 Advances in Microbiology, 2012, 2, 216-226 http://dx.doi.org/10.4236/aim.2012.23026 Published Online September 2012 (http://www.SciRP.org/journal/aim) Distribution of cry and cyt Genes among Indigenous Bacillus thuringiensis Isolates with Mosquitocidal Activity Ayyasamy Mahalakshmi1, Kabilan Sujatha1, Poornima Kani2, Rajaiah Shenbagarathai2* 1UGC-NRCBS, School of Biological Sciences, Madurai Kamaraj University, Madurai, India 2PG and Research Department of Zoology and Biotechnology, Lady Doak College, Madurai, India Email: *shenbagarathai@rediffmail.com Received June 11, 2012; revised July 5, 2012; accepted July 15, 2012 ABSTRACT Bacillus thuringiensis strains isolated from Madurai, TamilNadu, India were evaluated for their mosquitocid al activity, as well as cry and cyt genes diversity. It revealed that 99% of the parasporal crystal morphology of these isolates was spherical in nature and a variable percentage (0% - 100%) o f toxicity was observed agains t Culex quinquefasciatus and Aedes aegypti. PCR analysis revealed that 53% of the isolates were positive for the various cry and cyt genes tested, whereas 47% did not produce any PCR product for the cry gene analyzed. Diverse pattern of cry and cyt genes distribu- tion was observed even in the isolates from the same sample. B. thuringienis subsp. LDC-9 showed three-fold higher toxicity against Culex quinquefasciatus than that of B. thuringiensis var israelensis which might be used as a potential strain to control mosquitoes in near future after field evaluation. Keywords: Bacillus thuringiensis; Crystal Endotoxin; Culex quinquefasciatus; Aedes aegypti 1. Introduction The search for native strains to control dipteran species have an impact on the control of mosquitoes worldwide, as vector borne diseases are major public health prob- lems and their prevalence has dramatically increased worldwide [1]. Chemical insecticides have been proved to be very effective in vector control programme, but their adverse environmental effects, insecticidal resis- tance and resurgence have prompted the search for alter- native strategies for insect pest control [2]. Among the various alternatives, B. thuringiensis and B. sphaericus are the most potent and successful group of organisms for effective control of pests among the microorganisms [3]. The environmental safety of Bt. based products em- ployed in pest control methods are well documented [4]. B. thuringiensis is a gram positive organism that synthe- sizes crystalline inclusions (δ-endotoxins) during sporu- lation. These toxins are highly specific, completely de- gradable and harmless to humans, vertebrates and plants. Hence, researchers across the world are interested for screening new strains with increased levels of insecti- cidal toxicity with a broader spectrum of activ ity [5]. The various screening programmes resulted in the number of B. thuringiensis strains not only active against Lepidop- tera, Diptera, Coleoptera but also against Hymenoptera, Phthiraptera or Mallophaga, Acari, Nematheliminthes, Platyhelminthes and Sarcomastigophora [6-8]. The insecticidal activity of B. thuringiensis strains against Dipterans is attributed to the presence of Cry and Cyt proteins [9]. Cry toxins are activated by host prote- ases, which interact with specific receptors located on the host cell surface, resulted in the formation of a pre-pore oligomeric structure that is insertion competent. In con- trast, Cyt toxins directly interact with membrane lipids and insert into the membrane [10]. The mosquitocidal activity of a B. thuringiensis strain is due to the additive effect of each toxin and a complex synergistic interactio n among them. B. thuringiensis subsp. israelensis produces four Cry toxins such