Paper Menu >>
Journal Menu >>
 Vol.2, No.7, 753-758 (2010) doi:10.4236/health.2010.27114 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Neuronavigation and epilepsy surgery Martin B. Glaser1*, Konrad J. Werhahn2, Peter Grunert1, Clemens Sommer3, Wibke Müller-Forell4, Joachim Oertel1 1Department of Neurosurgery, Unive rsity Hospital Medical Cente r, Johannes Gutenberg University, Mainz, Germany; *Corresponding Aut hor: glaserm@uni-mainz.de 2Department of N e urology, University Hospital Medical Center, Johannes Gutenberg University, Mainz, Germany 3Department of Neuro p a th o l o gy, University Hospital Medical Center, Johannes Gutenberg University, Mainz, Germany 4Institution of Neuroradiology, University Hospital Medical Center, Johannes Gutenberg University, Mainz, Germany Received 16 February 2010; revised 11 March 2010; accepted 12 March 2010. ABSTRACT Resective epilepsy surgery is an elective ther- apy indicated in focal epilepsy patients who are resistant to pharmacotherapy. Every effort sho- uld be undertaken to perform the procedures as safe and less traumatic as possible. Neurona- vigation could represent a suitable tool to re- duce surgical morbidity and increase surgical radicality. Here, we present a series of 41 pa- tients who were operated on for medically in- tractable epilepsy using neuronavigation. Over- all, complication rate was 17% with a favourable seizure outcome of 88% (Engel’s class I/II). Our data suggest that neuronavigation is a valuable surgical technique to accomplish a favourable outcome in epilepsy surgery. Keywords: Neuronavigation; Epilepsy Surgery; Outcome 1. INTRODUCTION Epilepsy is a frequent condition. Approximately 40 mil- lion people are affected worldwide and the prevalence of epilepsy has been estimated to be around 0.7% [1]. The mean annual incidence of first unprovoked seizures in population-based studies is 56.8 per 100 000 person- years, 23.5 per 100 000 person-years for single unpro- voked seizures, and 33.3 per 100 000 person-years for epilepsy (recurrent unprovoked seizures). Partial sei- zures occur in 40-60%, two-thirds of which are temporal lobe epilepsies [2,3]. Clinically, focal epilepsy may first be suspected with a first witnessed report of a general- ized tonic-clonic seizure but often seizures may be more subtle consisting of a transient short lasting loss of con- sciousness with or without oral or manual automatisms or focal tonic or clonic movements affecting parts of the body. Seizures may lead to developmental retardation, social impairment (e.g. limited choice of profession, ability to obtain a driving licence) and even sudden un- expected death in epilepsy [4]. In most cases, conserva- tive treatment with antiepileptic drugs is successful in preventing clinical seizures, but up to 33% of patients will prove to be resistant to medical treatment [5]. Patients with focal epilepsy are generally surgical candidates, if medical treatment with at least two differ- ent anticonvulsive drugs in sufficient doses fails and disabling seizures persist. Bad prognostic factors for medical treatment in focal epilepsy are a structural lesion on Magnetic Resonance Imaging (MRI), particularly with dual pathology, post-stroke scars and vascular mal- formations having the best and cortical dysgenesis and hippocampal sclerosis the poorest outcome [3]. Optimal surgical results are obtained in patients with a circum- scribed seizure onset (especially temporal/temporome- sial) in video-EEG recordings, concordant focal