Paper Menu >>
Journal Menu >>
 Advances in Microbiology, 2012, 2, 327-331 http://dx.doi.org/10.4236/aim.2012.23039 Published Online September 2012 (http://www.SciRP.org/journal/aim) Choline Promotes Growth and Tabtoxin Production in a Pseudomonas syringae Strain Lucas A. Gallarato, Emiliano D. Primo, Ángela T. Lisa*, Mónica N. Garrido Departamento de Biología Molecular, Facultad de Ciencias Exactas, Físico-Químicas y Naturales, Universidad Nacional de Río Cuarto, Córdoba, Argentina Email: *tlisa@exa.unrc.edu.ar Received May 31, 2012; revised June 29, 2012; accepted July 11, 2012 ABSTRACT Some Pseudomonas syringae pathovars secrete tabtoxin, a monocyclic β-lactam antibiotic, responsible for chlorosis, the principal halo blight symptom in susceptible plants as oats, rye, barley, wheat and sorghum, among other. Here, we demonstrated that the production of tabtoxin in a P. syringae strain increased at least 150%, when choline, betaine or dimethylglycine were used as nitrogen source, or when choline was added as osmoprotectant in hyperosmolar culture media. Besides, we investigated the induction of phosphorylcholine phosphatase (PchP) activity when choline or its metabolites were used as nitrogen sources. PchP is an enzyme involved in Pseudomonas aeruginosa pathogenesis through its contribution to the breakdown of choline-containing compounds of the host cells. Considering these results and that the success of a pathogenic microorganism depends on its ability to survive and proliferate in its target tissue, we propose that choline is one of the plant signals that contribute to establishment of the infection by tabtoxin-produc- ing strains of P. syringae. Keywords: Choline; Pseudomonas syringae; Phytotoxins; Phosphorylcholine Phosphatase 1. Introduction In previous work, we demonstrated that the presence of choline as carbon and nitrogen sources in a culture me- dium of Pseudomonas aeruginosa induce at least three proteins, phosphorylcholine phosphatase (PchP), hemo- lytic phospholipase C (PlcH) and acetylcholinesterase (AchE) [1,2]. PchP is involved in the pathogenesis of P. aeruginosa through the coordinated and sequential action of PlcH and PchP on phosphatidylcholine or sphyngomye- lin and phosphorylcholine, respectively [2-5]. In this way, these enzymes serve to liberate osmoprotective agents and nutrients that are needed for the growth and sur- vival of P. aeruginosa in some phosphatidylcholine-rich environments as occurs in the lungs of cystic fibrosis patients. In P. aeruginosa PAO1 the gene encoded PchP (PA5292) was identified and named pchP [6]. A search for proteins similar to PchP was performed by the Blast Web interface (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to- wards the non-redundant protein sequences database demonstrated the presence of orthologs in other members of Pseudomonas genus and some mammals and plant pathogenic organisms. Previous experiments in our labo- ratory indicated that P. syringae pv. tomato DC3000 was able to grow in a basal salt medium with choline, betaine or dimethylglycine as carbon and nitrogen sources [7]. Under any of these conditions the bacteria produced an acid phosphatase activity with catalytic properties similar to those described