 Vol.2, No.7, 781-788 (2010) doi:10.4236/health.2010.27118 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Internal environment in cancer patients and proposal that carcinogenesis is adaptive response of glycolysis to overcome adverse internal conditions Mayumi Watanabe1, Kenya Miyajima2, Ittoku Matsui2, Chikako Tomiyama-Miyaji3, Eisuke Kainuma1, Masashi Inoue1, Hiroaki Matsumoto1, Yuh Kuwano1, Toru Abo1* 1Department of Immunology, Niigata University School of Medicine, Niigata, Japan; *Corresponding Aut hor: immunol2@med.niigata-u.ac.jp 2Yushima-Shimizuzaka Clinic, Tokyo, Japan 3School of Health Sciences, Faculty of Medicine, Niigata Univ e rsity, Niigata, Japan Received 4 March 2010; revised 16 March 2010; accepted 20 March 2010. ABSTRACT In a series of our recent studies, stress was found to induce simultaneously hypothermia and hyperglycemia. These conditions are bene- ficial to obtain prompt force which depends on the glycolysis pathway and to escape emer- gency. Since we have noticed that such condi- tions resemble the internal environment seen in some cancer patients, it was investigated whe- ther such conditions were accompanied with other patients. We selected patients with early and advanced cancer. Body temperature and other parameters including blood gas contents were examined. A difference was seen in body temperature, namely, many patients showed hypothermia, irrespective of cancer stages. Fur- ther characterization of other parameters show- ed that hypothermia and hyperglycemia existed in many patients. They had immunosuppressive state and anemia. Blood gas analysis showed that oxygen contents were low and carbon di- oxide contents were high in patients. These re- sults suggest a possibility that the internal en- vironment seen in patients is responsible to induce onset of disease and to maintain their cell growth, because cancer cells have an en- ergy system of predominant glycolysis. Al- though hypothermia, hypoxia and hyperglyce- mia are important to activate the glycolysis pathway and to escape from emergency, such responses suppress the mitochondrial pathway for long span and may result in carcinogenesis. Keywor ds: Cancer; Hypothermia; Hypoxia; Hyper glycemia; Glycolysis; Mitochondria 1. INTRODUCTION Many investigators and clinicians have felt that cancer might be a systemic disease although tumor masses are primarily present at local sites. If this is the case, we have to consider specific, common internal environment in cancer patients. In the course of the analysis of many parameters in cancer patients, we noticed that many cancer patients showed simultaneous hypothermia and hyperglycemia. In light of these findings, we then investigated the in- ternal environment in relation to stress-associated re- sponses [1,2 ]. Of interest was that both hypother mia and hyperglycemia were induced by stress. Such an internal environment might be beneficial for humans and animals to escape emergencies [3]. Namely, prompt output of force by white muscle fibers depends on the energy production system of glycolysis. Although the efficiency of energy production is low (2 ATP/glucose) in the gly- colysis pathway, the ATP synthesis in this system is much quicker (× 100) than that of the mitochondrial sys- tem (× 1) [4]. In a short span of time, hypothermia and hyperglyce- mia are therefore good conditions for escape from stress or emergencies. However, these conditions are not ap- propriate for energy production of the mitochondrial pathway (i.e., oxidative phosphorylation). Many patients with hypothermia and hyperglycemia suffer from gen- eral fatigue, emaciated conditions, diabetic disease, etc. In the present study, we investigated the internal en- vironment