as Cry4Aa, Cry4Ba, Cry10Aa, and Cry11Aa, Cyt1Aa and Cyt2Ba respectively [11,12]. The presence of the Cyt toxin synergizes and delays or pre- vents the development of resistance to Cry toxins by functioning as a Cry-membrane bound receptor [10]. However, information on the diversity and distribution of cry and cyt genes among mosquitocidal B. thuringiensis isolates from southern part of India is negligible [13,14]. Hence, the present study was envisaged to analyze the distribution of the cry and cyt genes of indigenous mos- quitocidal B. thuringiensis isolates. 2. Materials and Methods 2.1. Sampling Procedure Triplicate samples were collected with an internal di- *Corresponding a uthor. C opyright © 2012 SciRes. AiM  A. MAHALAKSHMI ET AL. 217 ameter of 2.5 cm from different locations of Madurai district, Tamilnadu, India situated at an altitud e of 100.58 meters above mean sea level, 9.3˚N latitude and 77˚E longitude with annu al rainfall of 85 cm, relative hu midity of 40% - 70% with mean maximum and minimum tem- perature of 37.5˚C and 20.9˚C respectively. A total of 360 soil samples were collected which included 60 sam- ples from the various ecological niches such as River bank, Subterranean, Urban, Agricultural land, animal contaminated soils and mountain regions as indicated in Table 1. Approximately, 10 g of soil samples were col- lected from four different sites of each location and pooled into one sample, homogenized by thorough mix- ing, sieved, air-dried up to 20% moisture content [15] and stored in sealed polythene bags within desiccators. A subsample of 1 g was used for further analysis. The stor- age time ranges from few days to three weeks with moisture level of 20%. Leaf samples such as Murraya koengii and Ricinis communis were obtained from 2.0 to 2.5 m above the ground, 0.3 m inside the outer leaf canopy and from the east side of each tree or shrub. Cross contamination be- tween samples was prevented by detaching leaves or nee- dles while they were enclosed in standard plastic sand- wich bags, which were immediately sealed for storage. Samples containing leaf litters, leaf dust and animal droppings (contaminated samples) were collected and stored at 4˚C until use. A total of 60 samples of leaf lit- ters were collected from Alagarkovil. Approximately 10 number of dead insect includes centipede, millipede, Spodoptera larva, pupa and Culex quinquefasciatus larva were assumed to be infected by B. thuringensis were collected from soil (sandy soil), plant surface (Ricinis communis leaf) and water bodies (stagnant water) using sterile forceps and was transferred in sterile polythene bags to the laboratory for analysis. 2.2. B. thuringiensis Isolation The insect samples except Culex quinquefasciatus were surface sterilized following the methodology described by Alves [16] which eliminated external contamination. The larvae were passed first in 70% alcohol for 2 sec, followed in 5% sodium hypochlorite for 3 min and fi- nally in sterile 10% sodium thiosulfate for 5 min. The specimens were then washed three times in sterile dis- tilled water and transferred aseptically into a sterile mor- tar and macerated with a sterile pestle. The macerate was resuspended in 10 mL sterile distilled water and 5 mL was heated at 80˚C for 3 min followed by co oling on ice for 5 - 10 min. The diluted samples (0.1 mL) were plated on nutrient agar and plates were incubated at 33˚C ± 2˚C for 24 h. In case of leaves, the leaf sections with an area of approximately 2 - 3 cm2 were trimmed and ground with a small mortar with 1 mL sterilized distilled water. Soil samples, animal contaminated soil samples, leaf litter samples, leaf dust, effluent samples were prepared as suspensions in 10 mL sterile distilled water. 