for the P. aeruginosa PchP. Tabtoxin is a monocyclic β-lactam produced by P. sy- ringae pv. tabaci, coronafaciens, and garcae, and it had also been detected in some P. syringae pv. tomato strains [8-10]. This toxin inhibits the target enzyme glutamine synthetase, which results in the abnormal accumulation of ammonia in plant cells, causing the characteristic chlorosis symptom in susceptible plants [11]. It was re- ported that both, growth of P. syringae and quantity of tabtoxin synthesized, were significantly affected by car- bon source, nitrogen source and amino acid supplements [12]. Here, we report that choline, a normal constituent of plant tissues, in addition to inducing a PchP activity in a phytopathogenic member of Pseudomon as, elevate the production of tabtoxin by this bacterium in both, iso and hyperosmolar conditions. 2. Material and Methods Pseudomonas syringae pv. coronafaciens S5 was isolated in a field south of Córdoba, Argentina and characterized in our laboratory. The bacterium was grown aerobically at 28˚C in a tabtoxin inducer medium (TMM) described *Corresponding author. C opyright © 2012 SciRes. AiM  L. A. GALLARATO ET AL. 328 in [12] and slightly modified in our laboratory. It con- tained: H2NaPO4 6.52 mM, HK2PO4 4.6 mM, FeSO4 20 mg· L –1, CaCl2 100 mg·L–1 and MgSO4.7H2O 8 mM and a reducing concentration of sucrose and NH4Cl (20 mM). When NH4Cl was replaced by choline, betaine, dime- thylglycine or individual amino acids, they were used at the same concentration (20 mM). Casamino acids were used at 0.25% (p/v). When indicated, some of the above mentioned compounds were added to TMM, as supple- mentary nitrogen source at final concentration of 1.0 mM. For tabtoxin determination by an agar diffusion bioas- say [9,13], agar discs (3 - 4 mm) were removed from M9 minimal medium + glucose + NH4Cl [14] agar plates before being spread with E. coli as indicator strain. As source of toxin, 40 µl of a culture filtrate (0.2 µm filter) of P. syringae pv. coronafaciens S5 grown in TMM with NH4Cl or the appropiate nitrogen source, was used. The aliquot culture filtrates were put into the agar holes, and after 3 hours at 4˚C to allow aliquot diffusion, the plates were spread with a suspension of the E. coli culture grown to 0.3 - 0.5 OD600, and then incubated for 16 hours at 37˚C. The presence of the toxin was revealed by a growth inhibition halo around the hole containing the culture filtrate. Glutamine was used to antagonize the toxin by mixing 25 μl of a 0.1% solution of the amino acid with cultures filtrates in the agar hole. In order to quantify results obtained with the agar dif- fusion test we defined the term: toxigenic activity (TA). TA was calculated as the relationship between the di- ameter of the produced inhibition halo (measured in cen- timeters) and the optical density (OD) of the 72 hours grown P. syringae S5 cultures used to be tested, since the final OD values of cultures grown in different conditions were slightly dissimilar. To confirm the chlorosis produced by the tabtoxin ac- tion, seven to ten day old plant leaves of Avena sativa were cut and put in sterilized Petri dishes with filter pa- per imbibed with sterile water. Leaves were inoculated with 10 