in cancer patients in detail and herein propose on adaptation theory, namely, that the onset of cancer is a phenomenon of a glycolytic adaptation response by living beings to overcome deteriorated internal condi- tions in the body. Cumulative evidence has shown that  M. Watanabe et al. / HEALTH 2 (2010) 781-788 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 782 cancer cells have a shift of glucose metabolism from oxidative phosphorylation to glycolysis, eventually re- sulting in few or defective mitochondria in the cyto- plasm [5-7]. Although many investigators have consid- ered that carcinogens such as ultrav iolet rays, food addi- tives, air pollution, etc. [8-12], induce multiple mutation steps in proto-oncogenes, there is an alternative possib il- ity that such mutation is a process of glycolytic adapta- tion by living beings, namely, cancer cells. Hypothermia, hypoxia and hyperglycemia, which are induced by con- tinuous stress (due to the lifestyle in patients), might be important factors which induce the adaptive response. 2. MATERIALS AND METHODS 2.1. Subjects Patients with early or advanced cancer were first exam- ined as to body temperature (n = 28). They were 54.3 ± 8.0 years old. Age-matched healthy controls (n = 27), 45.8 ± 11.0 years of age, were also examined. For detailed analysis of many parameters other than body temperature, patients with advanced cancer (n = 13) were then selected (Ta bl e 1 ). Details of the cancer pa- tients are listed in the table, including sex and age (52.1 ± 8.7 years old). At the time of analysis, these patients were receiving neither chemotherapy nor irradiation therapy. Age-matched healthy controls (n = 11), 46.7 ± 10.0 of age, were also examined. Table 1. Patients with Advanced Cancer. Case Type of Cancer Sex Age 1 chronic myelogenic leukemia F 68 2 ovarian cancer F 56 3 brain cancer M 50 4 stomach cancer F 63 5 parotid gland cance F 48 6 rectum cancer, metastasis to lung M 58 7 uterus cervical cancer F 36 8 rectum cancer, metastasis to lung M 45 9 bladder cancer M 52 10 lung cancer M 48 11 breast cancer F 56 12 malignant lymphoma M 42 13 stomach cancer M 55 Informed consent was obtained from all subjects. 2.2. Parameters Tested Blood for the analysis was obtained from a vein. Blood glucose was measured by Precision Xtra TM (Abott Ja- pan Co., Ltd., Chiba, Japan). Venous blood analysis of lactate and of the levels of pH, O2 and CO2 was also performed using i-STAT 300F (i-STAT Corporation, NJ, USA). To analyze the hematological parameters, leukocyte counts of fresh venous blood were determined by hem- ocytemeter and were stained by the Giemsa method. The contents of hemoglobin (Hb) and others in the blood were measured by Sodium Lauryl Sulfate (SLS)-Hb methods and hematocytemeter, respectively. 2.3. Statistical Analysis The difference between the values was determined by Student’s t-test, Mann-Whitney’s U test and Welch’s t-test. 3. RESULTS 3.1. Hypothermia and Hyperglycemia Seen in Cancer Patients Twenty-eight patients with early or advanc ed cancer and twenty-seven healthy subjects were examined as to body temperature (Figure 1(a)). It was found that there were many persons with hypothermia (36.1 ± 0.5˚C) among cancer patients in comparison with healthy persons (36.6 ± 0.4˚C), the difference being statistically significant (p < 0.01). We then analyzed many parameters in patients with advanced cancer (n = 13) and age-matched controls (n = 11) (Figure 1(b)). Hypothermia was confirmed in these patients with advanced cancer (35.9 ± 0.5˚C) in com- parison with controls (36.5 ± 0.3˚C). In addition to hy- pothermia, hyperglycemia was also detected in cancer patients (125.3 ± 28.5 mg/dL) in comparison with con- trols (106.3 ± 1 1.1 mg/dL). Since stress-associated responses simultaneously in- duce hypothermia and hyperglycemia, other stress-as- sociated parameters were also examined in this experi- ment (Figure 1(b), bottom). Although there was a ten- dency that some patients with advanced cancer had a high pulse rate (sympathetic nerve activ ation) and a high level of lactate, these were not common to all p atients (p > 0.05 in both pulse and lactate). 