5 mL of the resulting suspen sions were transferred in a fresh tube and incubated in the water bath at 80˚C for 3 min fol- lowed by cooling on ice for 5 - 10 min. The aliquots (0.2 ml) were spread on plates of nutrient agar and incubated at 33˚C ± 2˚C for 24 h. 2.3. Genomic DNA Isolation Total genomic DNA of B. thuringiensis was isolated by the method of Kalman et al., [17] with some modifica- tions. Cultures were grown in Luria-Bertani broth (10 g tryptone, 5 g yeast extract, 10 g NaCl·L–1) to an optical density of 0.8 at 600 nm. The cells were washed once in 0.5 mL of TES (10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 100 mM NaCl) and resuspended in 2 mL of SET (25% sucrose, 25 mM EDTA, 25 mM Tris-HCl (p H 8.0) with 2 mg o f ly s o z ym e · mL–1 and were incubated at 37˚C for 1 h. Then SDS was added to a final concentration of 1% and incubated at 50˚C for 5 min and 4˚C for overnight. The supernatant was then extracted thrice with phenol-chlo- roform-isoamylalcohol (25:24:1) and precipitated with ethanol. Following centrifugation, the washed DNA pel- lets were resuspended in 100 µl of 1X TE (10 mM Tris-HCl (pH 8.0), 1 mM EDTA). 2.4. PCR Amplification The family-specific primers (Table 1) for cry (cry2, cry 4-spe, cry10-spe and cry11-gen) and cyt (cyt1 gra1, cyt2 gra1) genes were used for screening cry an d cyt genes through Polymerase Chain Reaction (PCR) following the method of Ibarra et al., [18]. The PCR mix (25 µL) con- sisted of 30 ng of total genomic DNA, 1X buffer, 3 mM MgCl2, 0.2 mM each deoxynucleotide triphosphate, I U proofreading Taq DNA polymerase, 1.0 µM each primer set and 3% DMSO as PCR additive. Amplification was done in an Eppendorf PCR system (Master cycler Per- sonal 5332, Eppendorf, Hamburg, Germany) with a 3 min denaturation step at 95˚C, followed by 30 amplifica- tions cycles of 95˚C for 1 min, 52˚C for 1 min, 72˚C for 1 min and an extra extension step of 10 min at 72˚C. The amplified products were electrophoresed on agarose gel [19] and filed using Gel-Doc photodocumentor device (Geneline, Spectronics, India). B. thuringiensis subsp. kurstaki HD-1 and B. thuringiensis subsp. israelensis (gift from Bacillus Genetic Stock Center, Ohio) were used as positive controls and B. subtilis as a negative control. The distribution frequency of cry gene in B. thuringiensis strains from a certain origin is defined as the percentage of B. thuringiensis isolates containing this gene among all the isolates from that origin. Copyright © 2012 SciRes. AiM 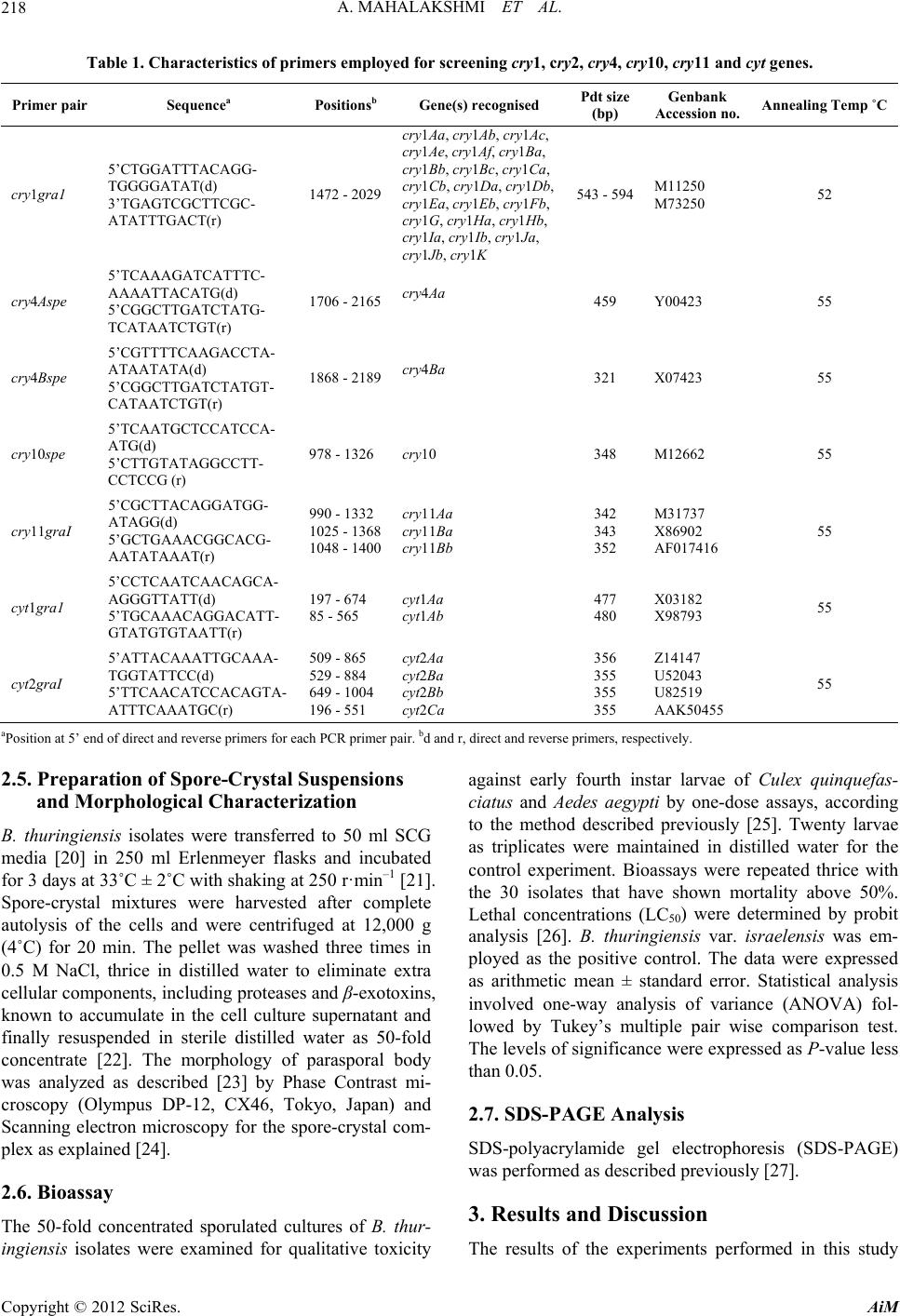 A. MAHALAKSHMI ET AL. Copyright © 2012 SciRes. AiM 218 Table 1. Characteristics of primers employed for screening cry1, cry2, cry4, cry10, cry11 and cyt genes. Primer pair Sequencea Positionsb Gene(s) recognised Pdt size (bp) Genbank Accession no. Annealing Temp ˚C cry1gra1 5’CTGGATTTACAGG- TGGGGATAT(d) 3’TGAGTCGCTTCGC- ATATTTGACT(r) 1472 - 2029 cry1Aa, cry1Ab, cry1Ac, cry1Ae, cry1Af, cry1Ba, cry1Bb, cry1Bc, cry1Ca, cry1Cb, cry1Da, cry1Db, cry1Ea, cry1Eb, cry1Fb, cry1G, cry1Ha, cry1Hb, cry1Ia, cry1Ib, cry1Ja, cry1Jb, cry1K 543 - 594M11250 M73250 52 cry4Aspe 5’TCAAAGATCATTTC- AAAATTACATG(d) 5’CGGCTTGATCTATG- TCATAATCTGT(r) 1706 - 2165cry4Aa 459 Y00423 55 cry4Bspe 5’CGTTTTCAAGACCTA- ATAATATA(d) 5’CGGCTTGATCTATGT- CATAATCTGT(r) 1868 - 2189cry4Ba 321 X07423 55 cry10spe 5’TCAATGCTCCATCCA- ATG(d) 5’CTTGTATAGGCCTT- CCTCCG (r) 978 - 1326 cry10 348 M12662 55 cry11graI 5’CGCTTACAGGATGG- ATAGG(d) 5’GCTGAAACGGCACG- AATATAAAT(r) 990 - 1332 1025 - 1368 1048 - 1400 cry11Aa cry11Ba cry11Bb 342 343 352 M31737 X86902 AF017416 55 cyt1gra1 5’CCTCAATCAACAGCA- AGGGTTATT(d) 5’TGCAAACAGGACATT- GTATGTGTAATT(r) 197 - 674 85 - 565 cyt1Aa cyt1Ab 477 480 X03182 X98793 55 cyt2graI 5’ATTACAAATTGCAAA- TGGTATTCC(d) 5’TTCAACATCCACAGTA- ATTTCAAATGC(r) 509 - 865 529 - 884 649 - 1004 196 - 551 cyt2Aa cyt2Ba cyt2Bb cyt2Ca 356 355 355 355 Z14147 U52043 U82519 AAK50455 55 aPosition at 5’ end of direct and reverse primers for each PCR primer pair. bd and r, direct and reverse primers, r espectively. 