 Journal of Sustainable Bioenergy Systems, 2012, 2, 49-59 http://dx.doi.org/10.4236/jsbs.2012.23008 Published Online September 2012 (http://www.SciRP.org/journal/jsbs) Microalgae: A Potential Source of Biodiesel Shalini Rajvanshi, Mahendra Pal Sharma Biofuel Research Laboratory, Alternate Hydro Energy Centre, Indian Institute of Technology Roorkee, Roorkee, India Email: rajvanshiroorkee@gmail.com, mpshafah@iitr.ernet.in Received July 12, 2012; revised August 16, 2012; accepted August 25, 2012 ABSTRACT The economic development of the world is highly dependent on fossil fuel supplies which are constrained not only by limited availability but also generate high levels of pollution. Looking at the limited fossil fuel associated with problems, concerted efforts have been started to search for alternative bio fuels like bio ethanol and biodiesel. Since the diesel is being used massively in industrial commercial, agriculture and other sectors. Therefore, the production and utilization of biodiesel from oil seeds crops has been getting renewed interest in recent years in the India to overcome the demerits of oil from oil seed crops. The production of biodiesel from microalgae has several advantages over the above re- sources due to higher algal biomass and oil productivities and the need of non-arable land for its growth. Industrial and municipal wastewaters can be potentially utilized fo r cultiv ation of micro algal o il th at can be used fo r the produ ction of biodiesel to completely displace petro diesel. The micro algal biomass has been reported to yield high oil contents and have the diesel production . Accordingly, lot of R & D work has been initiated for the growth, harvestin g, oil extraction and conversion to biodiesel. Keywords: Microalgal Species; Cultivation; Harvesting ; Oil Extraction and Biodiesel Production 1. Introduction Owing to the limited availability and associated envi- ronmental problems with fossil fuel utilization, the re- newable energy based biofuel viz biodiesel and bioetha- nol are viewed as future substitute fuels for diesel and gasoline respectively. Sugar based fuel alcohol produc- tion is not feasible unless methods are developed to con- vert lingo-cellulosic biomass into ethanol and there is no competition with food supplies. The biodiesel from edi- ble oils is also not a sustainable option due to heavy competition with seed plants and accordingly, non-edible oils like Jatropha , Pongamia, Neam oils etc. are ac- corded top priority for biodiesel p roduction in India. The plantations of Jatropha curcas are under cultivation on large land area in the country and hopes are raised for sustainable availability o f oil for conversion to biodiesel. Apart from these non-edible oil resources, microalgae is also becoming the focus as future source of biodiesel as these are found exceedingly rich in oil that can be con- verted to biodiesel using existing technology. Microalgae are prokaryotic (e.g. Cyanobacteria, Cyanophyceae) or eukaryotic (e.g. green algae) and diatoms (Bacillario- phyta) that can grow rapidly and live in harsh conditions due to their unicellular or simple multicellular structure [1,2]. A study has estimated that mo re t han 50,0 00 mic ro algal species exist, but only 30,000 are studied and ana- lyzed as yet [3]. If grown properly, the microalgal based biodiesel has potential to completely substitute diesel without competing with the food and other supplies of agricultural products. The oil yield from some microalgae is reported to ex- ceed 80% (on dry weight basis) compared to 40% - 50% from edible/non-edible oil seeds. An average annual productivity of micro algal biomass in a well designed production system located in a tropical zone may be about 1.535 kg·m–2 d–1 with biodiesel yield of 98.4 m3 per hectare. The other added advantage of microalgae is that unlike other oil crops, they grow rapidly and double their biomass within 24 h which could be as short as 3.5 h contrary to the time for oil crops (months together). The present paper reviews the possibilities of using different types of micro algal species as source of oil, techniques for algal growth, harvesting, oil extraction and conversion to biodiesel and its fu ture scope in India. 2. Literature Review Advantage of using microalgae for biodiesel production has been reported by a number of workers [4-11]. The interest in microalgae for biodiesel started in 1970s dur- ing the first oil crisis due to high oil yields. The average oil yield is repo rted between 1% and 70% but under cer- tain conditions, some species can yield up to 90% of dry biomass weight [12]. The variation in fatty acid compo- sition of oil from different algae species is reported by C opyright © 2012 SciRes. JSBS  S. RAJVANSHI, M. P. SHARMA 50 several authors [13-17]. In fact, several studies have re- ported the use of microalgae for the production of bio- diesel and other by products [18-22]. Moheimani [23] studied the effect of pH on algal growth in a plate photobioreactor. Kaewpintong [24] found better growth of microalgae in an airlift bioreactor due to better mixing of the microalgal culture. Thomas et