3.2. Immunosuppressive States and Anemia in Cancer Patients Immunoparameters were examined in cancer patients and 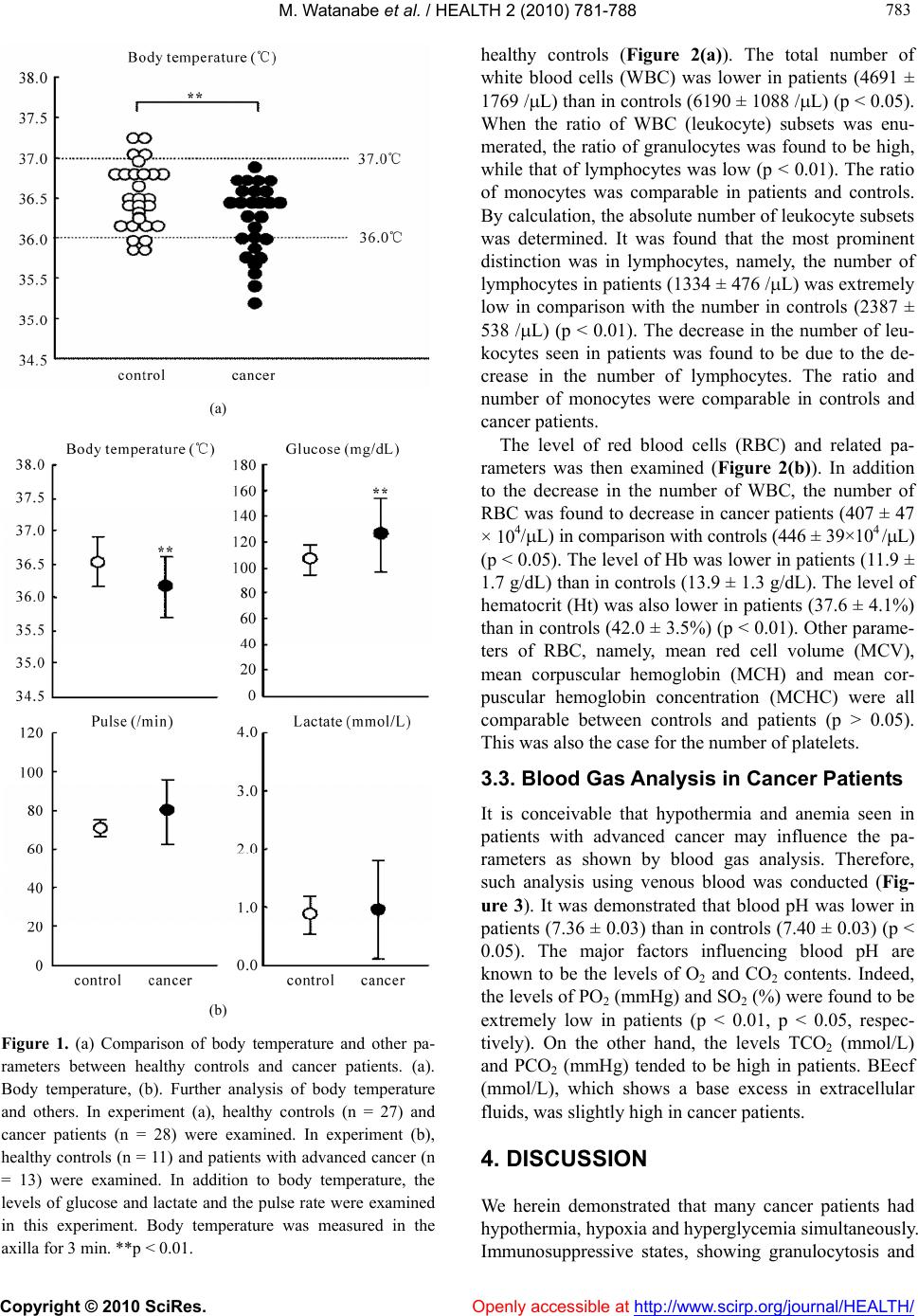 M. Watanabe et al. / HEALTH 2 (2010) 781-788 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 783 783 (a) (b) Figure 1. (a) Comparison of body temperature and other pa- rameters between healthy controls and cancer patients. (a). Body temperature, (b). Further analysis of body temperature and others. In experiment (a), healthy controls (n = 27) and cancer patients (n = 28) were examined. In experiment (b), healthy controls (n = 11) and patients with advanced cancer (n = 13) were examined. In addition to body temperature, the levels of glucose and lactate and the pulse rate were examined in this experiment. Body temperature was measured in the axilla for 3 min. **p < 0.01. healthy controls (Figure 2(a)). The total number of white blood cells (WBC) was lower in patients (4691 ± 1769 /L) than in controls (619 0 ± 1088 /L) (p < 0.05). When the ratio of WBC (leukocyte) subsets was enu- merated, the ratio of granulocytes was found to be high, while that of lymphocytes was low (p < 0.01). The ratio of monocytes was comparable in patients and controls. By calculation, the abso lute number of leukocyte subs ets was determined. It was found that the most prominent distinction was in lymphocytes, namely, the number of lymphocytes in patients (1334 ± 476 /L) was extremely low in comparison with the number in controls (2387 ± 538 /L) (p < 0.01). The decrease in the number of leu- kocytes seen in patients was found to be due to the de- crease in the number of lymphocytes. The ratio and number of monocytes were comparable in controls and cancer patients. The level of red blood cells (RBC) and related pa- rameters was then examined (Figure 2(b)). In addition to the decrease in the number of WBC, the number of RBC was found to decrease in cancer patients (407 ± 47 × 104/L) in comparison with controls (446 ± 39×104 /L) (p < 0.05). The level of Hb was lower in patients (11.9 ± 1.7 g/dL) than in controls (13.9 ± 1.3 g/dL). The level of hematocrit (Ht) was also lower in patients (37.6 ± 4.1%) than in controls (42.0 ± 3.5%) (p < 0.01). Other parame- ters of RBC, namely, mean red cell volume (MCV), mean corpuscular hemoglobin (MCH) and mean cor- puscular hemoglobin concentration (MCHC) were all comparable between controls and patients (p > 0.05). This was also the case for the number of platelets. 3.3. Blood Gas Analysis in Cancer Patients It is conceivable that hypothermia and anemia seen in patients with advanced cancer may influence the pa- rameters as shown by blood gas analysis. Therefore, such analysis using venous blood was conducted (Fig- ure 3). It was demonstrated that blood pH was lower in patients (7.36 ± 0.03 ) than in controls (7.40 ± 0.03) (p < 0.05). The major factors influencing blood pH are known to be the levels of O2 and CO2 contents. Indeed, the levels of PO2 (mmHg) and SO2 (%) were found to be extremely low in patients (p < 0.01, p < 0.05, respec- tively). On the other hand, the levels TCO2 (mmol/L) and PCO2 (mmHg) tended to be high in patients. BEecf (mmol/L), which shows a base excess in extracellular fluids, was slightly high in cancer patients. 