pathol- ogy on MRI (e.g. hippocampal sclerosis) and concordant neuropsychological findings [6,7]. The need for a device enabling precise introduction of instruments into deep intracerebral structures was first addressed by Zernov et al. [8] 1890. He constructed a frame which was fixed on the skull by screws. The posi- tion of deep structures were measured from external anatomical landmarks. Clark developed 1908 a frame which served as a stable coordinate system for calcula- tion of intracranial targets in relation to the frame [9,10]. In the second half of the last century these frames were refined. More and more indications were found along with the progress of the imaging modalities (x-ray, an- giogram, computed tomography, MRI). Frame based stereotactic systems are still the most accurate naviga- tional tools and very small targets like the subthalamic nucleus can be implanted with depth electrodes for the treatment of parkinsonism.  M. B. Glaser et al. / HEALTH 2 (2010) 753-758 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 754 One major disadvantage of the stereotactic frame is the restricted surgical field as long as the arc is in place. At the end of the 1980s the “frameless” navigation was developed, with first clinical applications in neurosur- gery at the beginning of th e 1990s [11]. Nowadays, frameless neuronavigation is an accepted tool in contemporary microneurosurgery [12-15]. Its application contributes to make surgical approaches smaller and less invasive [16]. Consequently neuronavi- gation was integrated also in epilepsy surgery [17]. The neuronavigation is basically a miniature of a GPS (general positioning system). The neuronavigation sys- tems are able to determine the position of the tip of a pointer in 3-D-space and to transfer the position into the appropriate CT or MRI data set in real time during the entire operation (in case of a microscope the focus cor- responds to the tip of the pointer). From the technical point of view we can distinguish between armbased and armless navigation. The latter have the advantage not to restrict the operative field. Different armless systems were realized using sonic, infrared, magnetic waves or visible light (see Figure 1). The transfer of the pointer tip in the appropriate images makes a registration before application necessary. Per point registration and surface registration were developed for this purpose. The navi- gation devices have higher flexibility but less accuracy in comparison to the frame based systems. Regarding navigation accuracy we have clearly to distinguish be- tween technical accuracy of the navigation system (how accurately the system determines the position in the 3-D- space), registration accuracy (how accurately is the data transfer from 3-D-space into the CT and MRI image space) and application accuracy depending of the intra- operative situation including brain shift [18]. Figure 1. Drawing of an armless neuronavigation system setup. For this study, we reviewed our surgical cases that were performed for pharmacoresistent focal epilepsy using a neuronavigation device. 