, pp. 758-757.
doi:10.1016/0038-1098(92)90158-6
[6] A. Lunden and J. O. Thomas, “High Conductivity Solid
State Conductors: Recent Trends and Applications,”
World Scientific, Singapore City, 1986.
[7] H. K. Liu, M. L. Hu, W. S. Tse, D. P. Wong and S. J. Lin,
“Raman Studies of Low Temperature Phase Transition in
LiKSO4,” Chinese Journal of Physics, Vol. 36, No. 3,
1998, pp. 542-548.
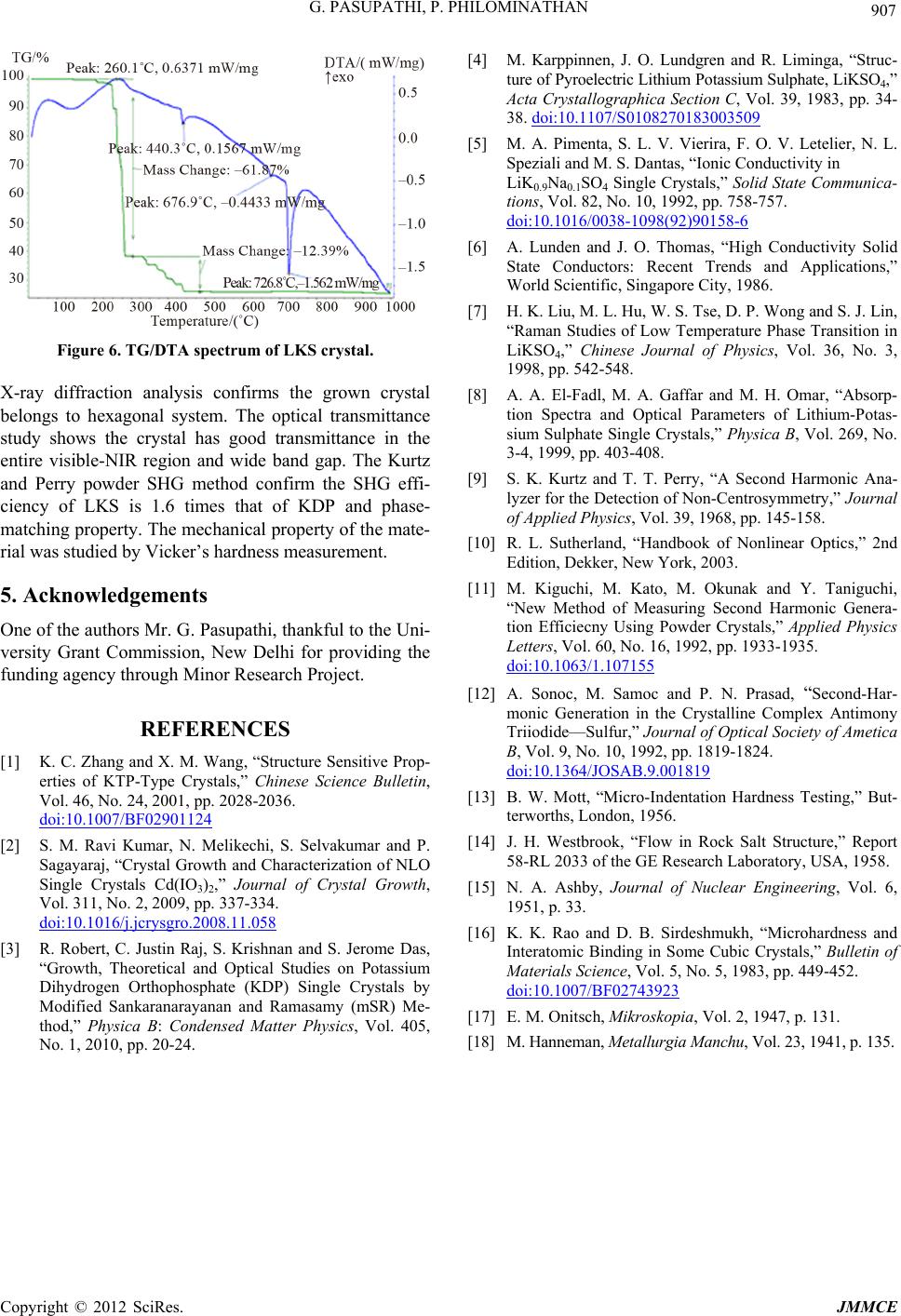
Figure 6. TG/DTA spectrum of LKS crystal.