4. DISCUSSION We herein demonstrated that many cancer patients had hypothermia, hypoxia and hyperglycemia simultaneously. Immunosuppressive states, showing granulocytosis and 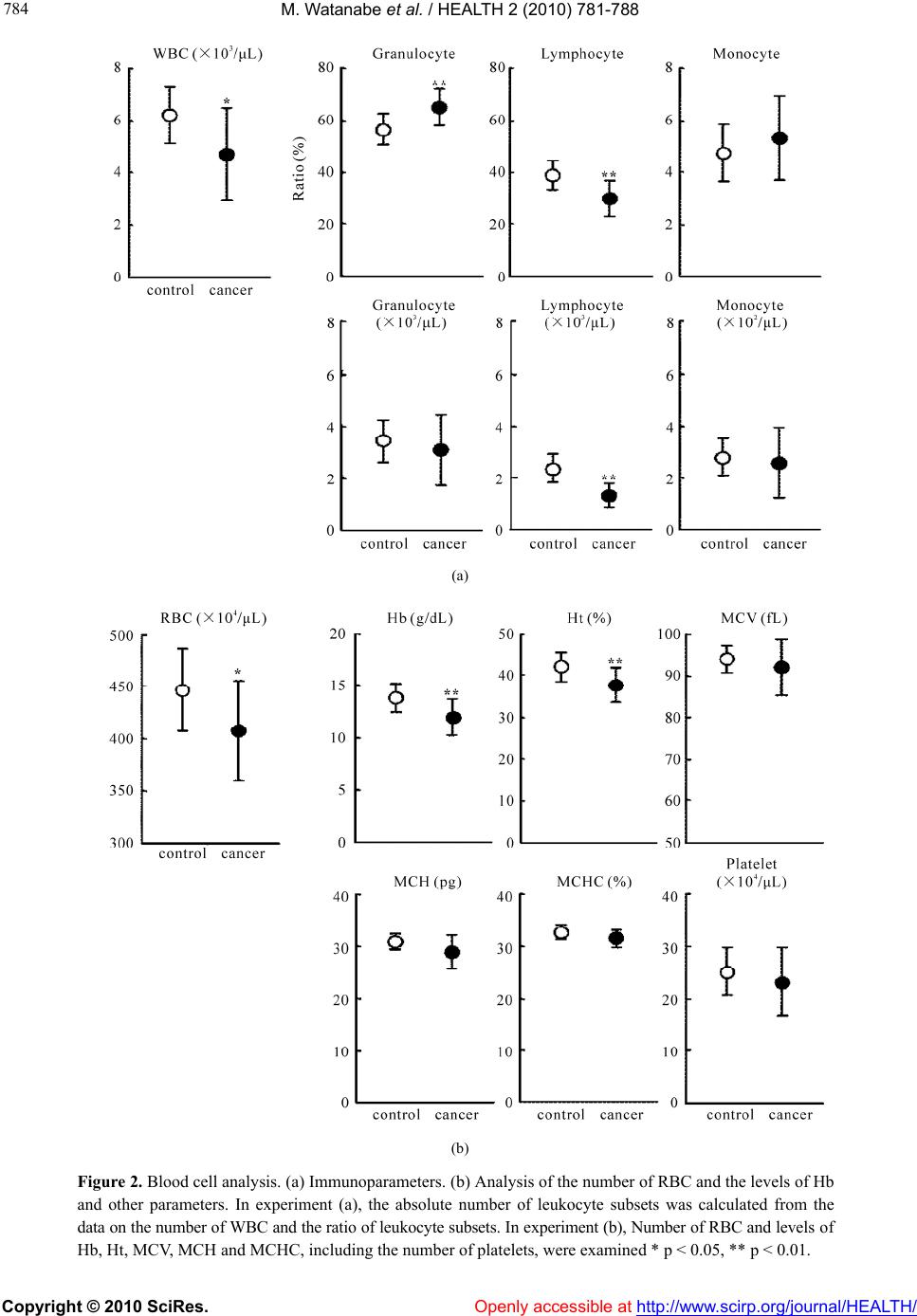 M. Watanabe et al. / HEALTH 2 (2010) 781-788 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 784 (a) (b) Figure 2. Blood cell analysis. (a) Immunoparameters. (b) Analysis of the number of RBC and the levels of Hb and other parameters. In experiment (a), the absolute number of leukocyte subsets was calculated from the data on the number of WBC and the ratio of leukocy te subsets. In experi ment (b), Number of RBC a nd leve l s o f b, Ht, MCV, MCH and MCHC, including the number of platelets, were examined * p < 0.05, ** p < 0.01. H Openly accessible at 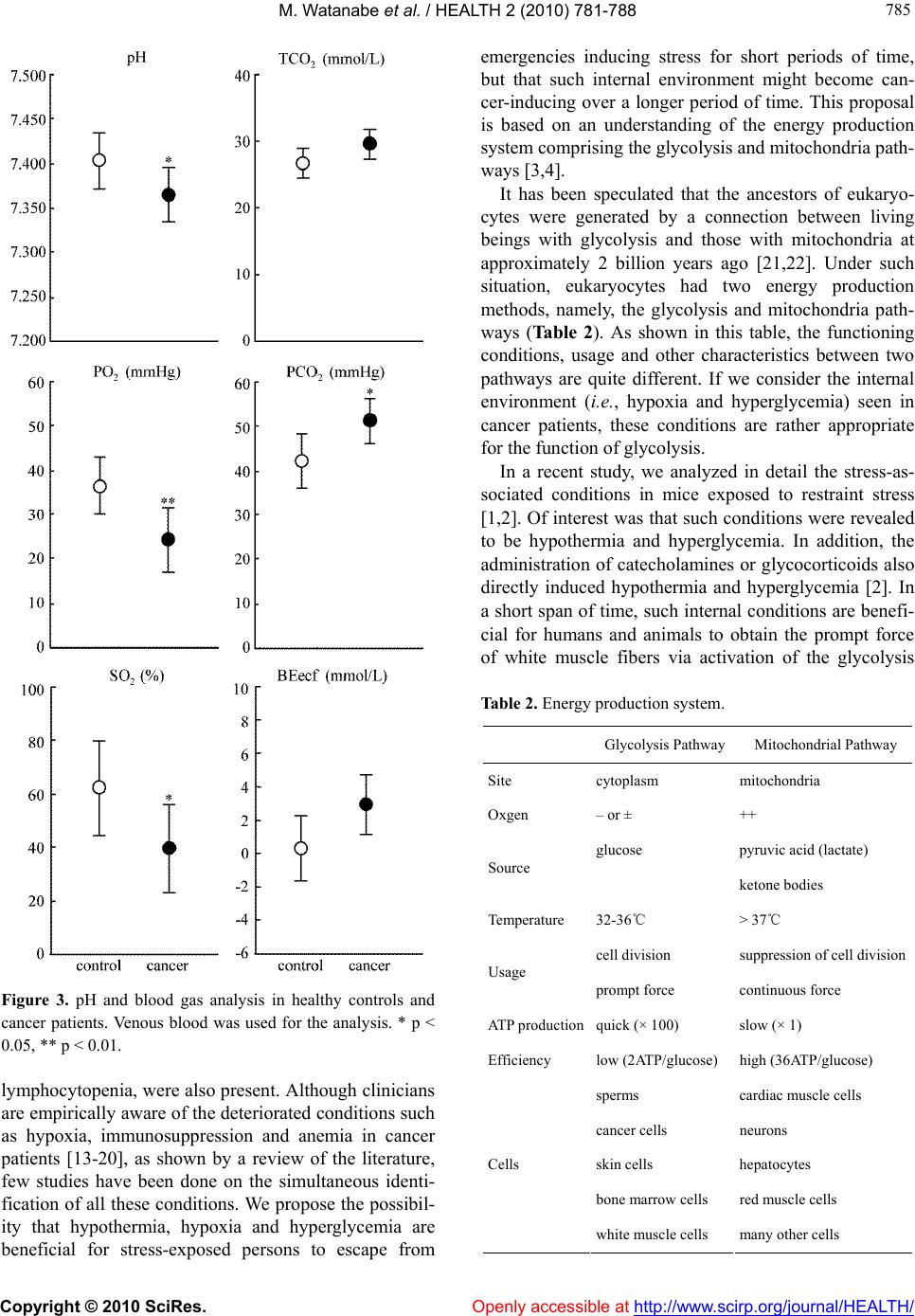 M. Watanabe et al. / HEALTH 2 (2010) 781-788 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 785 785 Figure 3. pH and blood gas analysis in healthy controls and cancer patients. Venous blood was used for the analysis. * p < 0.05, ** p < 0.01. lymphocytopenia, were also present. Although clinicians are empirically aware of the deteriorated conditions such as hypoxia, immunosuppression and anemia in cancer patients [13-20], as shown by a review of the literature, few studies have been done on the simultaneous identi- fication of all these conditions. We propose the possibil- ity that hypothermia, hypoxia and hyperglycemia are beneficial for stress-exposed persons to escape from emergencies inducing stress for short periods of time, but that such internal environment might become can- cer-inducing over a longer period of time. This proposal is based on an understanding of the energy production system comprising the glycolysis and mitochondria path- ways [3,4]. It has been speculated that the ancestors of eukaryo- cytes were generated by a connection between living beings with glycolysis and those with mitochondria at approximately 2 billion years ago [21,22]. Under such situation, eukaryocytes had two energy production methods, namely, the glycolysis and mitochondria path- ways (Ta b l e 2 ). As shown in this table, the functioning conditions, usage and other characteristics between two pathways are quite different. If we consider the internal environment (i.e., hypoxia and hyperglycemia) seen in cancer patients, these conditions are rather appropriate for the function of glycolysis. In a recent study, we analyzed in detail the stress-as- sociated conditions in mice exposed to restraint stress [1,2]. Of interest was that such conditio ns were revealed to be hypothermia and hyperglycemia. In addition, the administration of catecholamines or glycocorticoids also directly induced hypothermia and hyperglycemia [2]. In a short span of time, such internal conditions are benefi- cial for humans and animals to obtain the prompt force of white muscle fibers via activation of the glycolysis Table 2. Energ y prod uctio n system. Glycolysis Pathway Mitochondrial Pathway Site cytoplasm mitochondria Oxgen – or ± ++ glucose pyruvic acid (lactate) Source ketone bodies Temperature 32-36℃ > 37℃ cell division suppression of cell division Usage prompt force continuous force ATP productionquick (× 100) slow (× 1) Efficiency low (2ATP/gl ucos e) high (36ATP/glucose) sperms cardiac muscle cells cancer cells neurons skin cells hepatocytes bone marrow cells red muscle cells Cells white muscle cells many other ce lls  M. Watanabe et al. / HEALTH 2 (2010) 781-788 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 786 pathway [3]. As a result, such conditions realize the power needed to escape from stressful conditions such as those in emergencies. In other words, the internal condi- tions of hypothermia and hyperglycemia