2. MATERIALS AND METHODS In our retrospective study, we gathered the clinical data of all patients who had navigation assisted surgery for medically intractable epilepsy. We evaluated the charts of 41 patients who were treated in our institution from 09.2003 to 08.2009 and reviewed the postoperative clinical follow up as well as neuro-imaging data for the degree of resection and complications. Initially we used the Optical Tracking System (OTS®, Radionics, Burlington, Massachusetts, USA). In 31 cases, we navigated with the BrainLAB® System (BrainLAB, Heimstetten, Germany) and in a further 9 cases with the SonoWand® (Mison, Trondheim, Norway). In frameless Neuronavigation, after general anaesthe- sia has been induced and immediately before surgery the patient’s head is fixed in a three point fixation device and then referenced to the presurgical MRI (or other imaging modality such as computed tomography) by indicating to at least 4 defined landmarks so that the navigation system may locate the patient´s head in the three dimensional space. Hereafter the patient´s individ- ual anatomy is shown on a monitor according to the re- gion where a pointer is held on. The surgeon sees exactly where the targeted lesion is in relation to the skull sur- face to place the craniotomy on the ideal site. Moreover, he may check the position of his instrument any time during surgery. For selective amygdala-hippocampectomies, we used a supraorbital craniotomy via a subfrontal approach [19]. Temporal pole resections with amygdala-hippocampec- tomies were approached via a small anterior temporal craniotomy (diameter approx. 2.5 cm). For extratempo- ral lesionectomies neuronavigation was also employed to gain direct access with craniotomies as small as possible. “Keyhole” approaches were applied when possible, es- pecially in deeper seated lesions. 3. PATIENTS This series includes 41 consecutive patients with phar- macoresistent focal epilepsy with a mean age of 36 years (15-70 years). There were 17 male and 24 female indi- viduals. The mean duration of the epilepsy was 15.8 years. Most patients suffered from mesial temporal lobe epilepsy (n = 28, 17 left/11 right). All of them had been transferred from the department of neurology of the University Medical Center, Mainz, after video-EEG- monitoring for identification of the seizure onset region, 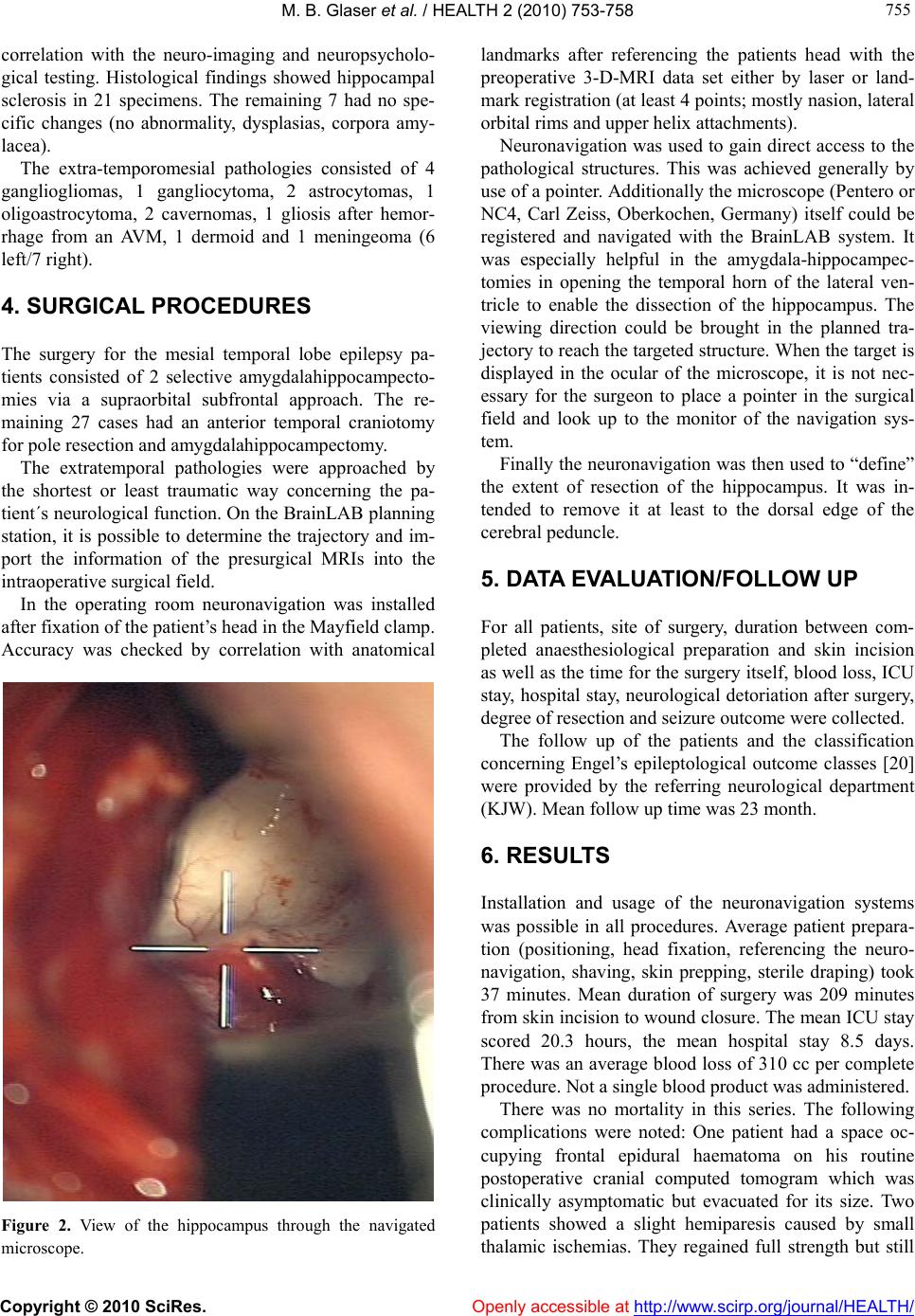 M. B. Glaser et al. / HEALTH 2 (2010) 753-758 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 755 755 correlation with the neuro-imaging and neuropsycholo- gical testing. Histological findings showed hippocampal sclerosis in 21 specimens. The remaining 7 had no spe- cific changes (no abnormality, dysplasias, corpora amy- lacea). The extra-temporomesial pathologies consisted of 4 gangliogliomas, 1 gangliocytoma, 2 astrocytomas, 1 oligoastrocytoma, 2 cavernomas, 1 gliosis after hemor- rhage from an AVM, 1 dermoid and 1 meningeoma (6 left/7 right). 4. SURGICAL PROCEDURES The surgery for the mesial temporal lobe epilepsy pa- tients consisted of 2 selective amygdalahippocampecto- mies via a supraorbital subfrontal approach. The re- maining 27 cases had an anterior temporal craniotomy for pole resection and amygda l ah i pp ocampectomy. The extratemporal pathologies were approached by the shortest or least traumatic way concerning the pa- tient´s neurological function. On the BrainLAB planning station, it is possible to determine the trajectory and im- port the information of the presurgical MRIs into the intraoperative surgical field. In the operating room neuronavigation was installed after fixation of the patient’s head in the Mayfield clamp. Accuracy was checked by correlation with anatomical Figure 2. View of the hippocampus through the navigated microscope. landmarks after referencing the patients head with the preoperative 3-D-MRI data set either by laser or land- mark registration (at least 4 points; mostly