X-ray diffraction analysis confirms the grown crystal
belongs to hexagonal system. The optical transmittance
study shows the crystal has good transmittance in the
entire visible-NIR region and wide band gap. The Kurtz
and Perry powder SHG method confirm the SHG effi-
ciency of LKS is 1.6 times that of KDP and phase-
matching property. The mechanical property of the mate-
rial was studied by Vicker’s hardness measurement.
[8] A. A. El-Fadl, M. A. Gaffar and M. H. Omar, “Absorp-
tion Spectra and Optical Parameters of Lithium-Potas-
sium Sulphate Single Crystals,” Physica B, Vol. 269, No.
3-4, 1999, pp. 403-408.
[9] S. K. Kurtz and T. T. Perry, “A Second Harmonic Ana-
lyzer for the Detection of Non-Centrosymmetry,” Journal
of Applied Physics, Vol. 39, 1968, pp. 145-158.
[10] R. L. Sutherland, “Handbook of Nonlinear Optics,” 2nd
Edition, Dekker, New York, 2003.
[11] M. Kiguchi, M. Kato, M. Okunak and Y. Taniguchi,
“New Method of Measuring Second Harmonic Genera-
tion Efficiecny Using Powder Crystals,” Applied Physics
Letters, Vol. 60, No. 16, 1992, pp. 1933-1935.
doi:10.1063/1.107155
5. Acknowledgements
One of the authors Mr. G. Pasupathi, thankful to the Uni-
versity Grant Commission, New Delhi for providing the
funding agency through Minor Research Project.
[12] A. Sonoc, M. Samoc and P. N. Prasad, “Second-Har-
monic Generation in the Crystalline Complex Antimony
Triiodide—Sulfur,” Journal of Optical Society of Ametica
B, Vol. 9, No. 10, 1992, pp. 1819-1824.
doi:10.1364/JOSAB.9.001819
REFERENCES
[1] K. C. Zhang and X. M. Wang, “Structure Sensitive Prop-
erties of KTP-Type Crystals,” Chinese Science Bulletin,
Vol. 46, No. 24, 2001, pp. 2028-2036.
doi:10.1007/BF02901124
[13] B. W. Mott, “Micro-Indentation Hardness Testing,” But-
terworths, London, 1956.
[14] J. H. Westbrook, “Flow in Rock Salt Structure,” Report
58-RL 2033 of the GE Research Laboratory, USA, 1958.
[2] S. M. Ravi Kumar, N. Melikechi, S. Selvakumar and P.
Sagayaraj, “Crystal Growth and Characterization of NLO
Single Crystals Cd(IO3)2,” Journal of Crystal Growth,
Vol. 311, No. 2, 2009, pp. 337-334.
doi:10.1016/j.jcrysgro.2008.11.058
[15] N. A. Ashby, Journal of Nuclear Engineering, Vol. 6,
1951, p. 33.
[16] K. K. Rao and D. B. Sirdeshmukh, “Microhardness and
Interatomic Binding in Some Cubic Crystals,” Bulletin of
Materials Science, Vol. 5, No. 5, 1983, pp. 449-452.
doi:10.1007/BF02743923
[3] R. Robert, C. Justin Raj, S. Krishnan and S. Jerome Das,
“Growth, Theoretical and Optical Studies on Potassium
Dihydrogen Orthophosphate (KDP) Single Crystals by
Modified Sankaranarayanan and Ramasamy (mSR) Me-
thod,” Physica B: Condensed Matter Physics, Vol. 405,
No. 1, 2010, pp. 20-24.
[17] E. M. Onitsch, Mikroskopia, Vol. 2, 1947, p. 131.
[18] M. Hanneman, Metallurgia Manchu, Vol. 23, 1941, p. 135.