al. [25] studied the growth of microalgal species that grow well in this medium containing carbon dioxide as a carbon source and nitrate as a nitrogen source and deter- mined the effect of nitrogen as well as the salt on the chemical compositions of the algae. Ugwu and Aoyagi [26] studied mass production of algae and have been done to develop a photobioreactor for algal culture. Weissman and Goebel [27] studied primary harvesting methods for biofuels production. Samson and Leduy [28] developed a flat reactor equipped with fluorescence lamps for the growth of mi- cro algal oil Further, Ortega and Roux [29] developed a outdoor flat panel reactor using thick transparent PVC materials. The design of vertical alveolar panels and flat plate reactors for mass cultivation of different algae was reported by Tredici and Materassi, Zhang et al., and Hoekema et al. [30-32]. Hu et al., Eriksen and Wang found that high dissolved oxygen (DO) levels can be reached in tubular photobioreactors [33-35]. 3. Classification of Microalgae Photosynthetic organisms growing in aquatic environ- ments include macroalgae, microalgae and emergents [36]. These primitive organisms with simple cellular structure and large surface to volume ratio are able to uptake large amount of nutrients. The photosynthesis in microalgae is similar to higher plants but is more effi- cient due to their simple cellular structure [37]. The mi- croalgae can be classified on the basis of their pigmenta- tion, life cycle and basic cellular structure as given in Table 1. The mass production of oil is focused mainly on mi- croalgae of 0.4 mm dia of diatoms and cyanobacteria rather than macroalgae e.g. Seaweed and is preferred for biodiesel production due to its less complex structure, fast growth and high oil content. Research & Develop- ment is also carried out to use the seaweeds for bio-en- ergy, perhaps, due to higher resource av ailability [39,40]. Botryococcus braunii, Chlorella, Dunaliella tertiolecta, Gracilaria, Pleurochrysis carterae, Sargassum, are some of the microalgal species currently studied for their suita- bility for biodiesel production [41-43]. 4. Algae Oil Extraction Techniques Oil extraction from algae is one of the costly processes that can determine the sustainability of algae-based bio- diesel. Oil extraction methods can be broadl y classified as: Each of these methods has drawbacks: The mechanical press generally requires drying of th e algae, which is an energy intensive step. The use of chemical solvents poses safety and health issues. Supercritical extraction requires high pressure equip- ment that is both expensive and energy intensive. Table 2 compares oil yields of microalgae with other oil feedstocks. It is seen from that there are significant variations in biomass productivity, oil yield and biodiesel productivity. Microalgae are more advantageous due to higher biomass productivity, oil and biodiesel yield. The table shows that low, medium and high oil content micro-algae have high oil yield/ha/year and hence higher biodiesel productivities (l/ha/yr) which is much more than the productivities of oil seed crops. This is one of the most important reasons that microalgae have attracted the attention of researchers in India to scientifically grow, harvest, extract oil and convert it to biodiesel. 5. Technology for Growing Algae The following technologies are used for the production of algae: Table 1. Classification of microalgae [38]. S No Name of microalgae Known speciesStorage material Habitat 1 Diatoms (Bacillariophyceae) 100,000 Chyrsolaminarin (polymer of car bo hydrates) and TAGs Oceans, fresh and brackish water 2 Green algae (Chlorophyceae) 8000 Starch and TAGs Freshwater 3 Blue-green algae (Cyanophyceae) 2000 Starch and TAGs Different habitats 4 Golden algae (Chrysophyceae) 1000 TAGs and carbohydrates Freshwater Copyright © 2012 SciRes. JSBS 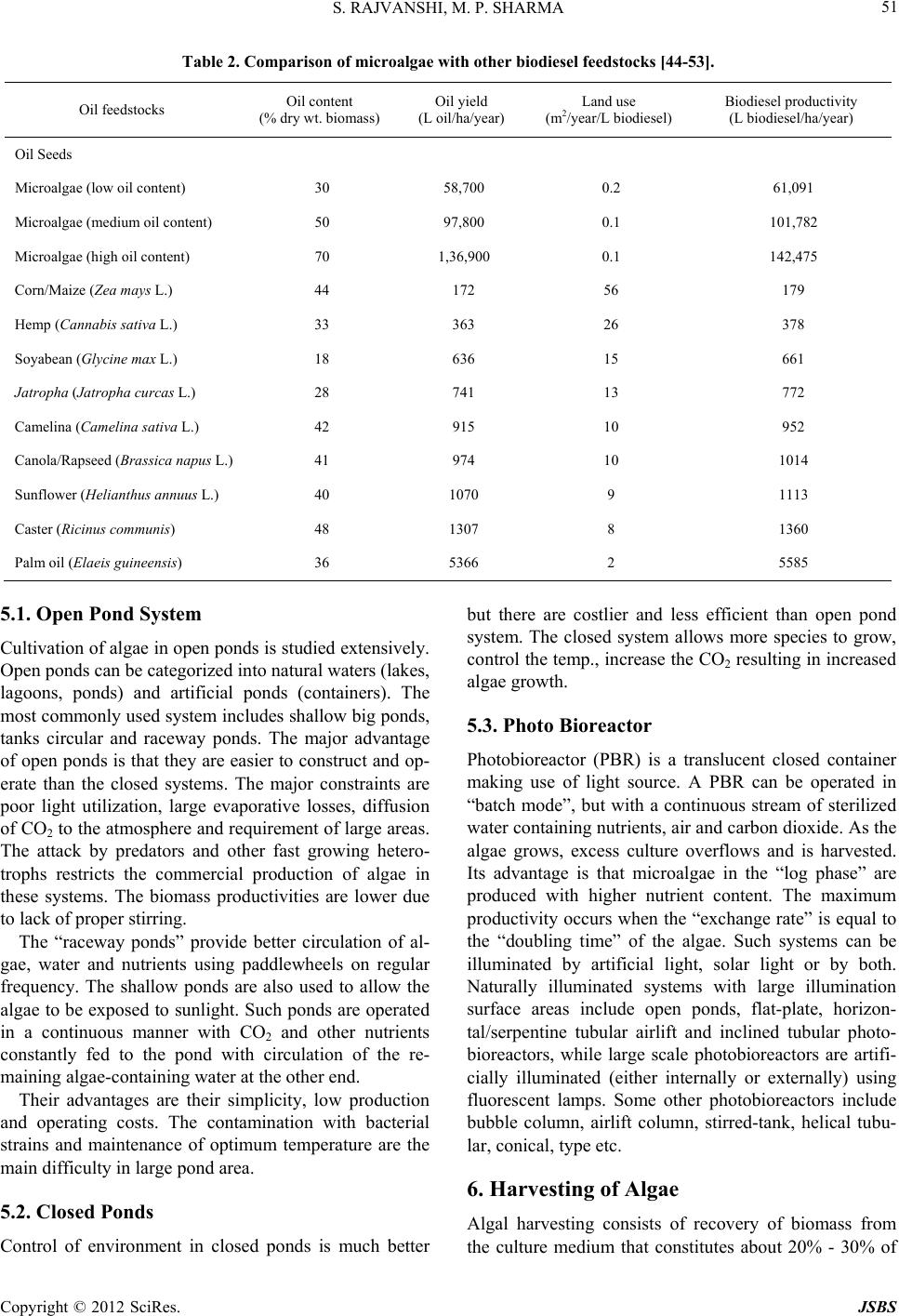 S. RAJVANSHI, M. P. SHARMA 51 Table 2. Comparison of microalgae with other biodiesel feedstocks [44-53]. Oil feedstocks Oil content (% dry wt. biomass) Oil yield (L oil/ha/year) Land use (m2/year/L biodiesel) Biodiesel productivity (L biodiesel/ha/year) Oil Seeds Microalgae (low oil conte nt ) 30 58,700 0.2 61,091 Microalgae (medium oil content) 50 97,800 0.1 101,782 Microalgae (high oil content) 70 1,36,900 0.1 142,475 Corn/Maize (Zea mays L.) 44 172 56 179 Hemp (Cannabis sativa L.) 33 363 26 378 Soyabean (Glycine max L.) 18 636 15 661 Jatropha (Jatropha curcas L.) 28 741 13 772 Camelina (Camelina sativa L.) 42 915 10 952 Canola/Rapseed (Brassica napus L. )41 974 10 1014 Sunflower (Helianthu s annuus L.) 40 1070 9 1113 Caster (Ricinus communis) 48 1307 8 1360 Palm oil (Elaeis guineensis) 36 5366 2 5585 5.1. Open Pond System Cultivation of alg ae in open p onds is studied ex tensively. Open ponds can be categorized into natural waters (lakes, lagoons, ponds) and artificial ponds (containers). The most commonly used system includes shallow big ponds, tanks circular and raceway ponds. The major advantage of open ponds is that they are easier to con struct and op- erate than the closed systems. The major constraints are poor