nasion, lateral orbital rims and upper helix attachments). Neuronavigation was used to gain direct access to the pathological structures. This was achieved generally by use of a pointer. Additionally the microscope (Pentero or NC4, Carl Zeiss, Oberkochen, Germany) itself could be registered and navigated with the BrainLAB system. It was especially helpful in the amygdala-hippocampec- tomies in opening the temporal horn of the lateral ven- tricle to enable the dissection of the hippocampus. The viewing direction could be brought in the planned tra- jectory to reach the targeted structure. When the target is displayed in the ocular of the microscope, it is not nec- essary for the surgeon to place a pointer in the surgical field and look up to the monitor of the navigation sys- tem. Finally the neuronav igation was then used to “define” the extent of resection of the hippocampus. It was in- tended to remove it at least to the dorsal edge of the cerebral peduncle. 5. DATA EVALUATION/FOLLOW UP For all patients, site of surgery, duration between com- pleted anaesthesiological preparation and skin incision as well as the time for the surgery itself, blood loss, ICU stay, hospital stay, neurological detoriation after surgery, degree of resection and seizure outcome were collected. The follow up of the patients and the classification concerning Engel’s epileptological outcome classes [20] were provided by the referring neurological department (KJW). Mean follow up time was 23 month. 6. RESULTS Installation and usage of the neuronavigation systems was possible in all procedures. Average patient prepara- tion (positioning, head fixation, referencing the neuro- navigation, shaving, skin prepping, sterile draping) took 37 minutes. Mean duration of surgery was 209 minutes from skin incision to wound closure. The mean ICU stay scored 20.3 hours, the mean hospital stay 8.5 days. There was an average blood loss of 310 cc per co mplete procedure. Not a single bl ood pr o duct was ad ministered. There was no mortality in this series. The following complications were noted: One patient had a space oc- cupying frontal epidural haematoma on his routine postoperative cranial computed tomogram which was clinically asymptomatic but evacuated for its size. Two patients showed a slight hemiparesis caused by small thalamic ischemias. They regained full strength but still  M. B. Glaser et al. / HEALTH 2 (2010) 753-758 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 756 have a deficit in fine motor skills. A further two patients had incomplete oculomotor palsies which resolved without sequelae. One patient developed a severe gener- alized vasospasm 10 days after subtotal frontal lobec- tomy. He has no focal neurological deficit but a relevant lack of motivation. One rhinoliquorrhoea occurred after a supraorbital approach via the opened frontal sinus. The liquorrhoea ceased after temporary lumbar drainage. Postsurgical imaging showed complete removal of the extratemporal pathologies in 9 of the 13 cases. The de- gree of hippocampal resection was noted in relation to the brain stem: a relatively short resection of the hippo- campus only to the middle of the cerebral peduncle was performed in 5 cases, to the dorsal margin of the cerebral peduncle in 20 cases and in a further 3 cases beyond. The neuronavigation was sufficiently exact in all cases at the beginning of the procedure. Accuracy was as reliable with laser patient registration as with registration via anatomical landmarks. The calculated mean