2.5. Preparation of Spore-Crystal Suspensions and Morphological Characterization B. thuringiensis isolates were transferred to 50 ml SCG media [20] in 250 ml Erlenmeyer flasks and incubated for 3 days at 33˚C ± 2˚C with shaking at 250 r·min–1 [21]. Spore-crystal mixtures were harvested after complete autolysis of the cells and were centrifuged at 12,000 g (4˚C) for 20 min. The pellet was washed three times in 0.5 M NaCl, thrice in distilled water to eliminate extra cellular components, including proteases and β-exotoxins, known to accumulate in the cell culture supernatant and finally resuspended in sterile distilled water as 50-fold concentrate [22]. The morphology of parasporal body was analyzed as described [23] by Phase Contrast mi- croscopy (Olympus DP-12, CX46, Tokyo, Japan) and Scanning electron microscopy for the spore-crystal com- plex as explained [24]. 2.6. Bioassay The 50-fold concentrated sporulated cultures of B. thur- ingiensis isolates were examined for qualitative toxicity against early fourth instar larvae of Culex quinquefas- ciatus and Aedes aegypti by one-dose assays, according to the method described previously [25]. Twenty larvae as triplicates were maintained in distilled water for the control experiment. Bioassays were repeated thrice with the 30 isolates that have shown mortality above 50%. Lethal concentrations (LC50) were determined by probit analysis [26]. B. thuringiensis var. israelensis was em- ployed as the positive control. The data were expressed as arithmetic mean ± standard error. Statistical analysis involved one-way analysis of variance (ANOVA) fol- lowed by Tukey’s multiple pair wise comparison test. The levels of significance were expressed as P-value less than 0.05. 2.7. SDS-PAGE Analysis SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was perform ed as described previously [2 7]. 3. Results and Discussion The results of the experiments performed in this study  A. MAHALAKSHMI ET AL. 219 are presented and discussed in the following sections. 3.1. Environmental Distribution of B. thuringiensis The strains with typical B. thuringiensis colony mor- phology (fried egg appearance) were noted in 495 of the 540 samples collected from various ecological niches as shown in Figure 1. B. thuringiensis occurrence was highest in the agricu ltural so il (93%). Similar observ ation of the ubiquitous distribution of B. thuringiensis was previously reported [21]. B. thuringiensis abundance in soil might be due to the high levels of insect activity and a large amount of nutrients in the soil, allowing optimum survival and enrichment as previously reported [28]. While comparing B.t diversity from various samples, many isolates were selected from soil samples (89%) than from insect and leaf samples (67%) with respect to the biological origin as noted in Table 2. This observa- tion deviated from studies of Wang et al. [29] wherein they have reported that the average frequency of B. thur- ingiensis isolates from soil samples were 29.8% only. As B. thuringiensis is an insect pathogen, the soil sam- ples were further analyzed based on the presence and absence of plants and insects, in order to correlate how environment influenced the densities of B. thuringiensis. The analysis of samples sites based on the presence and absence of plant communities revealed that B. thur- ingiensis index of 0.5 was observed in agricultural fields, which included plants such as Oryza