light utilization, large evaporative losses, diffusion of CO2 to the atmo spher e and r equirement of large areas. The attack by predators and other fast growing hetero- trophs restricts the commercial production of algae in these systems. The biomass productivities are lower due to lack of proper stirri ng . The “raceway ponds” provide better circulation of al- gae, water and nutrients using paddlewheels on regular frequency. The shallow ponds are also used to allow the algae to be exposed to sunlight. Such ponds are operated in a continuous manner with CO2 and other nutrients constantly fed to the pond with circulation of the re- maining algae-containing water at the other end. Their advantages are their simplicity, low production and operating costs. The contamination with bacterial strains and maintenance of optimum temperature are the main difficulty in large pond area. 5.2. Closed Ponds Control of environment in closed ponds is much better but there are costlier and less efficient than open pond system. The closed system allows more species to grow, control the temp., increase the CO2 resu lting in increased algae growth. 5.3. Photo Bioreactor Photobioreactor (PBR) is a translucent closed container making use of light source. A PBR can be operated in “batch mode”, but with a continuous stream of sterilized water containing nutrients, air and carbon dioxide. As the algae grows, excess culture overflows and is harvested. Its advantage is that microalgae in the “log phase” are produced with higher nutrient content. The maximum productivity occurs when the “exchange rate” is equal to the “doubling time” of the algae. Such systems can be illuminated by artificial light, solar light or by both. Naturally illuminated systems with large illumination surface areas include open ponds, flat-plate, horizon- tal/serpentine tubular airlift and inclined tubular photo- bioreactors, while large scale photobioreactors are artifi- cially illuminated (either internally or externally) using fluorescent lamps. Some other photobioreactors include bubble column, airlift column, stirred-tank, helical tubu- lar, conical, type etc. 6. Harvesting of Algae Algal harvesting consists of recovery of biomass from the culture medium that constitutes about 20% - 30% of Copyright © 2012 SciRes. JSBS  S. RAJVANSHI, M. P. SHARMA 52 the total biomass production cost. Most common har- vesting methods include sedimentation, centrifugation, filtration, ultra-filtration or combination of flocculation- flotation. Flocculation is used to aggregate the microalgal cells to increase the effective particle size and hence ease the sedimentation, centrifugal recovery and filtration [54]. These techniques are summarized in Table 3. High-density algal cultures can be concentrated by chemical flocculation or centrifugation using aluminum sulphate, ferric chloride etc to coagulate and precipitate the cells to settle down at the bottom or to float to the surface. Algal biomass is finally recovered by siphoning off the supernatant or skimming the cells off the surface. Once the algae is harvested and dried, several methods like mechanical solvent extraction and chemical methods can be applied for oil extraction, the choice of which depends upon the particle size of algal biomass. However, solvent extraction is usu ally applied to get hig h oil yields from algae. The oil yields from different microalgae are given in Table 4 which shows that Nannochloropsis species has highest while Tetraselmis suecica minimum oil yield. 7. Physicochemical Properties of Oil To assess the potential of biod iesel as a substitute of die- sel fuel, the properties of biodiesel such as density, vis- cosity, flash point, cold filter plugging point, solidifying point, and heating value were determined. A comparison of these properties of biodiesel from microalgal oil with diesel and ASTM biodiesel standard is given in Table 5. It can be seen that most of these parameters comply with the limits established by ASTM related to b iodiesel qual- ity. The microalgal biodiesel showed much lower cold filter plugging point of –11˚C in compared to than diesel while the viscosity and acid value is higher than diesel. The Table 5 shows that the fuel properties of micro- algal biodiesel are comparable to diesel fuel. 8. Biodiesel Production from Algal Oil Out of the four oil modification methods, the most prom- ising method to overcome the problem of high viscosity is transesterification which is a multi step reaction con- sisting of three reversible steps, where triglycerides are converted to diglycerides, diglycerides to monoglyc- erides and monoglycerides to esters (biodiesel) and glyc- erol as by-product. Transesterification of Microalgal Oil to Biodiesel Transesterification does not alter the fatty acid composi- tion of the feedstocks and hence the composition of bio- diesel. The effect of FFA on biodiesel yield and adoption of suitable transesterification process is reviewed in Ta- ble 6 which indicates that selection of base or acid-base catalyzed process and other conditions is based on the FFA contents of the oil and accordingly the time and other parameters