devia- tion was 1.7 mm. It was possible to reach all lesions/ structures that were aimed for. It was extremely helpful in localization of the temporal horn in amygdala-hippo- campectomies. Neuronavigation overestimated the de- gree of resection of the hippocampus, possibly due to brain shift after CSF loss-especially after opening of the lateral ventricle. Postoperative seizure outcome was favourable after amygdala-hippocampectomy with 21 patients Engel’s class I and 6 patients Engel’s class II. One patient was seizure free for 3.5 years and developed pharmacoresis- tent temporal lobe epilepsy again so that re-resection is being considered. In the patients group with the extratemporal resections, 11 patients became seizure free (Engel’s class I). Two patients did not profit at all and have still the same sei- zure frequency in comparison to the presurgical state (partial tumor resections). In total, antiepileptic drugs were discon tinued in 8 pa- tients and reduced in 5. The majority of 29 patients is still under medication, similar to presurgical status. 7. DISCUSSION For decades, atraumatic surgery for medically refractory epilepsy has been the objective in order to improve pa- tients functions and at the same time effectively reduce seizures. Neuronavigation contributes to that aim by minimizing the craniotomies and reach the target in the planned trajectory [13]. On the other hand, there are only few publications concerning neuronavigation and resective epilepsy sur- gery [17,21-25]. Previous reports on neuronavigation in epilepsy sur- gery were published without discussing its advantages and pitfalls or without giving any clinical data [26-28]. Wurm et al. [24] published the largest series of 140 patients who underwent surgery for medically intractable epilepsy. After the procedure for miscellaneous patholo- gies surgeons answered a questionnaire to assess the impact of the neuronavigation. They concluded that the application of the navigation system was effectively and safe in terms that the targets, even small in size, could be located precisely and electrodes could be placed accu- rately as well. Moreover the approach could be indi- vidually tailored. In a previous series of Oertel et al. [22] neuronaviga- tion seemed to be helpful in avoidance of complications (8% vs. 22%). In 93% the surgeon rated the application of the neuronavigation as “helpful”. A comparison of the complications in various studies is compiled in Table 2, seizure outcome in Table 3. In our series complication rate and seizure outcome are comparable to larger series [29]. The application was safe. There were no complications with direct referral to the use of the navigation system. The time for prepara- tion of the navigation was acceptable: in our evaluation the total time from anaesthesia induction to skin incision was 37 minutes. In comparison to that the installation of the neuronavigation equipment alone took additional 26 minutes in another study [30]. Surgery itself was not prolonged. Table 1. Usefulness of neuronavigation. Presurgical pla nni ng /st rategy Helpful for studying patients indiv idual anatomy Determination of craniotomy si te Helpful, especially over convexity Locating lesions Helpful, especially in subcortical p ath olo gie s Amygdala-hippocam pectomies Extreme helpful in access the temporal horn Resection control Var i a ble (brain shift), often overestimation, consider alternatives ( e.g. ultr asound ) Delicate site of surgery Helpful, shows eloquent structu res as well  M. B. Glaser et al. / HEALTH 2 (2010) 753-758 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 757 757 Table 2. Epilepsy surgery and complications (perm. = permanent; trans. = transient). Complications Acar et al. Oertel et al. Cho et al. Glaser et al. Sindou et al. without Navigation CSF fistula 2 trans. 