μl aliquots of a supernatant from a of P. syringae S5 culture grown for 72 hours using a syringe without a needle. A positive test for tabtoxin produced chlorosis after 2 days [15]. PchP and acid phosphatase activities were measured in whole cells as in [3,6]. One unit of PchP was defined as the amount of enzyme that released 1 μmol of p-nitro- phenol from p-nitrophenyl phosphate or 1 μmol of phos- phate (Pi) from phosphorylcholine per minute at 37˚C. Protein concentration was determined according to [16], using bovine serum albumin as the standard. All our data were analyzed by GraphPad Prism soft- ware (version 4.00 for Windows GraphPad Software, San Diego California USA, www.graphpad.com). Differ- ences were considered significant at p < 0.05. 3. Results Choline and metabolic derivatives increased tabtoxin production and PchP activity in P. syringae S5. The growth of P. syringae S5 in a minimal medium (TMM medium, see Material and Methods) with choline, betaine or dimethylglycine as the sole carbon and nitrogen sources was very low, and scarce tabtoxin production was detected (data not shown). The use of sucrose and 4 NH as carbon and nitrogen sources, respectively, en- hanced the production of tabtoxin as was reported earlier for a different strain [12]. When NH4 + was replaced by 20 mM choline, TA increased approximately 55% - 65% relative to the TA measured from supernatants of su- crose/ 4 NH grown cells (control culture, which was con- sidered the 100%, Figure 1(a)). The derivatives of cho- line such as betaine and dimethylglycine, also induced TA with higher values than that found in control culture, although to a lesser extent compared with choline. To test the specificity of choline and its derivatives on tabtoxin production, other nitrogen sources such as amino acids were used. As shown in Figure 1(a), only casaminoacids were as effective as 4 in the TA pro- duced, whereas glycine, alanine and serine only produced a TA of 85%, 48% and 46% of the TA measured in the control. The amino acids methionine, threonine and as- partic and glutamic acids, in spite of supporting growth of cells, failed to increase production of the toxin. When the control culture (sucrose/4) was supplemented with 1 mM of the amino acids, the TA remained similar to the control (Figure 1(b)). However, the TA increased 50% - 60% over the control when was supple- mented with 1 mM of choline. NH NH 4 NH NH PchP activity was measured in the cells grown in the above culture conditions. The PchP activity determined in sucrose/4 grown cells was 0.65 ± 0.17 U. mg of proteins–1 (means ± SEM, n = 6). Only cells grown in the presence of choline and its derivatives as nitrogen source, showed a significant increment. Choline and dimethyl- glycine produced an increased activity of 4 - 5-fold times and betaine 3 - 4 fold, compared with the activity meas- ured in control cells. In P. syringae it was demonstrated that choline pro- vided consistently better osmoprotection than betaine in hyperosmolarity [17,18]. Thus, it was determined if tab- toxin production and PchP activity were affected in cells cultured under hyperosmotic conditions and if choline influenced this condition. P. syringae S5 was grown until stationary