sativa (Rice), Bras- sica oleracea (Cabbage), Zea mays (Corn), Pennisetum glaucum (pearl millet), Pisum sativum var. sativum (Garden pea), Vigna mungo (Black gram) and Brassica napus (Rapeseed) (Table 2). On the other hand, analysis of soil samples based on the presence and absence of intense insect activity revealed that B. thuringiensis in- dex of 0.89 was isolated from soil samples without in- sects (Table 3). This observation did not correlate with the relationship between insect environments and densi- ties of B. thuringiensis as indicated in the earlier studies [30,31]. The isolated B. thuringiensis strains were screened for crystal inclusions using Phase Contrast Microscopy. A total of 417 strains were selected among 1956 spore formers based on the crystal inclusions after Phase Con- trast Microscopic observation. 99% of these isolates showed spherical parasporal inclusions (Figure 2). This observation was different from the earlier reports of Figure 1. Map showing sample collected sites; closed circle blue dots indicates sample collected sites. Copyright © 2012 SciRes. AiM 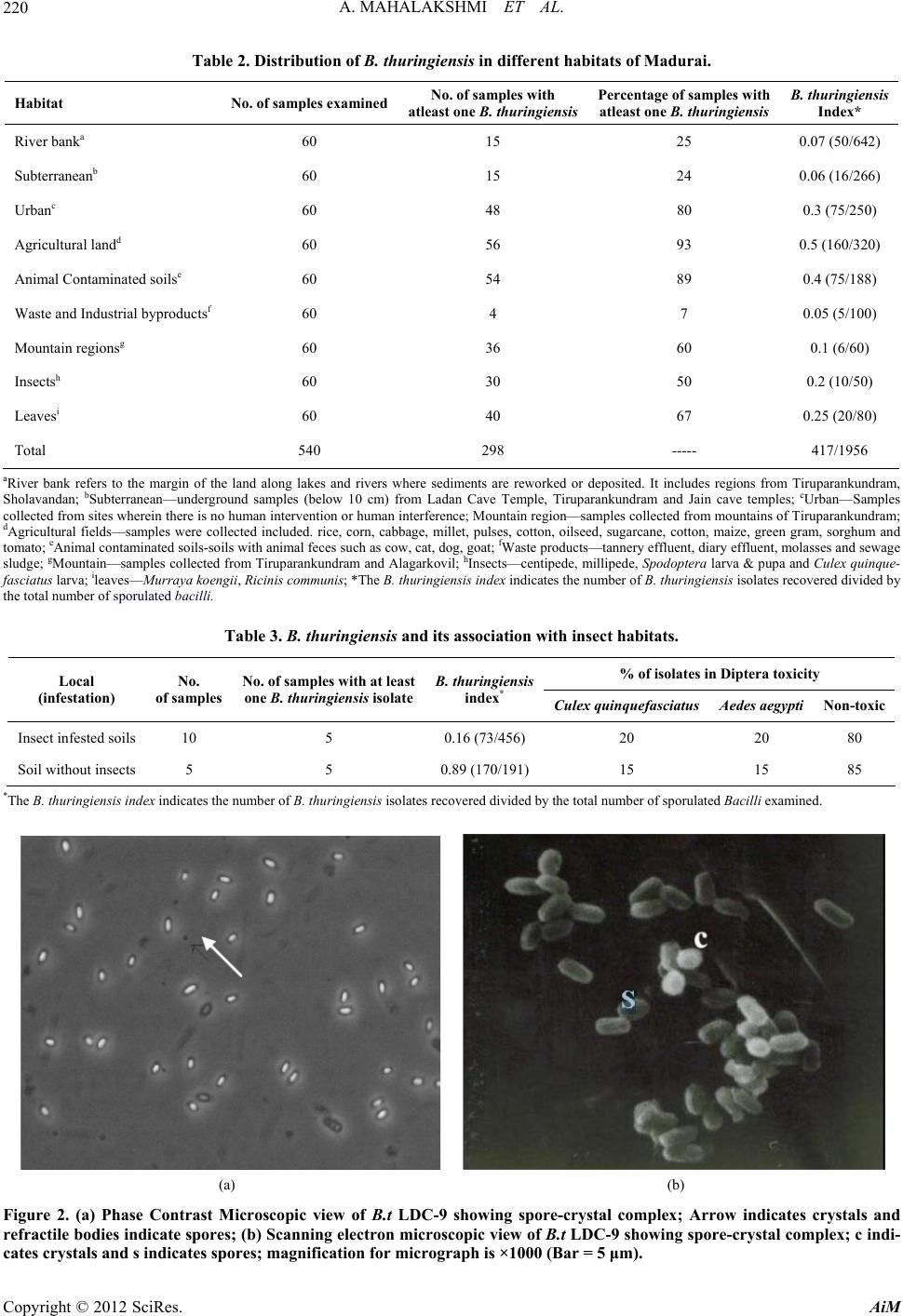 A. MAHALAKSHMI ET AL. 220 Table 2. Distribution of B. thuringiensis in different habitats of Madurai. Habitat No. of samples examinedNo. of samples with atleast one B. thuringiensis Percentage of samples with atleast one B. thuringiensis B. thuringiensis Index* River banka 60 15 25 0.07 (50/642) Subterraneanb 60 15 24 0.06 (16/266) Urbanc 60 48 80 0.3 (75/250) Agricultural landd 60 56 93 0.5 (160/320) Animal Contaminated soilse 60 54 89 0.4 (75/188) Waste and Industrial byproductsf 60 4 7 0.05 (5/100) Mountain regionsg 60 36 60 0.1 (6/60) Insectsh 60 30 50 0.2 (10/50) Leavesi 60 40 67 0.25 (20/80) Total 540 298 ----- 417/1956 aRiver bank refers to the margin of the land along lakes and rivers where sediments are reworked or deposited. It includes regions from Tiruparankundram, Sholavandan; bSubterranean—underground samples (below 10 cm) from Ladan Cave Temple, Tiruparankundram and Jain cave temples; cUrban—Samples collected from sites wherein there is no human intervention or human interference; Mountain region—samples collected from mountains of Tiruparankundram; dAgricultural fields—samples were collected included. rice, corn, cabbage, millet, pulses, cotton, oilseed, sugarcane, cotton, maize, green g ram, sorghum and tomato; eAnimal contaminated soils-soils with animal feces such as cow, cat, dog, goat; fWaste products—tannery effluent, diary effluent, molasses and sewage sludge; gMountain—samples collected from Tiruparankundram and Alagarkovil; hInsects—centipede, millipede, Spodoptera larva & pupa and Culex quinque- fasciatus larva; ileaves—Murraya koengii, Ricinis communis; *The B. thuringiensis index indicates the number of B. thuringiensis isolates recovered divided by the total number of sporulated bacilli. Table 3. B. thuringiensis and its association with insect habitats. % of isolates in Diptera toxicity Local (infestation) No. of samples No. of samples with at least one B. thuringiensis isolate B. thuringiensis index* Culex quinquefasciatus Aedes aegypti Non-toxic Insect infested soils 10 5 0.16 (73/456) 20 20 80 Soil without insects 5 5 0.89 (170/191) 15 15 85 *The B. thuringiensis index indicates the number of B. thuringiensis isolates recovered divided by the total numbe r o f sporulated Bacilli examined. (a) (b) Figure 2. (a) Phase Contrast Microscopic view of B.t LDC-9 showing spore-crystal complex; Arrow indicates crystals and refractile bodies indicate spores; (b) Scanning electron microscopic view of B.t LDC-9 showing spore-crystal complex; c indi- cates crystals and s indicates spores; magnification for micrograph is ×1000 (Bar = 5 μm). Copyright © 2012 SciRes. AiM  A. MAHALAKSHMI ET AL. Copyright © 2012 SciRes. AiM 221 Bernhard et al., [32] wherein strains with bipyramidal crystals were predominant in Eastern Asia, except South- east habitats. The differences in the distribution of mor- phology of parasporal body might be due to the genetic variation caused by the differences in the environmental conditions or to habitat effects [33]. 