1 trans. Visual field defects 4 perm. Not investigated 1 4 perm. Not investigated CN palsy 1 trans. 2 trans. Motor deficit 1 perm. 1 trans. 2 trans. 2 perm. Aphasia 1 trans. 1 perm. 1 trans. Postop. haematoma 1 1 3 Infection 3 n = 39 n = 38 n = 46 n = 41 n = 100 Table 3. Seizure-outcome after epilepsy surgery. Engel’s class Acar et al. Oertel et al. Cho et al. Glaser et al. Sindou et al. without Navigation I 37 (95%) 20 (53%) 28 (61%) 32 (78%) 85 (85%) II 2 (5%) 10 (22%) 4 (10%) 9 (9%) III 6 (13%) 2 (5%) 2 (2%) IV 2 (4%) 3 (7%) 4 (4%) n = 39 n = 38 n = 46 n = 41 n = 100 8. CONCLUSIONS Based on these results and our experience in the use of neuronavigation, we conclude that the application of a navigation system in epilep sy cases is safe and helpful in finding the targeted structure and in minimizing trauma to the patient by smaller craniotomies. REFERENCES [1] Hauser, W.A., Annegers, J.F. and Kurland, L.T. (1991) Prevalence of epilepsy in Rochester, Minnesota: 1940- 1980. Epilepsia, 32(4), 429-445. [2] Olafsson, E., Ludvigsson, P., Gudmundsson, G., Hes- dorffer, D., Kjartansson, O. and Hauser, W.A. (2005) In- cidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: A prospective study. The Lancet Neurology, 4(10), 627- 634. [3] Semah, F., Picot, M.C., Adam, C., Broglin, D., Arzi- manoglou, A., Bazin, B., Cavalcanti, D. and Baulac, M. (1998) Is the underlying cause of epilepsy a major prog- nostic factor for recurrence? Neurology, 51(5), 1256- 1262. [4] Kloster, R. and Engelskjon, T. (1999) Sudden unexpected death in epilepsy (SUDEP): A clinical perspective and a search for risk factors. Journal of Neurology, Neurosur- gery & Psychiatry, 67(4), 439-444. [5] Sillanpaa, M. and Schmidt, D. (2006) Natural history of treated childhood-onset epilepsy: Prospective, long-term population-based study. Brain, 129(Pt3), 617-624. [6] Tonini, C., Beghi, E., Berg, A.T., Bogliun, G., Giordano, L., Newton, R.W., Tetto, A., Vitelli, E., Vitezic, D. and Wiebe, S. (2004) Predictors of epilepsy surgery outcome: A meta-analysis. Epilepsy Research, 62(1), 75-87. [7] Berg, A.T., Vickrey, B.G., Langfitt, J.T., Sperling, M.R., Walczak, T.S., Shinnar, S., Bazil, C.W., Pacia, S.V. and Spencer, S.S. (2003) The multicenter study of epilepsy surgery: Recruitment and selection for surgery. Epilepsia, 44(11), 1425-1433. [8] Zernov, D. (1890) L’encephalometre. revue générale de clinique et de thérapeutique, 19, 320. [9] Horsley, V.R.C. (1908) The structure and functions of the cerebellum examined by a new method. Brain, 31(1), 45-124. [10] Kirschner, M. (1933) Die Punktionstechnik und die Elek- trokoagulation des Ganglion Gasseri. Über gezielte Op- erationen. Langenbecks Archiv für klinische Chirurgie, 176, 581-620. [11] Grunert, P., Darabi, K., Espinosa, J. and Filippi, R. (2003) Computer-aided navigation in neurosurgery. Neurosur- gical Review, 26(2), 73-99. [12] Enchev, Y. (2009) Neuronavigation: Geneology, reality, and prospects. Neurosurgical Focus, 27(3), E11. [13] Ganslandt, O., Behari, S., Gralla, J., Fahlbusch, R. and Nimsky, C. (2002) Neuronavigation: Concept, techniques  M. B. Glaser et al. / HEALTH 2 (2010) 753-758 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 758 