phase, in TMM with different osmolarity pro- duced by raising the sucrose concentration to 0.75 M. A series of nitrogenous sources were used (20 mM 4 NH , 20 mM choline, or 20 mM 4 plus 1 mM choline). At the end of growth, final OD was measured, and cells were used to measure PchP activity and the culture su- pernatants to test the production of tabtoxin. When NH 4 NH Copyright © 2012 SciRes. AiM 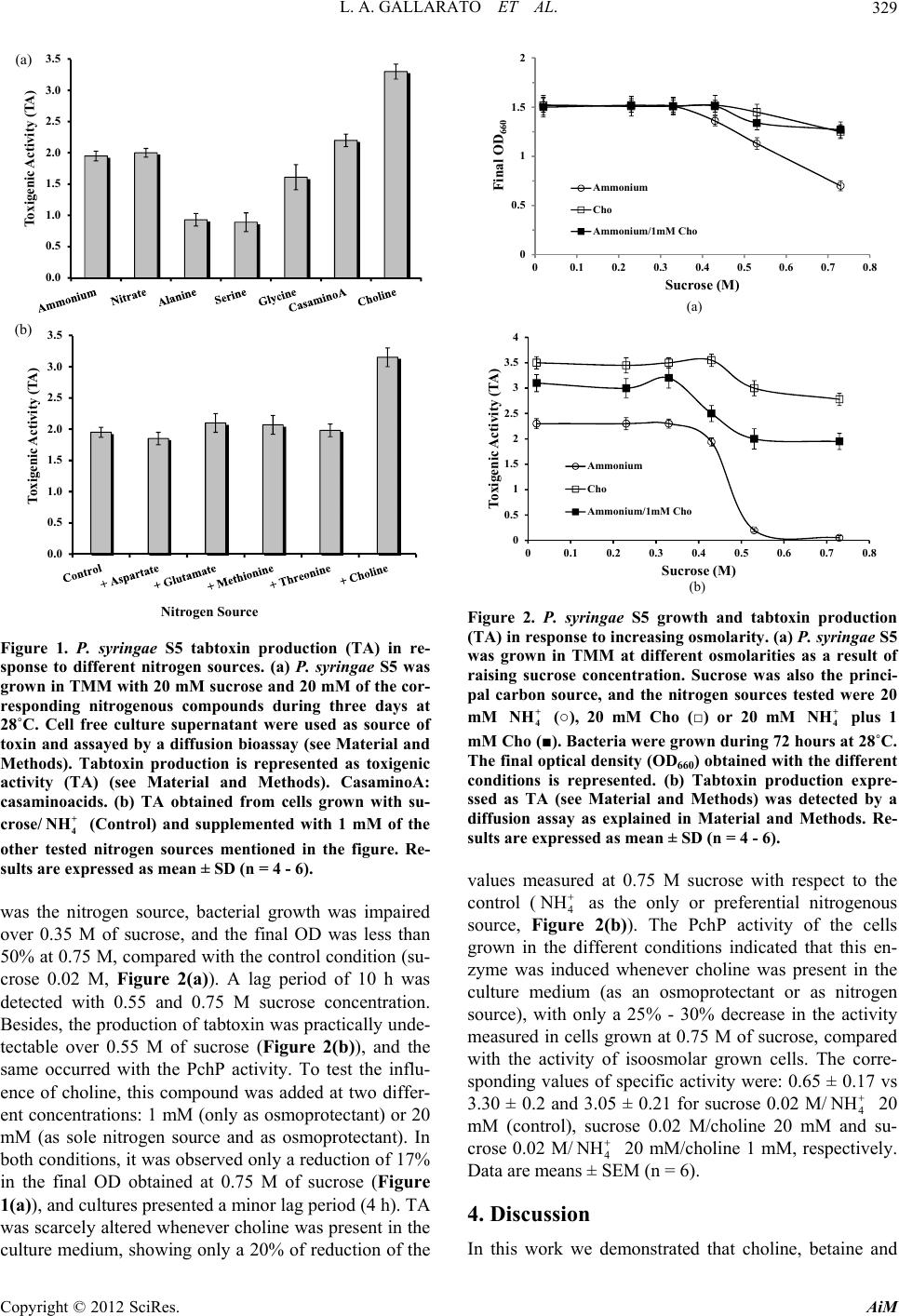 L. A. GALLARATO ET AL. 329 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 Toxigenic Activity (TA) Nitrogen Source B 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 Toxigenic Activity (TA) A (a) (b) 0 0.5 1 1.5 2 00.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 Final OD 660 Sucrose (M) A Ammoni um Cho Ammonium/1mM Cho Figure 1. P. syringae S5 tabtoxin production (TA) in re- sponse to different nitrogen sources. (a) P. syringae S5 was grown in TMM with 20 mM sucrose and 20 mM of the cor- responding nitrogenous compounds during three days at 28˚C. Cell free culture supernatant were used as source of toxin and assayed by a diffusion bioassay (see Material and Methods). Tabtoxin production is represented as toxigenic activity (TA) (see Material and Methods). CasaminoA: casaminoacids. (b) TA obtained from cells grown with su- crose/ (Control) and supplemented with 1 mM of the other tested nitrogen sources mentioned in the figure. Re- sults are expressed as mean ± SD (n = 4 - 6). + 4 NH was the nitrogen source, bacterial growth was impaired over 0.35 M of sucrose, and the final OD was less than 50% at 0.75 M, compared with the control condition (su- crose 0.02 M, Figure 2(a)). A lag period of 10 h was detected with 0.55 and 0.75 M sucrose concentration. Besides, the production of tabtoxin was practically unde- tectable over 0.55 M of sucrose (Figure 2(b)), and the same occurred with the PchP activity. To test the influ- ence of choline, this compound was added at two differ- ent concentrations: 1 mM (only as osmoprotectant) or 20 mM (as sole nitrogen source and as osmoprotectant). In both conditions, it was observed only a reduction of 17% in the final OD obtained at 0.75 M of sucrose (Figure 1(a)), and cultures presented a minor lag period (4 h). TA was scarcely altered whenever choline was present in the culture medium, showing only a 20% of reduction of the 0 0.5 1 1.5 2 2.5 3 3.5 4 0 0.10.20.30.40.50.60.70.8 Toxigenic Activity (TA) Sucrose (M) (a) Ammoni um Cho Ammonium/1mM Cho (b) Figure 2. P. syringae S5 growth and tabtoxin production (TA) in response to increasing osmolarity. (a) P. syringae S5 was grown in TMM at different osmolarities as a result of raising sucrose concentration. Sucrose was also the princi- pal carbon source, and the nitrogen sources tested were 20 mM (○), 20 mM Cho (□) or 20 mM plus 1 mM Cho (■). Bacteria were grown during 72 hours at 28˚C. The final optical density (OD660) obtained with the different conditions is represented. (b) Tabtoxin production expre- ssed as TA (see Material and Methods) was detected by a diffusion assay as explained in Material and Methods. Re- sults are expressed as mean ± SD (n = 4 - 6). + 4 NH + 4 NH NH values measured at 0.75 M sucrose with respect to the control (4 as the only or