do not seem to be a failure of our body r es po nses. If a certain person is exposed to stress for a long time, the internal conditions of hypothermia and hyperglyce- mia then suppress the mitochondria pathway which pro- duces continuous force and energy for protein synthesis. Such a person might be suffering from general fatigue, emaciated conditions, diabetic disease and other difficul- ties. This notion seems to be important for understanding the mechanisms involved in the onset of many diseases. In addition, we propose another possibility of hypo- thermia, hypoxia and hyperglycemia which may be as- sociated with the onset of malignancy. As shown in Ta- ble 3, many investigators and clinicians have believed that carcinogens are key factors which induce cancer via the multiple mutation steps of proto-oncogenes [23-27]. However, such carcinogens do not seem to be always present in actual cases. Given this fact, such cases may be rather rare. We propose herein the possibility th at the internal environment (i.e., hypoxia and hyperglycemia) induced by continuous stress results in an adaptation response in which normally dividing cells become can- cer cells (i.e., living beings with glycolysis). In such cases, stress may be caused by overwork, mental stress, obesity, etc., namely, their lifestyle. We consider that these cases are of higher frequency than those due to carcinogens in carcinogenesis. Immunosuppression and anemia in cancer patients as revealed in the present study might result from such stress via the activation of sympathetic nerves [28]. Interaction of leukocyte sub sets with catecholamines or glucocorticoids (hypothalamic- pituitary-adrenal axis) was reported previously [29]. Table 3. Transformation to Cancer Cells. Direct Cause Secondary Response Frequency ultraviolet food additives radiation air pollution Carcinogens other carcinogens mutation of proto- oncogenes or other genes by carcinogens Less hypothermia hypoxia Stress from lifestyle hyperglycemia mutation of proto- oncogenes or other genes as adaptation to “living beings of glycolysis” High O. Warlburg has reported that cancer cells contained a few mitochondria in the cytoplasma and produce energy mainly by the g lycolysis p athway [7]. Recent cu mulativ e evidence also supports this earlier observation [30-35]. The functions of oncog enes are eventually related to not only the system of cell-proliferation but also to the sys- tem of energy production. The nature of the predominant function of glycolysis in cancer cells is utilized by PET scans [36,37]. The energy produced by glycolysis is used not only to obtain prompt elicitation but also for cell dividing energy (see Table 2 again). In other words, the initial stress-associated response of hypothermia, hy- poxia and hyperglycemia is estimated as allostasis (i.e., change of the internal environment to overcome stress). However, continuous stress then turns to allostatic load and induces the carcinogenesis as adaptation responses (i.e., break of “homeostasis” in our body). These con- cepts on “allostasis” were proposed by B. S. McEwen, F. S. Dhabhar and their colleagues [38-41]. Stressful life events were reported to be related to such allostatic load [42,43]. In our recent study using hyperthermia equipment [44, 45], many cancer patients could live in good conditions without further tumor enlargement when they exposed to mild hyperthermia (i.e., the maximum rectum tempera- ture is 38.0ºC for 15-30 min). In some cases, tumor re- gression resulted from mild hyperthermia [45]. At this time, the values of pH, PO2, PCO2 and other factors im- proved. Immunosuppression and anemia seen in cancer patients were also alleviated. These results suggest that