of conversions are selected. Table 3. Algal harvesting techniques. S. No. Algae harvest method Relative cost Algal species 1 Foam fractionation Very high Scenedesmus, Chlorella 2 Ozone flocculation Very high 3 Centrifugation Very high Scenedesmus, Chlorella 4 Electrofloatation High 5 Inorganic chemical flocculation High Oxidation ponds 6 Polyelectrolyte flocculation High Dunaliella 7 Filtration High Spirulina, Coelastrum 8 Microstrainers Unknown Spirulina 9 Tube settling Low Micractinium 10 Diecrete sedimentation Low Coelastrum 11 Phototactic autoconcentration Very low Euglena, Dunaliella 12 Autoflocculation NA Micractinium 13 Bioflocculation NA Micractinium 14 Tilapia-enhanced sedimentation NA Scenedesmus, Chlorella Copyright © 2012 SciRes. JSBS  S. RAJVANSHI, M. P. SHARMA 53 Table 4. Oil from different microalgal species [7]. Type of microalgae Oil content (% dry wt. basis) Botryococcus braunii 25 - 75 Chlorella sp. 28 - 32 Crypthecodinium cohnii 20 Cylindrotheca sp. 16 - 37 Dunaliella primolecta 23 Isochrysis sp. 25 - 33 Monallanthus salina >20 Nannochloris sp. 20 - 35 Nannochloropsis sp. 31 - 68 Neochloris oleoabu n d ans 35 - 54 Nitzschia sp. 45 - 47 Phaeodactylum tricornutum 20 - 30 Schizochytrium sp. 50 - 77 Tetraselmis suecica 15 - 23 Table 5. Comparison of properties of micro algal biodiesel with diesel and ASTM biodiesel standard. Properties Biodiesel from microalgae oil Diesel fuel ASTM biodiesel standard Density (kg·L –1) 0.864 0.838 0.84 - 0.90 Viscosity (mm2·s–1, cP at 40˚C) 5.2 1.9 - 4.1 3.5 - 5.0 Flash Point (˚C) 155 60 Min 100 Solidifying point (˚C) –12 –50 to 10 - Cold filter plugging point (˚C) –11 –3.0 (max –6.7) Summer max 0, Winter max < –15 Acid value (mg KOH·g–1) 0.374 Max 0.5 Max 0.5 Heating value (MJ·kg–1) 41 40 - 45 - H/C ratio 1.81 1.81 - Table 6 reviews the different types of transesterifica- tion reactions depending on the presence of free fatty acids (FFA) contents in the oil. Table shows that very little wo rk is available on trans- esterification of microalgal oil to biodiesel. Depending upon the FFA contents in oil, the base catalysed process is applied to the oil with FFA < 1% while two step proc- esses is applied to high FFA oils as per details reported in our paper [70, 7 1] . It is reported that biodiesel from oils with a high FFA has higher cetane number and energy contents, but lower cloud and pour points and higher viscosity. These result shows that the fatty acid profile of the oil influences the quality of the biodiesel considerably. Table 7 gives the fatty acid profile of some of the vegetable oil used for biodiesel production. The vegetable oil and their bio- diesel with high content of oleic acid are the most suit- able biofuel due to their greater stability and better fuel characteristics. Oxidation stability, an important issue in the biodiesel due to the presence of polyunsaturated compounds, is influenced by factors such as presence of air, heat, traces of metal, peroxides, light, or structural features of the compounds, mainly, the presence of double bonds [74]. Biodiesel produced from oils with high concentrations of saturated fatty acid, has better stability. Therefore, vege- table oils rich in linoleic and linolenic acids such as soy- bean and sunflower tend to give methyl ester fuels with poor oxidation stability (Table 7) whereas nonpoly- unsaturated fuels, such as palm and olive methyl ester generally show good stability [75]. 9. Status of Biodiesel Producti on from Microalgal in India As mentioned above, the microalgae have the highest oil yielding potential which is about 6 - 10 times more than vegetable oil. The algae production can be increased utilizing waste water from domestic and industrial sec- tors that contain considerable nutrients necessary for its growth. Further, the stability of biodiesel from micro- algae is the added advantage of fuel characteristics which persist for longer period of time unlike biodiesel from oil seed crops. Extensive work has been done by Indian scientists on utilization of microalgae for food and the pharmaceutical applications. The lists of organizations/institutions who are working on various aspects of microalgae such as microalgae collected from natural vegetation which is used for the production of biogas and biofuel in India are given in Table 8. 10. Conclusions The microalgae are considered as one of the most pro- mising feedstocks for future bio-diesel production in India. The advantages of microalgae are their wide- spread availability, higher oil yields and reduced pres- sure on cultivable land. The difficulty in efficient bio- diesel production from algae lies not only in the extrac- tion of the oil, but also developing an algal strain with a high lipid content and fast growth rate. Once the microalgal oil starts to be unavailable the ap- plication of type of transesterification well established conversion process may be suitably used for converting to biodiesel which has fuel properties similar to petro diesel. Apart from technologies developed for algal cul- Copyright © 2012 SciRes. JSBS 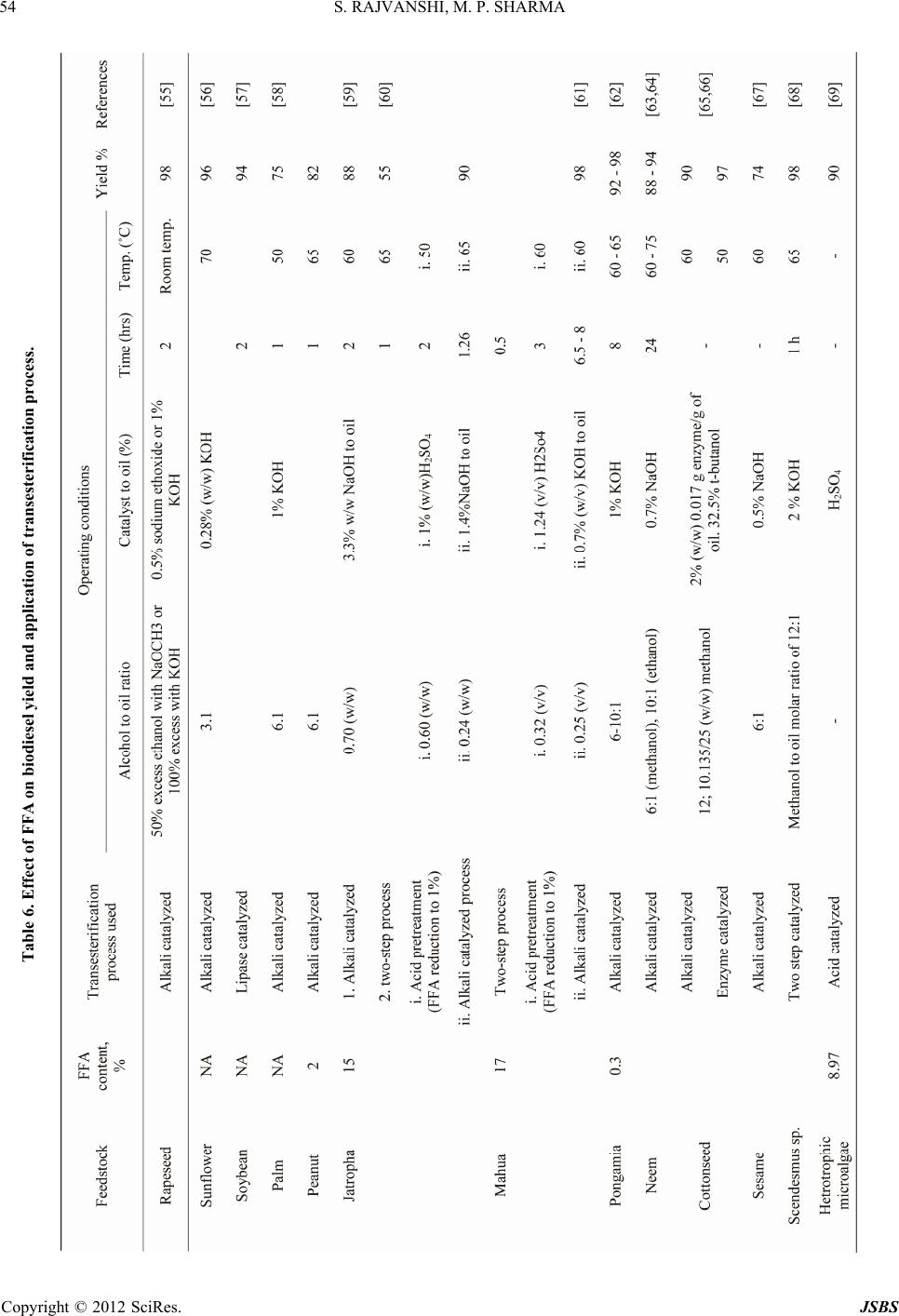 S. RAJVANSHI, M. P. SHARMA 54 Table 6. Effect