preferential nitrogenous source, Figure 2(b)). The PchP activity of the cells grown in the different conditions indicated that this en- zyme was induced whenever choline was present in the culture medium (as an osmoprotectant or as nitrogen source), with only a 25% - 30% decrease in the activity measured in cells grown at 0.75 M of sucrose, compared with the activity of isoosmolar grown cells. The corre- sponding values of specific activity were: 0.65 ± 0.17 vs 3.30 ± 0.2 and 3.05 ± 0.21 for sucrose 0.02 M/4 NH 20 mM (control), sucrose 0.02 M/choline 20 mM and su- crose 0.02 M/4 NH 20 mM/choline 1 mM, respectively. Data are means ± SEM (n = 6). 4. Discussion In this work we demonstrated that choline, betaine and Copyright © 2012 SciRes. AiM  L. A. GALLARATO ET AL. 330 dimethylglycine were more efficient for promoting tab- toxin production as compared to 4 and a series of amino acids previously used as the best nitrogen sources for tabtoxin production [12]. The ability of P. syringae S5 to use choline as an alternative nitrogen source for the growth and as an osmoprotectant was an expected result. In this regard, it was reported [17,18] that choline pro- vided consistently better osmoprotection than betaine when it was supplied at 1 mM in the culture media, and that choline was capable of osmoprotectant activity even as at a concentration as low as 50 µM. Nevertheless, it was surprising that choline was also able to repress the inhibitory effect of high osmolarity on tabtoxin produc- tion. In some other P. syringae pathovars it had been reported that a variety of nutritional and environmental factors are involved in phytotoxins production. Thus, it has been demonstrated that specific plant-released sig- nals are detected by the toxin-producing bacteria and are responsible for the expression of coronatine and syrin- gomycin phytotoxin genes [8,19]. NH Our observation of the ability of P syringae S5 to in- duce the PchP activity by choline and its derivatives un- der iso or hyperosmolar conditions, support the role pro- posed for the P. aeruginosa enzyme in the metabolism of phosphorus and nitrogen and in different environmental conditions [1,2,4]. The P. aeruginosa PchP kinetic prop- erties studied with its natural substrate phosphorylcholine suggested the great utility of this enzyme for the estab- lishment and colonization of the bacteria in different tis- sues of the host [2,4]. Interestingly, the pchP gene is highly conserved in the Pseudomonas group, with 18 putative orthologs found so far, including three different pathovars of P. syringae (P. syringae pv. tomato str. DC3000, P. syringae pv. syringae B728a and P. syringae phaseolicola 1448A) and in the multihost P. aeruginosa PA14 (http://www.pseudomonas.com/). From the results presented here we propose that cho- line, and probably its derivatives may be considered one of the plant-released signals that contribute to an accurate establishment of the infection provoked by tabtoxin pro- ducer P. syringae strains. Thus, during the endophytic phase of P. syringae S5, the action of PchP on apoplast phosphorylcholine, or the coordinated action of a bacte- rial lecithinase and PchP on the structural phospholipids of vegetable cells would