a slight shift of glucose metabolism from the glycolysis pathway to the mitochondria pathway (i.e., oxidative phosphorylation) might be important to cure malignancy. Local, strong hyperthermia (e.g., 42ºC) was not effective and rather acted as severe stress in cancer patients. In other words, systemic improvement of the internal envi- ronment is critical to cure malignancies. However, we do not recommend patients to use expensive equipment for hyperthermia. The most important things for spontane- ous regression of cancer are as follow: changing harmful lifestyle (e.g., overwork), using hot-water bottle at sleeping time, taking a deep breath several times a day, dietary consideration and control of the fear. We have herein proposed the possibility that stress- associated conditions are beneficial for humans to es- cape from emergencies in a short span of time, but that the resulting hypothermia and hyperglycemia act as fac- tors which induce the generation of cancer cells. In other words, the onset of malignancy might be a return to “living beings with glycolysis at 2 billion years ago”. Cancer cells eventually contain only a few mitochondria in the cytoplasm. However, we could not neglect a mi- tochondrial function in tumor cell growth [46,47].  M. Watanabe et al. / HEALTH 2 (2010) 781-788 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 787 787 A hypothesis by J. S. Fang, R. J. Gillies and R. A. Gaten was proposed that evolution of carcinogenesis is an adaptation response to hypoxia and acidosis, showing the interaction of cancer cells and microenvironments in the surrounding tissu es [48,49]. At that time, a paracrine signaling between epithelial and stromal cells is known to be important for tumor initiatio n and prog ression [50]. We were also able to reveal the systemic, internal envi- ronment of hypoxia, acidosis and other conditions in actual cancer patients. However, a further research is required to support our proposal definitely. Such re- search includes an animal experiment using mice with cancer and a cancer cell culture experiment under condi- tions of hypothermia, hypoxia and hyperglycemia 5. ACKNOWLEDGEMENTS This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan. The au- thors thank Mrs Yuko Kaneko for preparation of the manuscript and Mr Taiki Hashimoto and Ms Kaori Yamamoto (Yushima-Shimizuzaka clinic) for arrangement of the clinical research. REFERENCES [1] Watanabe, M., Tomiyama-Miyaji, C., Kainuma, E., et al. (2008) Role of-adrenergic stimulus in stress-induced modulation of body temperature, blood glucose and in- nate immunity. Immunology Letters, 115(1), 43-49. [2] Kainuma, E., Watanabe, M., Tomiyama-Miyaji, C., et al. (2009) Association of glucocorticoid with stress-induced modulation of body temperature, blood glucose and in- nate immunity. Psychoneuroendocrinology, 34(10), 1459- 1468. [3] Kainuma, E., Watanabe, M., Tomiyama-Miyaji, C., et al. (2009) Proposal of alternative mechanism responsible for the function of high-speed swimsuits. Biomedical Re- search, 30(1), 69-70. [4] Voet, D. and Voet, J.G. (2004) Glycolysis. In: D. Voet, J.G. Vo e t , Ed s . , Biochemistry, 3rd Edition, J. Wiley & Sons, New Yo rk, 607-647. [5] Dang, C.V. and Semenza, G.L. (1999) Oncogenic altera- tions of metabolism. Trends in Biochemical Science, 24(2), 68-72. [6] Shaw, R.J. (2006) Glucose metabolism and cancer. Cur- rent Opinion in Cell Biology, 18(6), 598-608. [7] Warburg, O. (1956) On the origin of cancer cells. Science, 123(3191), 309-314. [8] Nijjar, T., Bassett, E., Garbe, J., et al. (2005) Accumula- tion and altered localization of telomere-associated pro- tein TRF2 in immortally transformed and tumor-derived human breast cells. Oncogene, 24(20), 