3.2. Identification of cry and cyt Gene Composition of B. thuringiensis Isolates and Their Distribution in Different Sources Identification o f B.t cry and cyt genes by PCR has proven to be a very useful method for strain characterization, offering several advantages in terms of rapidity and re- producibility [34]. Selected 417 strains were character- ized for the presence or absence of the specific cry2, cry4, cry10, cry11, cyt1 and cyt2 genes by PCR. The most frequent cry genes were cry4 (50%) [53% cry4a + 47% cry4b] and cry2 (25%) followed by cry11 (10%) and cry10 (15%) genes. Similar occurrence of cry4 and cry2 gene diversity was reported by Ibanez et al. [35]. Our study revealed that cry11 (10%) whereas Bravo et al., [36] indicated that 38% of the tropical strains were with cry11 and cyt genes. Cytolytic genes, cyt1 (9%) and cyt2 (7%) genes were the least identified. Strains with multi- ple cry genes were found at a lesser frequency (less than 3%) than strains with single cry genes. Isolates with a cry gene harbouring cyt1 and or cyt2 gene were less than 2% (Figure 3). Interestingly, few strains such as B. thur- ingiensis LDC-9, LDC-14 and LDC-21 in our B. thur- ingiensis collection harbored more than one cry gene as reported earlier [37]. On the other hand, 47% of the strains with crystal inclusions failed to give PCR product when assayed with the primers used in this study. It did not necessarily imply that these strains were devoid of genes coding for insecticidal properties, as all of them did produce crystals [36]. These strains might contain other cry, cyt or non insecticidal parasporal inclusions as suggested by Uemori et al. [37]. Further, the cry gene frequency of these isolates were correlated to the source of the sample and classified into three groups. The first group contained the most common cry genes (cry2, cry4 and cry11) with high frequencies noted in the soils, compared to other sources. The second group included cry10 gene, found only in the samples derived from the insects, while absent in other samples. The third group contained the cytolytic cyt1 and cyt2 genes, present only in soil and insect samples (Figures 4 and 5). These re- sults are in close agreement with Wang et al., [29], where the strains from different sources differed in their cry gene content. 3.3. Correlation with Insect Toxicity All the selected B. thuringiensis isolates with dipteran specific cry genes such as cry 2, cry4, cry10 and cry11 were tested for its mean toxic mortalities using three rep- licates against Culex quinquefasciatus and Aedes aegypti as noted (Table 4). The spore-crystal complex of B. thuringiensis isolates, which promoted 50% or more lar- val mortality, were considered as active strains as re- ported by Hossain et al., [30]. Toxicity tests revealed that only 7% of the B. thuringiensis isolates were pathogenic (>50%) to dipteran larvae. Nontoxic B. thuringiensis is more common than toxic B. thuringiensis as reported by earlier studies of Ohba [38]. The variation in toxicity was not related to cry gene content in all cases, as some strains sharing the same cry gene but significantly dif- fered in their insecticidal potency. In this study, for ex- Figure 3. Frequency of single mosquitocidal cry or cyt genes in indigenous Bacillus thuringiensis isolates.  