of FFA on biodiesel yield and application of transesterification pr oc e ss. Copyright © 2012 SciRes. JSBS 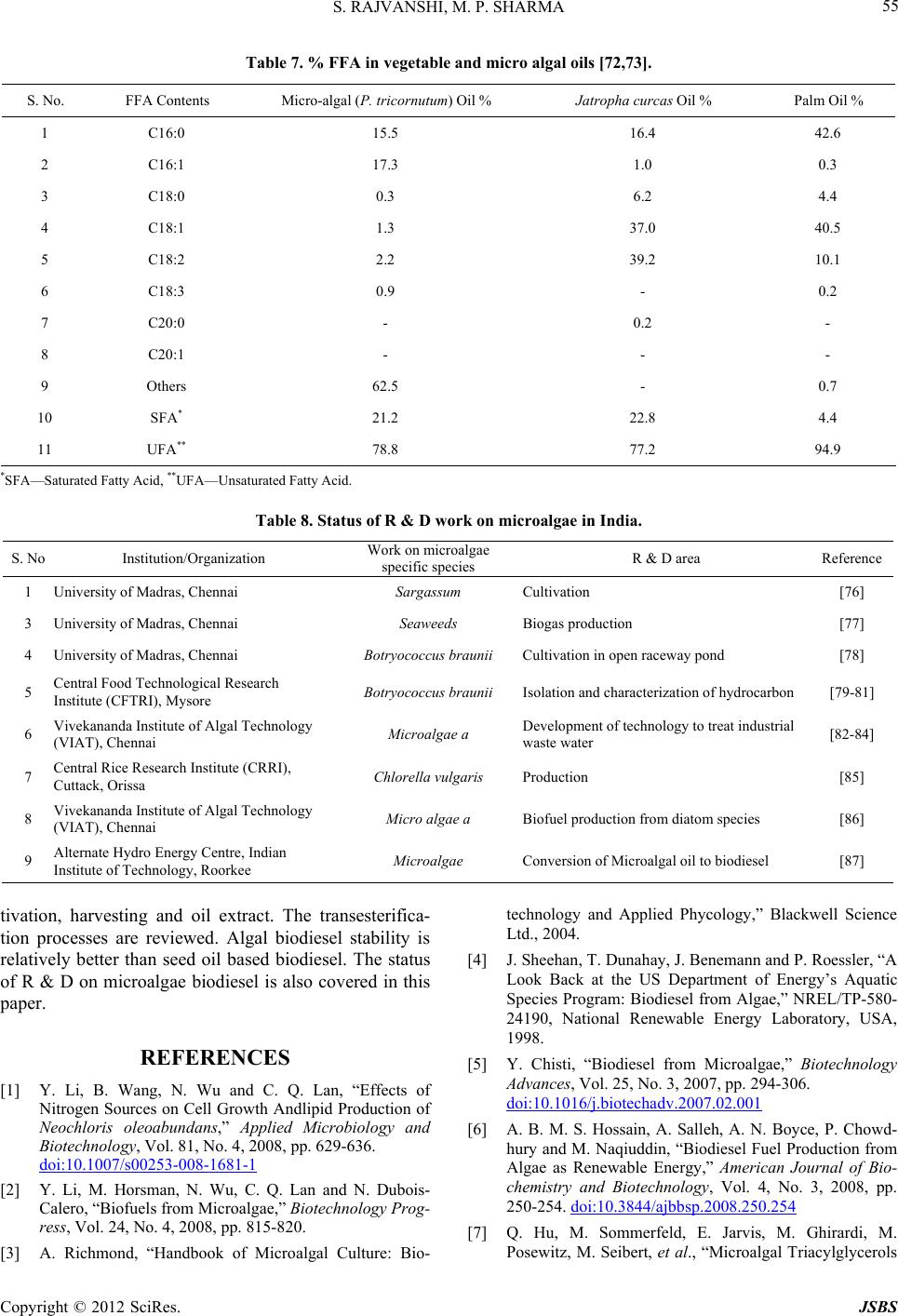 S. RAJVANSHI, M. P. SHARMA 55 Table 7. % FFA in vegetable and micro algal oils [72,73]. S. No. FFA Contents Micro-algal (P. tricornutum) Oil % Jat r o p ha curcas Oil % Palm Oil % 1 C16:0 15.5 16.4 42.6 2 C16:1 17.3 1.0 0.3 3 C18:0 0.3 6.2 4.4 4 C18:1 1.3 37.0 40.5 5 C18:2 2.2 39.2 10.1 6 C18:3 0.9 - 0.2 7 C20:0 - 0.2 - 8 C20:1 - - - 9 Others 62.5 - 0.7 10 SFA* 21.2 22.8 4.4 11 UFA** 78.8 77.2 94.9 *SFA—Saturated Fatty Acid, **UFA—Unsaturated Fatty Acid. Table 8. Status of R & D work on microalgae in India. S. No Institution/Organization Work on microalgae specific species R & D area Reference 1 University of Madras, Chennai Sargassum Cultivation [76] 3 University of Madras, Chennai Seaweeds Biogas production [77] 4 University of Madras, Chennai Botryococcus braunii Cultivation in open raceway pond [78] 5 Central Food Technological Research Institute (CFTRI), Mysore Botryococcus braunii Isolation and charac terization of hydro c arbon [79-81] 6 Vivekananda Institute of Alga l Technology (VIAT), Chennai Microalgae a Development of technology to treat industria l waste water [82-84] 7 Cen t r al R i ce Research Instit ut e ( CR RI), Cuttack, Orissa Chlorella vulgaris Production [85] 8 Vivekananda Institute of Alga l Technology (VIAT), Chennai Micro algae a Biofuel pr o duction from di a t om species [86] 9 Alternate Hydro Energy Centre, Indian Institute of Technology, Roorkee Microalgae Conversion of M icroalgal oil to bi odiesel [87] tivation, harvesting and oil extract. The transesterifica- tion processes are reviewed. Algal biodiesel stability is relatively better than seed oil based biodiesel. The status of R & D on microalgae biodiesel is also covered in this paper. REFERENCES [1] Y. Li, B. Wang, N. Wu and C. Q. Lan, “Effects of Nitrogen Sources on Cell Growth Andlipid Production of Neochloris oleoabundans,” Applied Microbiology and Biotechnology, Vol. 81, No. 4, 2008, pp. 629-636. doi:10.1007/s00253-008-1681-1 [2] Y. Li, M. Horsman, N. Wu, C. Q. Lan and N. Dubois- Calero, “Biofuels from Microalgae,” Biotechnology Prog- ress, Vol. 24, No. 4, 2008, pp. 815-820. [3] A. Richmond, “Handbook of Microalgal Culture: Bio- technology and Applied Phycology,” Blackwell Science Ltd., 2004. [4] J. Sheehan, T. Dunahay, J. Benemann and P. Roessler, “A Look Back at the US Department of Energy’s Aquatic Species Program: Biodiesel from Algae,” NREL/TP-580- 24190, National Renewable Energy Laboratory, USA, 1998. [5] Y. Chisti, “Biodiesel from Microalgae,” Biotechnology Advances, Vol. 25, No. 3, 2007, pp. 294-306. doi:10.1016/j.biotechadv.2007.02.001 [6] A. B. M. S. Hossain, A. Salleh, A. N. Boyce, P. Chowd- hury and M. Naqiuddin, “Biodiesel Fuel Production from Algae as Renewable Energy,” American Journal of Bio- chemistry and Biotechnology, Vol. 4, No. 3, 2008, pp. 250-254. doi:10.3844/ajbbsp.2008.250.254 [7] Q. Hu, M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert, et al., “Microalgal Triacylglycerols Copyright © 2012 SciRes. JSBS  S. RAJVANSHI, M. P. SHARMA 56 as Feedstocks for Biofuels Production: Perspectives and Advances,” The Plant Journal, Vol. 54, No. 4, 2008, pp. 621-639. doi:10.1111/j.1365-313X.2008.03492.x [8] L. Rodolfi, G. C. Zittelli, N. Bassi, G. Padovani, N. Biondi, G. Bonini, et al., “Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-Cost Photobioreactor,” Biotech- nology and Bioengineering, Vol. 102, No. 1, 2009, pp. 100-112 . doi:10.1002/bit.22033 [9] J. N. Rosenberg, G. A. Oyler, L. Wilkinson and M. J. Betenbaugh, “A Green Light for Engineered Algae: Re- directing Metabolism to Fuel a Biotechnology Revolu- tion,” Current Opinion in Biotechnology, Vol. 19, No. 5, 2008, pp. 430-436. doi:10.1016/j.copbio.2008.07.008 [10] P. M. Schenk, S. R. T. Hall, E. Stephens, U. C. Marx, J. H. Mussgnug, C. Posten, et al., “Second Generation Biofuels: High-Efficiency Microalgae for Biodiesel Pro- duction,” Bioenergy Research, Vol. 1, No. 1, 2008, pp. 20-43. doi:10.1007/s12155-008-9008-8 [11] K. Tsukahara and S. Sawayama, “Liquid Fuel Production Using Microalgae,” Journal of the Japan Petroleum Insti- tute, Vol. 48, No. 5, 2005, pp. 251-259. doi:10.1627/jpi.48.251 [12] P. Spolaore, C. C. Joannis, E. Duran and A. Isambert, “Commercial Applications of Microalgae,” Journal of Bioscience and Bioengineering, Vol. 101, No. 2, 2006, pp. 87-96. doi:10.1263/jbb.101.87 [13] J. Pratoomyot, P. Srivilas and T. Noiraksar, “Fatty Acids Composition of 10 Microalgal Species,” Journal of Science and Technology, Vol. 27, No. 6, 2005, pp. 1179-1187. [14] L. Gouveia and A. C. Oliveira, “Microalgae as a Raw Material for Biofuels Production,” Journal of Industrial Microbiology and Biotechnology, Vol. 36, No. 2, 2009, pp. 269-274. doi:10.1007/s10295-008-0495-6 [15] F. Natrah, V. F. M Yoso, V. M. Shari, F. Abas and N. S. Mariana, “Screening of Malaysian Indigenous Microalgae for Antioxidant Properties and Nutritional Value,” Jour- nal of Applied Phycology, Vol. 19, No. 6, 2007, pp. 711- 718. doi:10.1007/s10811-007-9192-5 [16] T. M. Maata, A. A. Martins and