enable the hydrolysis of phos- phorylcholine to obtain choline and phosphorus, which can be used by the bacterium as nutrients. Choline may be derived for the synthesis of the osmoprotector betaine, whose accumulation would allow tolerance to fluctua- tions in osmolarity in the environment. In the saprophytic phase, the existence of choline in root exudates just like its presence in the plant tissues would favor the survival of the bacterium until it is able to infect again a plant. Our proposal is in agreement with the studies reported by [17,18], who suggested that P. syringae has evolved to survive in relatively choline-rich habitats. Further invest- tigations would be necessary to determine if choline is one of the specific plant-released signals contributing to establishment of the infection by some P. syringae path- ovars. 5. Acknowledgements We thank M. Woelker and the Microbiologist student L. Ruffinatto for their technical assistance, and L. Barberis for providing the Escherichia coli strain used for tabtoxin bioassay. ATL is a CareerMember of the Consejo Nacio- nal de Investigaciones Científicas y Técnicas (CONICET). LAG and EDP would like to acknowledge fellowship support from CONICET and the Ministerio de Ciencia y Tecnología, Córdoba (MinCyT-Cba). This work was su- pported by grants from CONICET, Agencia Córdoba Ciencia and SECYT-UNRC of Argentina. REFERENCES [1] A. T. Lisa, C. H. Casale and C. E. Domenech, “Choli- nesterase, Acid Phosphatase and Phospholipase C of Pseudomonas aeruginosa under Hyperosmotic Condi- tions in a High-Phosphate Medium,” Current Microbiol- ogy, Vol. 28, No. 2, 1994, pp. 71-76. doi:10.1007/BF01569049 [2] A. T. Lisa, G. I. Lucchesi and C. E. Domenech, “Patho- genicity of Pseudomonas aeruginosa and Its Relationship to the Choline Metabolism through the Action of Choli- nesterase, Acid Phosphatase, and Phospholipase C,” Cur- rent Microbiology, Vol. 29, No. 4, 1994, pp. 193-199. doi:10.1007/BF01570153 [3] M. A. Salvano and C. E. Domenech, “Kinetic Properties of Purified Pseudomonas aeruginosa Phosphorylcholine Phosphatase Indicated That This Enzyme May Be Util- ized by the Bacteria to Colonize in Different Environ- ments,” Current Microbiololy, Vol. 39, No. 1, 1999, pp. 1-8. doi:10.1007/PL00006819 [4] A. T. Lisa, P. R. Beassoni, M. J. Massimelli, L. H. Otero and C. E. Domenech, “A Glance on Pseudomonas aer- uginosa Phosphorylcholine Phosphatase, an Enzyme Whose Synthesis Depends on the Presence of Choline in Its En- vironment,” In: A. Méndez-Vilas, Ed., Communicating Current Research and Educational Topics and Trends in Applied Microbiology, Vol. 1, Badajoz, 2007, pp. 255- 262. [5] C. E. Domenech, L. H. Otero, P. R. Beassoni and A. T. Lisa, “Phosphorylcholine Phosphatase: A Peculiar En- zyme of Pseudomonas aeruginosa,” Enzyme Research, Vol. 2011, 2011, Article ID: 561841. [6] M. J. Massimelli, P. R. Beassoni, M. A. Forrellad, J. L. Barra, M. N. Garrido, C. E. Domenech and A. T. Lisa, “Identification, Cloning and Expression of Pseudomonas aeruginosa Phosphorylcholine Phosphatase Gene,” Current Microbiology, Vol. 50, No. 5, 2005, pp. 251-256. doi:10.1007/s00284-004-4499-9 Copyright © 2012 SciRes. AiM  L. A. GALLARATO ET AL. Copyright © 2012 SciRes. AiM 