A. MAHALAKSHMI ET AL. 222 Figure 4. Frequency of multiple mosquitocidal cry or cyt gene in indigenous B. thuringiensis isolates. ample, strains (B. thuringiensis LDC-14 and B. thur- ingiensis LDC-21) displayed the cry 4 gene but the mor- talities produced by th e spore-crytsal complex were 96% and 24% respectively. This might be explained by a variation in the level of gene expression, which can strongly influence the insect toxicity as reported by Mar- tinez and Caballero [39]. Strain B. thuringiensis LDC-9 alone with unique combinations of cry (cry4a, cry4b, cry10, cry11) and cyt genes (cyt1 and cyt2) demonstrated three-fold higher mosquitocidal activity (6 ng ·mL–1) than B. thuringiensis israelensis (19 ng ·mL–1). 3.4. Protein Profiling B. thuringiensis LDC-9 was noted to be the toxigenic strain based on the mosquitocidal activity and SDS- PAGE of spore-crystal suspensions of selected strain as shown (Figure 6). B. thuringiensis LDC-9 exhibited pro- tein profile which is distinct from B. thuringiensis sp. israelensis as reported earlier [18]. The present study resulted in identification of a novel B. thuringiensis LDC-9 with higher mosquitocidal activity than the earlier reported B. thuringiensis strains. Hence, this strain is likely to be a viable mosquito control agent after field evaluation and toxicity analysis against other aquatic insects including dragonflies, damselflies, mayflies, stone- flies, caddisflies, water beetles or bug s and other inverte- brates such as Daphnia, Cyclops, rotifers and crusta- ceans. 4. Conclusion B. thuringiensis presents great genetic and molecular diversity even in isolates from the same soil sample. Moreover, the diversity and activity of the isolates might have a relationship with the geographical origin of the samples. The results obtained here indicate that the B. thuringiensis LDC-9 may be a potential control agent Copyright © 2012 SciRes. AiM  A. MAHALAKSHMI ET AL. 223 (a) (b) Figure 5. (a) Mosquitocidal gene distribution based on the nature of the soil samples; (b) Mosquitocidal gene distribution based on the origin of the samples. Copyright © 2012 SciRes. AiM  A. MAHALAKSHMI ET AL. 224 Table 4. Larvicidal activity of indigenous B. thuringiensis strains against Culex quinquefasciatus and Aedes aegypti. Source Number of StrainsMortality (%) of Culex quinquefasciatus Mortality (%) of Aedes aegypti River bank (21), Subterranean (1), Urban (33) , Agricultural land (95), Animal contaminated soils (47), Wa ste and Industrial byproducts (2), Insects (3), Leaves (13) 215 0.0 0.0 River bank (20), Subterranean (9), Urban (36) , Agricultural land (36), Animal contaminated soils (18), Wa ste and Industrial byproducts (2), Mountain regions (1), Insects (2), Leaves (2) 126 13.71 ± 3.59a 13.71 ± 3.59 River bank (5), Subterranean (5), Urban (5), Agricultural land (21), Animal contam inated soils (4), Waste and Industrial byproducts (1), Insects (2), Mountain regions (2) , Insects (2), Leaves (2) 49 30 ± 5.14b 30 ± 5.14b River bank (2), Subterranean (1), Urban (1), Agricultural land (4), Animal contaminated soils (3), Insects (1), Mountain regions (2), Leaves (1) 15 50.6 ± 6.28c 50 .6 ± 6.28c River bank (2), Agricultural land (3), Animal contaminated soils (3), Insects (2), Mountain regions (1), Leaves (1) 12 69.5 ± 6.80d 69.5 ± 6.80d Agricultural soil sample ( 1) 1 100 ± 0e 100 ± 0e Mortality is expressed as average ± standard deviation. Different alphabets in superscripts indicates significant difference at P < 0.05. Number in parenthesis indicate the number of B.t strains employed in this study (To include strain s employed in this study). Figure 6. Protein profile of spore-crystal complex of selected B. thuringiensis isolates; Lane 1: B.t LDC-7; Lane 2: B.t LDC-21; Lane 3: B.t LDC-43; Lane 4: B.t LDC-52; Lane 5: B.t LDC-64; Lane 6: B.t LDC-9; Lane 7: B.t subsp. israelensis; Lane 8: B.t LDC-71; Lane 9: B.t LDC-127; Lane 10: B.t subsp. kurstaki HD-1; Lane M: Marker. that could be used in control programmes against mos- quitoes. 