3369-3376. [9] Rhiemeier, V., Breitenbach, U., Richter, K.H., et al. (2006) A novel aspartic proteinase-like gene expressed in stratified epithelia and squamous cell carcinoma of the skin. American Journal of Pathology, 168(4), 1354-1364. [10] Arora, A., Kalra, N., Shukla, Y. (2006) Regulation of p21/ras protein expression by diallyl sulfide in DMBA induced neoplastic changes in mouse skin. Cancer Let- ters, 242(1), 28-36. [11] Ikuta, S., Edamatsu, H., Li, M., Hu, L. and Kataoka, T. (2008) Crucial role of phospholipase C epsilon in skin inflammation induced by tumor-promoting phorbol ester. Cancer Research, 68(1), 64-72. [12] Rountree, C.B., Senadheera, S., Mato, J.M., Crooks, G.M. and Lu, S.C. (2008) Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-de- ficient mice. Hepatology, 47(4), 1288-1297. [13] Koksal, Y., Caliskan, U. and Unal, E. (2009) Hypother- mia in a child with Hodgkin disease. Journal of Pediatric Hematology Oncology, 31(2), 136-138. [14] Nduka, C.C., Puttick, M., Coates, P., Yong, L., Peck, D. and Darzi, A. (2002) Intraperitoneal hypothermia during surgery enhances postoperative tumor growth. Surgical Endoscopy, 16(4), 611-615. [15] Cortesi, E., Gascón, P., Henry, D., et al. (2005) Standard of care for cancer-related anemia: Improving hemoglobin levels and quality of life. Oncology, 68(Suppl 1), 22-32. [16] Heras, P. , Argyriou, A.A., Papapetropoulos, S., Karagian- nis, S., Argyriou, K. and Mitsibounas, D. (2005) The impact of weekly dosing of epoetin alfa on the haemato- logical parameters and on the quality of life of anemic cancer patients. European Journal of Cancer Care, 14(2), 108-112. [17] Mock, V. and Olsen, M. (2003) Current management of fatigue and anemia in patients with cancer. Seminars in Oncology Nursing, 19(4 Suppl 2), 36-41. [18] Fairclough, D.L., Gagnon, D.D., Zagari, M.J., Marschner, N. and Dicato, M. (2003) Evaluation of quality of life in a clinical trial with nonrandom dropout: The effect of epoetin alfa in anemic cancer patients. Quality of Life Research, 12(8), 1013-1027. [19] Tchekmedyian, N.S. (2002) Anemia in cancer patients: significance, epidemiology, and current therapy. Oncol- ogy, 16(9 Suppl 10), 17-24. [20] Ishiko, O., Sugawa, T., Tatsuta, I., et al. (1987) Ane- mia-inducing substance (AIS) in advanced cancer: in- hibitory effect of AIS on the function of erythrocytes and immunocompetent cells. Japanese Journal of Cancer Research, 78(6), 596-606. [21] Sagan, L. (1967) On the origin of mitosing cells. Journal of Theoretical Biology, 14(3), 255-274. [22] Margulis, L. and Stolz, J.F. (1984) Cell symbiosis [cor- rection of symbiosis] theory: Status and implications for the fossil record. Advances in Space Research, 4(12), 195-201. [23] Osinsky, S., Zavelevich, M. and Vaupel, P. (2009) Tumor hypoxia and malignant progression. Experimental Oncol- ogy, 31(2), 80-86. [24] Denko, N.C. (2008) Hypoxia, HIF1 and glucose metabo- lism in the solid tumour. Nature Reviews Cancer, 8(9), 705-713. [25] Susnow, N., Zeng, L., Margineantu, D. and Hockenbery, D.M. (2009) Bcl-2 family proteins as regulators of oxi- dative stress. Seminars in Cancer Biology, 19(1), 42-49. [26] Moreno-Sánchez, R., Rodríguez-Enríquez, S., Saavedra, E., Marín-Hernández, A. and Gallardo-Pérez, J.C. (2009) The bioenergetics of cancer: Is glycolysis the main ATP supplier in all tumor cells? Biofacto rs, 35(2), 209-225.  