331 [7] P. R. Beassoni, “Relación Entre Estructura y Función de la Fosforilcolina Fosfatasa de Pseudomonas aeruginosa y otras Bacterias del Género Pseudomonas,” Ph.D. Disser- tation, Universidad Nacional de Río Cuarto, Córdoba, 2006. [8] C. L. Bender, F. Alarcon-Chaidez and D. C. Gross, “Pse- udomonas syringae Phytotoxins: Mode of Action, Regu- lation, and Biosynthesis by Peptide and Polyketide Syn- thetases,” Microbiology and Molecular Biology Reviews, Vol. 63, No. 2, 1999, pp. 266-292. [9] M. S. H. Hwang, R. L. Morgan, S. F. Sarkar, P. W. Wang and D. S. Guttman, “Phylogenetic Characterization of Virulence and Resistance Phenotypes of Pseudomonas syringae,” Applied Environmental Microbiology, Vol. 71, No. 9, 2005, pp. 5182-5191. doi:10.1128/AEM.71.9.5182-5191.2005 [10] E. Arrebola, F. M. Cazorla, A. Perez-García and A. de Vicente, “Chemical and Metabolic Aspects of Antime- tabolite Toxins Produced by Pseudomonas syringae Path- ovars,” Toxins, Vol. 3, No. 9, 2011, pp. 1089-110. doi:10.3390/toxins3091089 [11] M. Kimura, H. Anzai and I. Yamaguchi, “Microbial Tox- ins in Plant-Pathogen Interactions: Biosynthesis, Resis- tance Mechanisms, and Significance,” Journal of General Applied Microbiology, Vol. 47, No. 4, 2001, pp. 149-160. doi:10.2323/jgam.47.149 [12] F. Dehbi, D. Harzallah and L. Larous, “Effects of Nu- tritional Factors on Production of Tabtoxin, a Phytotoxin, by Pseudomonas syringaepv. Tabaci,” Mededelingen (Rijk- suniversiteit te Gent. Fakulteit van de Landbouwkundige en Toegepaste Biologische Wetenschappen), Vol. 66, No. 2a, 2001, pp. 241-247. [13] B. Völksch and H. Weingart, “Toxin Production by pathovars of Pseudomonas syringae and Their Antago- nistic Activities against Epiphytic Microorganism,” Jour- nal of Basic Microbiology, Vol. 38, No. 2, 1998, pp. 135- 145. doi:10.1002/(SICI)1521-4028 [14] J. Sambrook and D. W. Russell, Molecular Cloning: A Laboratory Manual,” 3rd Edition, NY Laboratory Press, Cold Spring Harbor, 2001. [15] Y. P. Salch and P. D. Shaw, “Isolation and Characteriza- tion of Pathogenicity Genes of Pseudomonas syringae pv. Tabaci,” Journal of Bacteriology, Vol. 179, No. 6, 1988, pp. 2584-2591. [16] M. M. Bradford, “A Rapid and Sensitive Method for the Quantization of Microgram Quantities of Protein Utiliz- ing the Principle of Protein-Dye Binding,” Analytical Biochemistry, Vol. 72, 1976, pp. 248-254. doi:10.1016/0003-2697(76)90527-3 [17] C. Chen and G. A. Beattie, “Characterization of the Os- moprotectant Transporter OpuC from Pseudomonas sy- ringae and Demonstration That Cystathionine-β-Synthase domains Are Required for Its Osmoregulatory Function,” Journal of Bacteriology, Vol. 189, No. 19, 2007, pp. 6901-6912. doi:10.1128/JB.00763-07 [18] C. Chen and G. A. Beattie, “Pseudomonas syringae BetT Is a Low-Affinity Choline Transporter That Is Responsi- ble for Superior Osmoprotection by Choline over Glycine Betaine,” Journal of Bacteriology, Vol. 190, No. 8, 2008, pp. 2717-2725. doi:10.1128/JB.01585-07 [19] A. Brencic and S. C. Winans, “Detection of and Response to Signals Involved in Host-Microbe Interactions by Plant-Associated Bacteria,” Microbiology and Molecular Biology Review, Vol. 69, No. 1, 2005, pp. 155-164. doi:10.1128/MMBR.69.1.155-194.2005 |