N. S. Caetano, “Micro- algae for Biodiesel Production and Other Applications: A Review,” Renewable and Sustainable Energy Reviews, Vol. 14, No. 1, 2010, pp. 217-232. [17] S. Otles and R. Pire, “Fatty Acid Composition of Chlore- lla and Spirulina microalgae Species,” Journal of AOAC International, Vol. 84, No. 6, 2001, pp. 1708- 1714. [18] D. Bilanovic, A. Andargatchew, T. Kroeger and G. Shelef, “Freshwater and Marine Microalgae Sequestering of CO2 at Different C and N Concentrations—Response Surface Methodology Analysis,” Energy Conversion and Manage- ment, Vol. 50, No. 2, 2009, pp. 262-267. doi:10.1016/j.enconman.2008.09.024 [19] G. Hodaifa, M. E. Martínezb and S. Sánchezc, “Use of Industrial Wastewater from Oliveoil Extraction for Bio- mass Production of Scenedesmus obliquus,” Bioresource Technology, Vol. 99, No. 5, 2008, pp. 1111-1117. doi:10.1016/j.biortech.2007.02.020 [20] E. Jacob-Lopes, L. M. C. F. Lacerda and T. T. Franco, “Biomass Production and Carbon Dioxide Fixation by Aphanothece Microscopica Na¨geli in a Bubble Column Photobioreactor,” Biochemical Engineering Journal, Vol. 40, No. 1, 2008, pp. 27-34. doi:10.1016/j.bej.2007.11.013 [21] E. L. Jacob, C. H. G. Scoparo, L. M. C. F. Lacerda and T. T. Franco, “Effect of Light Cycles (Night/Day) on CO2 Fixation and Biomass Production by Microalgae in Photo- bioreactors,” Journal of Chemical Engineering and Pro- cessing, Vol. 48, No. 1, 2009, pp. 306-310. doi:10.1016/j.cep.2008.04.007 [22] M. Murakami, F. Yamada, T. Nishide, T. Muranaka, N. Yamaguchi and Y. Takimoto, “The Biological CO2 Fix- ation Using Chlorella sp. with High Capability in Fixing CO2,” Studies in Surface Science and Catalysis, Vol. 114, 1998, pp. 315-320. doi:10.1016/S0167-2991(98)80763-5 [23] N. R. Moheimani, “The Culture of Coccolithophorid Algae for Carbon Dioxide Bioremediation,” PhD Thesis. Murdoch University, Perth, 2005. [24] K. Kaewpintong, A. Shotiprunk, S. Powtongsook and P. Pavasout, “Photoautotrophic High Density Cultivation of Vegitative Cells of Haematococcus plevialis in Airlift Bioreactor,” Bioresource Technology, Vol. 98, No. 2, 2007, pp. 288-295. doi:10.1016/j.biortech.2006.01.011 [25] W. H. Thomas, T. G. Tornabene and J. Weissman, “Screening for Lipid Yielding Microalgae: Activities for 1983,” Final Subcontract Report, US Department of Energy, Washington DC, 1984. [26] C. U. Ugwu, H. Aoyagi and H. Uchiyama, “Photo- bioreactors for Mass Cultivation of Algae,” Bioresource Technology, Vol. 99, No. 10, 2007, pp. 4021-4028. [27] J. C. Weissman and R. P. Goebel, “Design and Analysis of Microalgal Open Pond Systems for the Purpose of Pro- ducing Fuels,” A Subcontract Report, US DOESERI, 1987. [28] R. Samson and A. Leduy, “Biogas Production from Anae- robic Digestion of Spirulina maxima Algal Biomass,” Biotechnology and Bio-Engineering, Vol. 24, No. 8, 1982, pp. 1919-1924. doi:10.1002/bit.260240822 [29] R. D. Ortega and J. C. Roux, “Production of Chlorella Biomass in Different Types of Flat Bioreactors in Temp- erate Zones,” Elsevier Biomass, Vol. 10, No. 2, 1986, pp. 141-156. [30] M. R. Tredici and R. Materassi, “The Italian Experience in the Development of Reactors for the Mass Cultivation of Photoautotrophic Microorganisms”, Journal of Applied Phycology, Vol. 4, No. 3, 1992, pp. 221-231. doi:10.1007/BF02161208 [31] M. L. Ghirardi, J. P. Zhang, J. W. Lee, T. Flynn, M. Seibert, E. Greenbaum, et al., “Microalgae: A Green Source of Renewable H2,” Trends in Biotechnology, Vol. 18, No. 12, 2000, pp. 506-511. doi:10.1016/S0167-7799(00)01511-0 [32] S. Hoekema, M. Bijmans, M. Janssen, J. Tramper and R. H. Wijffels, “A Pneumatically Agitated Flat-Panel Photo- bioreactor with Gas Re-Circulation: Anaerobic Photo- heterotrophic Cultivation of a Purple Non-Sulfur Bac- terium”, International Journal of Hydrogen Energy, Vol. Copyright © 2012 SciRes. JSBS  S. RAJVANSHI, M. P. SHARMA 57 27, No. 11-12, 2002, pp. 1331-1338. doi:10.1016/S0360-3199(02)00106-4 [33] Q. Hu, H. Guterman and A. Richmond, “A Flat Inclined Modular Photobioreactor for Outdoor Mass Cultivation of Phototrophs,” Biotechnology & Bioengineering, Vol. 51, No. 1, 1996, pp. 51-60. doi:10.1002/(SICI)1097-0290(19960705)51:1<51::AID-B IT6>3.0.CO;2-# [34] Q. Hu, N. Kurano, M. Kawachi, I. Iwasaki and A. Miyachi, “Ultrahigh-Cell-Density Culture of Amarine Alga Chlorococcum Littorale in a Flat-Plate Photobioreactor,” Applied Microbiology and Biotechnology, Vol. 49, No. 6, 1998, pp. 655-662. doi:10.1007/s002530051228 [35] N. Eriksen, “The Technology of Microalgal Culturing,” Biotechnology Letters, Vol. 30, No. 9, 2008, pp. 1525- 1536. doi:10.1007/s10529-008-9740-3 [36] B. Wang, Y. Li, N. Wu and C. Lan, “CO2 Bio-Mitigation Using Microalgae,” Applied Microbiology and Biotech- nology, Vol. 79, No. 5, 2008, pp. 707-718. doi:10.1007/s00253-008-1518-y [37] R. E. Lee, “Phycology,” Cambridge University Press, New York, 1980. [38] Wikipedia, “The Free Encyclopedia”. http://www.oilgae.org/algae_FAQ [39] L. Lewis, “Seaweed to Breathe New Life into Fight against Global Warming,” The Times Online, London, 2008. [40] S. J. Horn, “Production of Biogas and Bioethanol from Brown Macroalgae,” Seaweed Biofuels, 2009, p. 