M. Watanabe et al. / HEALTH 2 (2010) 781-788 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 788 [27] Vousden, K.H. and Ryan, K.M. (2009) P53 and metabo- lism. Nature Reviews Cancer, 9(10), 691-700. [28] Abo, T. and Kawamura, T. (2002) Immunomodulation by the autonomic nervous system: Therapeutic approach for cancer, collagen diseases, and inflammatory bowel dis- eases. Thera peutic A pheresis, 6(5), 348-357. [29] Sagiyama, K., Tsuchida, M., Kawamura, H., et al. (2004) Age-related bias in function of natural killer T cells and granulocytes after stress: Reciprocal association of ster- oid hormones and sympathetic nerves. Clinical and Ex- perimental Immunology, 135(1), 56-63. [30] Weinberg, R.A. (2007) The Biology of Cancer. 1st Edi- tion, Garland Science, New York. [31] Kondoh, H. (2008) Cellular life span and the Warburg effect. Experimental Cell Research, 314(9), 1923-1928. [32] Nijsten, M.W.N. and van Dam, G.M. (2009) Hypothesis: Using the Warburg effect against cancer by reducing glucose and providing lactate. Medical Hypotheses, 73(1), 48-51. [33] Heiden, M.G.V., Cantley, L.C. and Thompson, C.B. (2009) Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science, 324(5930), 1029-1033. [34] Máximo, V., Lima, J., Soares, P. and Sobrinho-Simões, M. (2009) Mitochondria and cancer. Virchows Arch, 454(5), 481-495. [35] Lee, H.-C. and Wei, Y.-H. (2009) Mitochondrial DNA instability and metabolic shift in human cancers. Interna- tional Journal of Molecular Sciences, 10(2), 674-701. [36] Gambhir, S.S. (2002) Molecular imaging of cancer with positron emission tomography. Nature Reviews Cancer, 2(9), 683-693. [37] Vesselle, H., Schmidt, R.A., Pugsley, J.M., et al. (2000) Lung cancer proliferation correlates with [F-18] fluoro- deoxyglucose uptake by positron emission tomography. Clinical Cancer Research, 6(10), 3837-3844. [38] McEwen, B.S. (2000) Allostasis and allostatic load: Im- plications for neuropsychopharmacology. Neuropsycho- pharmacology, 22(2), 108-124. [39] McEwen, B.S. (2004) Protection and damage from acute and chronic stress, allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences, 1032(1), 1-7. [40] Sephton, S.E., Dhabhar, F.S., Keuroghlian, A.S., et al. (2009) Depression, cortisol, and suppressd cell-mediated immunity in metastatic breast cancer. Brain Behavior and Immunity, 23(8), 1148-1155. [41] Du, J., Wang, Y., Hunter, R., et al. (2009) Dynamic regu- lation of mitochondrial function by glucocorticoids. Pro- ceedings of the National Academy of Sciences of the United States of America, 106(9), 3543-3548. [42] Bellingrath, S., Weigl, T. and Kudielka, B.M. (2009) Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers. St res s, 12(1), 37-48. [43] Alexander, J.L., Dennerstein, L., Woods, N.F., et al. (2007) Role of stressful life events and menopausal stage in wellbeing and health. Expert Review of Neurothera- peutics, 7(Suppl 11), 93-113. [44] Tomiyama-Miyaji, C., Watanabe, M., Ohishi, T., et al. (2007) Modulation of the endocrine and immune systems by well-controlled hyperthermia equipment. Biomedical Research, 28(3), 119-125. [45] Ohishi, T., Nukuzuma, C., Seki, A., et al. (2009) Alkali- zation of blood pH is responsible for survival of cancer patients by mild hyperthermia. Biomedical Research, 30(2), 95-100. [46] Deberardinis, R.J., Sayed, N., Ditsworth, D. and Thomp- son, C.B. (2008) Brick by brick: Metabolism and tumor cell growth. Current Opinion in Genetics and Develop- ment, 18(1), 54-61. [47] Frezza, C. and Gottlieb, E. (2009) Mitochondria in can- cer: not just innocent bystanders. Seminars in Cancer Bi- ology, 19(1), 4-11. [48] Gillies, R.J. and Gatenby, R.A. (2007) Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer and Metastasis Reviews, 26(2), 311-317. [49] Fang, J.S., Gillies, R.D. and Gatenby, R.A. (2008) Adap- tation to hypoxia and acidosis in carcinogenesis and tu- mor progression. Seminars in Cancer Biology, 18(5), 330-337. [50] Hu, M. and Polyak, K. (2008) Microenvironmental regu- lation of cancer development. Current Opinion in Genet- ics and Development, 18(1), 27-34.
|