and applications. Neurol India, 50(3), 244-255. [14] Wagner, W., Gaab, M.R., Schroeder, H.W. and Tschilt- schke, W. (2000) Cranial neuronavigation in neurosur- gery: Assessment of usefulness in relation to type and site of pathology in 284 patients. Minim Invasive Neuro- surg 43(3), 124-131. [15] Spetzger, U., Laborde, G. and Gilsbach, J.M. (1995) Frameless neuronavigation in modern neurosurgery. Minim Invasive Neurosurg, 38(4), 163-166. [16] Winkler, D., Lindner, D., Strauss, G., Richter, A., Schober, R. and Meixensberger, J. (2006) Surgery of cavernous malformations with and without navigational support-a comparative study. Minimally Invasive Neurosurgery, 49(1), 15-19. [17] Stone, S.S. and Rutka, J.T. (2008) Utility of neuronaviga- tion and neuromonitoring in epilepsy surgery. Neurosur- gical Focus, 25(3), E17. [18] Nimsky, C., Ganslandt, O., Hastreiter , P., Wang, R., Benner, T., Sorensen, A.G. and Fahlbusch, R. (2005) Preoperative and intraoperative diffusion tensor imaging- based fiber tracking in glioma surgery. Neurosurgery 56(1), 130-137. [19] Reisch, R., Stadie, A., Kockro, R., Gawish, I., Schwandt, E. and Hopf, N. (2009) The minimally invasive supraor- bital subfrontal key-hole approach for surgical treatment of temporomesial lesions of the dominant hemisphere. Minim Invasive Neurosurg, 52(4), 163-169. [20] Engel, J.V.N.P. Jr., Rasmussen, T.B. and Ojemann, L.M. (Ed.), (1993) Outcome with respect to epileptic seizures. Raven Press, New York. [21] Miyagi, Y., Shima, F., Ishido, K., Araki, T., Taniwaki, Y., Okamoto, I. and Kamikaseda, K. (2003) Inferior tempo- ral sulcus approach for amygdalohippocampectomy gui- ded by a laser beam of stereotactic navigator. Neurosur- gery 52(5), 1117-1123. [22] Oertel, J., Gaab, M.R., Runge, U., Schroeder, H.W., Wagner, W. and Piek, J. (2004) Neuronavigation and complication rate in epilepsy surgery. Neurosurgical Re- view, 27(3), 214-217. [23] Wurm, G., Wies, W., Schnizer, M., Trenkler, J. and Holl, K. (2000) Advanced surgical approach for selective amy- gdalohippocampectomy through neuronavigation. Neu- rosurger y, 46(2), 1377-1382. [24] Wurm, G., Ringler, H., Knogler, F., Schnizer, M. (2003) Evaluation of neuronavigation in lesional and non-le- sional epilepsy surgery. Computer Aided Surgery, 8(4), 204-214. [25] Hirabayashi, H., Chitoku, S., Hoshida, T. and Sakaki, T. (1999) Accuracy and availability of the computed as- sisted neurosurgery navigation system during epilepsy surgery. Stereotact Funct Neurosurg, 72(2-4), 117-124. [26] Acar, G., Acar, F., Miller, J., Spencer, D.C. and Burchiel, K.J. (2008) Seizure outcome following transcortical se- lective amygdalohippocampectomy in mesial temporal lobe epilepsy. Stereotact Funct Neurosurg, 86(5), 314- 319. [27] Fahlbusch, R., Ganslandt, O. and Nimsky, C. (2000) In- traoperative imaging with open magnetic resonance im- aging and neuronavigation. Child’s Nervous System, 16(10-11), 829-831. [28] Wheatley, B.M. (2008) Selective amygdalohippocam- pectomy: The trans-middle temporal gyrus approach. Neurosurg Focus, 25(3), E4. [29] Sindou, M.G.M., Isnard, J., Ryvlin, P., Fischer, C. and Mauguière, F. (2006) Temporo-mesial epilepsy surgery: outcome and complications in 100 consecutive adult pa- tients. Acta Neurochir (Wien), 148(1), 39-45 [30] Willems, P.W.A., Taphoorn, M.J.B., Burger, H., van der Sprenkel, J.W.B. and Tulleken, C.A.F. (2006) Effective- ness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: A randomized controlled trial. Journal of Neurosurgery, 104(3), 360-368. |