104. [41] http://www.algaefuels.org/algae_FAQ.htm [42] http://www.unapcaem.org/publication/bioenergy [43] http://www.netl.doe.gov/publications/proceedings/03/carb on-seq/PDFs/158 [44] A. A. A. Kheira and N. M. M. Atta, “Response of Jatropha curcas L. to Water Deficit: Yield, Water Use Efficiency and Oilseed Characteristics,” Biomass and Bioenergy, Vol. 33, No. 10, 2008, pp. 1343-1350. doi:10.1016/j.biombioe.2008.05.015 [45] K. Cenciani, M. C. B. Oliveira, B. J. Feigl and C. C. Cerri, “Sustainable Production of Biodiesel by Microalgae and Its Application in Agriculture,” African Journal of Micro- biology Research, Vol. 5, No. 26, 2011, pp. 4638-4645. [46] L. A. Kulay and G. A. Silva, “Comparative Screening LCA of Agricultual Stages of Soy and Castor Beans,” 2nd International Conference on Life Cycle Management, Barcelona, 5-7 September 2005, pp. 5-7. [47] Mobius Biofuels, Limited Liability Compa ny , 2008. http://www.mobiusbiofuels.com/biodiesel.htm [48] D. C. Nielsen, “Oilseed Productivity under Varying Water Availability,” Proceedings of 20th Annual Central Plains Irrigation Conference and Exposition, Akron, 1 January 2008, pp. 30-33. [49] C. L. Peterson and T. Hustrulid, “Carbon Cycle for Rapeseed Oil Biodiesel Fuels,” Biomass and Bioenergy, Vol. 14, No. 2, 1998, pp. 91-101. doi:10.1016/S0961-9534(97)10028-9 [50] U. Mubee, Zia-ul-Islam, M. W. Hussain and K. A. Malik, “Future of Your Fuel Tank,” Department of Biological Sciences, Lahore, 5 June 2010, p. 131. [51] L. Reijnders and M. A. J. Huijbregts, “Biogenic Green- house Gas Emissions Linked to the Life Cycles of Biodiesel Derived from European Rapeseed and Brazilian Soybeans,” Journal of Cleaner Production, Vol. 16, No. 18, 2008, pp. 1943-1948. doi:10.1016/j.jclepro.2008.01.012 [52] J. Vollmann, T. Moritz, C. Karg, S. Baumgartner and H. Wagentrist, “Agronomic Evaluation of Cameli na Gen otypes Selected for Seed Quality Charact eristics,” Industrial Crops and Products, Vol. 26, No. 3, 2007, pp. 270-277. doi:10.1016/j.indcrop.2007.03.017 [53] M. Zappi, R. Hernandez, D. Sparks, J. Horne, M. Brough, D. C. Swalm, et al., “A Review of the Engineering Aspects of the Biodiesel Industry,” MSU E-TECH La- boratory Report, 2003. [54] M. E. Grima, E. H. Belari, A. G. A. Fernandez, A. R. Medina and Y. Chisti, “Recovery of Microalgal Biomass and Metabolites: Process Options and Economics,” Bio- technology Advances, Vol. 20, No. 7-8, 2003, pp. 491- 515. doi:10.1016/S0734-9750(02)00050-2 [55] R. A. Korus, D. S. Hoffman and N. Bam, “Transes- terification Process to Manufacture Ethyl Ester of Rape Oil,” Proceedings of 1st Biomass Conference of the Ameri- cas: Energy, Environment, Agriculture and Industry, Vol. II, National Renewable Energy Laboratory (NREL), Golden, 1993, pp. 815-826. [56] G. Antolin, F. V. Tinaut, Y. Briceno, V. Castano, C. Perez and A. I. Ramirez, “Optimisation of Biodiesel Production by Sunflower Oil Transesterification,” Bio- resource Technology , Vol. 83, No. 2, 2002, pp. 111-114. doi:10.1016/S0960-8524(01)00200-0 [57] W. Du, Y. Y. Xu, J. Zeng and D. H. Liu, “Novozym 435-Catalysed Transesterification of Crude Soybean Oils for Biodiesel Production in Solvent-Free Medium,” Bio- technology and Applied Biochemistry, Vol. 40, No. 2, 2004, pp. 187-190. doi:10.1042/BA20030142 [58] D. Darnoko and M. Cheryan, “Kinetics of Palm Oil Transesterification in a Batch Reactor,” Journal of Ameri- can Oil Chemist Society, Vol. 77, No. 12, 2000, pp. 1263- 1267. doi:10.1007/s11746-000-0198-y [59] M. Ahmad, S. Rashid, M. A. Khan, M. Zafar, S. Sultana and S. Gulzar, “Optimization of Base Catalyzed Transes- terification of Peanut Oil Biodiesel,” African Journal of Biotechnology, Vol. 8, No. 3, 2009, pp. 441-446. [60] H. J. Berchmans and S. Hirata, “Biodiesel Production from Crude Jatropha curcas L. Seed Oil with a High Content of Free Fatty Acids,” Bioresource Technology, Vol. 99, No. 6, 2008, pp. 1716-1721. doi:10.1016/j.biortech.2007.03.051 [61] S. V. Ghadge and H. Raheman, “Biodiesel Production from Mahua (Madhuca indica) Oil Having High Free Fatty Acids,” Biomass and Bioenergy, Vol. 28, No. 6, 2005, pp. 601-605. doi:10.1016/j.biombioe.2004.11.009 [62] S. K. Kamree and A. Chadha, “Preparation of Biodiesel Copyright © 2012 SciRes. JSBS  S. RAJVANSHI, M. P. SHARMA 58 from Crude Oil of Pongamia pinnata,” Bioresource Tech- nology, Vol. 96, No. 13, 2005, pp. 1425-1429. doi:10.1016/j.biortech.2004.12.011 [63] L. C. Meher, V. S. S. Dharmagadda and S. N. Naik, “Optimisation of Alkali-Catalysed Transesterifcation of Pongamia pinnata Oil for Production of Biodiesel,” Bioresource Technology, Vol. 97, No. 12, 2006, pp. 1392- 1397. doi:10.1016/j.biortech.2005.07.003 [64] A. Karmakar, S. Karmakar and S. Mukherjee, “Properties of Various Plants and Animals Feedstocks fo Biodiesel Production,” Bioresource Technology, Vol. 101, No. 19, 2010, pp. 7201-7210. doi:10.1016/j.biortech.2010.04.079 [65] C. He, P. Baoxiang, W. Dezheng and W. Jinfu, “Biodiesel Production by the Transesterification of Cot- tonseed Oil by Solid Acid Catalysts,” Frontiers of Chemi- cal Engineering in China, Vol. 1, No. 1, 2007, pp. 11-15. [66] D. Royon, M. Daz, G. Ellenrieder and S. Locatelli, “Enzymatic Production of Biodiesel from Cottonseed Oil Using t-Butanol as a Solvent,” Bioresource Technology, Vol. 98, No. 3, 2006, pp. 648-653. doi:10.1016/j.biortech.2006.02.021 [67] A. Saydut, M. Z. Duz, C. Kaya, A. B. Kafadar and C. Hamamci, “Transesterified Sesame (Sesamum indicum L.) Seed Oil as a Biodiesel Fuel,” Bioresource Technology, Vol. 99, No. 14, 2008, pp. 6656-6660. doi:10.1016/j.biortech.2007.11.063 [68] L. Chen, T. Liu, W. Zhang, X. Chen and J. Wang, “Biodiesel Production from Algae Oil High in Free Fatty Acids by Two-Step Catalytic Conversion,” Bioresource Technology, Vol. 111, 2012, pp. 208-214. doi:10.1016/j.biortech.2012.02.033 [69] X. Miao and Q. Wu, “Biodiesel Production from Hetero- trophic Microalgal Oil,” Bioresource Technology, Vol. 97, No. 6, 2006, pp. 841-846. doi:10.1016/j.biortech.2005.04.008 [70] S. Jain, M. P. Sharma and S. Rajvanshi, “Evaluation of Engine Performance on Biodiesel from WCO,” 4th Inter- national Conference on Energy Informatics & Cyber- netics (EIC), Orlando, 29 June-2 July 2008. [71] S. Jain and M. P. Sharma, “Kinetics of Acid Base Ctalyzed Transesterification of Jatropha curcas Oil,” Bioresource Technology, Vol. 101, No. 20, 2010, pp. 7701-7706. doi:10.1016/j.biortech.2010.05.034 [72] C. C. Akoh, S. S. Chang, G. G. Lee and J. J. Shaw, “Enzymatic Approach to Biodiesel Production,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 22, 2007, pp. 8995-9005. doi:10.1021/jf071724y [73] M. Cartens, E. G. Molina, A. M. Robles, A. Giménez and M. J. G. Ibanez, “Eicosapentaenoic Acid (20:5n-3) from the Marine Microalgae Phaeodactylum tricornutum,” Jour- nal of American Oil Chemical Society, Vol. 73, No. 8, 1996, pp. 1025-1031. doi:10.1007/BF02523411 [74] D. Bajpai and V. K. Tyagi, “Biodiesel: Source, Pro- duction, Composition, Properties and Its Benefits,” Jour- nal of Oleo Science, Vol. 55, No. 10, 2006, pp. 487-502. doi:10.5650/jos.55.487 [75] M. J. Ramos, C. M. Fernández, A. Casas, L. Rodríguez and A. Pérez, “Influence of Fatty Acid Composition of Raw Materials on Biodiesel Properties,” Bioresource Technology, Vol. 100, No. 1, 2009, pp. 261-268. doi:10.1016/j.biortech.2008.06.039 [76] R. Rengasamy, “Demonstration and Extension of Culture and Cultivation of Alginophytes, Sargassum polycystem C. Agardh and S. wightii,” Department of Science, 2008- 2011. [77] R. Rengasamy, “Potential of Seaweed and Sea Grass for Biogas Production,” Aguagri, New Delhi, 2008-2009. [78] R. Rengasamy, “Optimization of Conditions for Mass Culture of Botryococcus braunii under Open Race Way Ponds,” Aban Informatics Pvt. Ltd., Chennai, 2008-2009. [79] C. Dayananda, R. Sarada, S. Bhattacharya and G. A. Ravishankar, “Effect of Media and Culture Conditions on Growth and Hydrocarbon Production by Botryococcus braunii,” Proc ess Biochemistry, Vol. 40, No. 9, 2005, pp. 3125-3131. doi:10.1016/j.procbio.2005.03.006 [80] C. Dayananda, R. Sarada, P. Srinivas, T. R. Shamala and G. A. Ravishankar, “Presence of Methyl Branched Fatty Acids and Saturated Hydrocarbons in Botryococcene Producing Strain of Botryococcus braunii,” Acta Physi- ologiae Plantarum, Vol. 28, No. 3, 2006, pp. 251-256. doi:10.1007/BF02706538 [81] U. Tripathi, R. Sarada and G. A. Ravishankar, “A Culture Method for Micro Algal Forms Using Two-Tier Vessel Providing Carbon-Dioxide Environment: Studies on Growth and Carotenoids Production,” Journal of Microbiology and Biotechnology, Vol. 17, No. 4, 2001, pp. 325-329. doi:10.1023/A:1016682120171 [82] V. Sivasubramanian, V. V. Subramanian, P. A. Raju and M. Muthukumaran, “Phycoremediation of Oil Drilling Waste at Kakinada,” International Conference on Algal Biomass, Resources and Utilization, Stella Maris College, Chennai, 27-30 July 2009. [83] R. Ranjithkumar, V. V. Subramanian and V. Sivasubra- manian, “Phycoremediation of Acidic Effluent from a Confectionary Industry near Chennai,” International Con- ference on Algal Biomass, Resources and Utilization, Stella Maris College, Chennai, 27-30 July 2009. [84] P. H. Rao, R. R. Kumar, B. G. Raghavan, V. V. Subra- manian and V. Sivasubramanian, “Phycoremediation of Effluent from a Leather Processing Chemical Industry,” International Conference on Algal Biomass, Resources and Utilization, Stella Maris College, Chennai, 27-30 July 2009. [85] S. Chinnasamy, B. Ramakrishnan, A. Bhatnagar and K. C. Das, “Biomass Production Potential of a Wastewater Alga Chlorella vulgaris ARC 1 under Elevated Levels of CO2 and Temperature,” International Journal of Molecular Sciences, Vol. 10, No. 2, 2009, pp. 518-532. doi:10.3390/ijms10020518 [86] T. V. Ramachandra, M. M. Durga and B. Karthic k, “Milk- ing Diatoms for Sustainable Energy: Biochemical Engi- neering versus Gasoline-Secreting Diatom Solar Panels,” Industrial & Engineering Chemistry Research, Vol. 48, No. 19, 2009, pp. 8769-8788. doi:10.1021/ie900044j [87] S. Rajvanshi, S. Jain and M. P. Sharma, “Micro Algae as Copyright © 2012 SciRes. JSBS  S. RAJVANSHI, M. P. SHARMA Copyright © 2012 SciRes. JSBS 59 Potential Source of Biodiesel in India,” 6th International Conference on Sustainable Development of Energy Water and Environment System, Dubrovnik, 25-29 September 2011.
|