5. Acknowledgements We acknowledge University Grants Commission (No.F.3- 35/2003 (SR)) and Department of Biotechnology-BIF (BT/ B125/001/200 6) for provid ing financial su pport and infra structure facility respectively. Authors are grateful to Dr. W. Isabel for providing us the litter samples for the study. They also express their gratitude to Dr. A. H. Bishop, Greenwich University, London for the help ren- dered towards scanning electron microscopy employed in this study. REFERENCES [1] L. Regis, M. H. Silva-Filha, C. Nielsen-LeRoux and J. F. Charles, “Bacterial Larvicides of Dipteran Disease Vec- tors,” Trends in Parasitology, Vol. 17, No. 8, 2001, pp. 377-380. doi:10.1016/S1471-4922(01)01953-5 [2] B. A. Federici, H. W. Park, D. K. Bideshi, M. C. Wirth, J. J. Johnson, Y. Sakano and M. Tang, “Developing Re- combinant Bacteria for Control of Mosquito Larvae,” Journal of the American Mosquito Control Association, Vol. 23, No. 2, 2007, pp.164-175. doi:10.2987/8756-971X(2007)23[164:DRBFCO]2.0.CO; 2 [3] G. I. Giraldo-Calderón, M. Pérez, C. A. Morales and C. B. Ocampo, “Evaluation of the Triflumuron and the Mixture of Bacillus thuringiensis plus Bacillus sphaericus for Copyright © 2012 SciRes. AiM  A. MAHALAKSHMI ET AL. 225 Control of the Immature Stages of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) in Catch Ba- sins,” Biomedica, Vol. 28, No. 2, 2008, pp. 224-233. [4] K. V. Frankenhuyzen, “Insecticidal Activity of Bacillus thuringiensis Crystal Proteins,” Journal of Invertebrate Pathology, Vol. 101, No. 1, 2009, pp. 1-16. doi:10.1016/j.jip.2009.02.009 [5] N. Raddadi, A. Belaouis, I. Tamagnini, B. M. Hansen, N. B. Hendriksen, A. Boudabous, A. Cherif and D. Daffon- chio, “Characterization of Polyvalent and Safe Bacillus thuringiensis Strains with Potential Use for Biocontrol,” Journal of Basic Microbiology, Vol. 49, No. 3, 2009, pp. 293-303. doi:10.1002/jobm.200800182 [6] G. Armengol, M. C. Escobar, M. E. Maldonado and S. Orduz, “Diversity of Colombian Strains of Bacillus thur- ingiensis with Insecticidal Activity against Dipteran and Lepidopteran Insects,” Journal of Applied Microbiology, Vol. 102, No. 1, 2007, pp. 77-88. doi:10.1111/j.1365-2672.2006.03063.x [7] N. Crickmore, D. R. Zeigler, J. Feitelson, E. Schnepf, J. Van Rie, D. Lereclus, J. Baum and D. H. Dean, “Revision of the Nomenclature for the Bacillus thuringiensis Pesti- cidal Crystal Proteins,” Microbiology and Molecular Bi- ology Reviews, Vol. 62, No. 3, 1998, pp. 807-813. [8] N. Crickmore, D. R. Zeigler, E. Schnepf, J. Van Rie, D. Lereclus, J. Baum, A. Bravo and D. H. Dean, “Bacillus thuringiensis Toxin Nomenclature,” 2009. http://www.lifesci.sussex.ac.uk/Home/Neil_Crickmore/Bt [9] M. Soberon, L. E. Fernández, C. Pérez, S. S. Gill and A. Bravo, “Mode of Action of Mosquitocidal Bacillus thur- ingiensis Toxins,” Toxicon, Vol. 49, No. 5, 2007, pp. 597-600. doi:10.1016/j.toxicon.2006.11.008 [10] A. Bravo, S. S. Gill and M. Soberón, “Mode of Action of Bacillus thuringiensis Cry and Cyt Toxins and Their Po- tential for Insect Control,” Toxicon, Vol. 49, No. 4, 2007, pp. 423-435. doi:10.1016/j.toxicon.2006.11.022 [11] A. Guerchicoff, R. A. Ugalde and C. P. Rubinstein, “Identification and Characterization of a Previously Un- described cyt Gene in Bacillus thuringiensis subsp. is- raelensis,” Applied and Environmental Microbiology, Vol. 63, No. 7, 1997, pp. 2716-2721. [12] D. Wu, J. J. Johnson and B. A. Federeci, “Synergism of Mosquitocidal Toxicity between CytA and CryIV Pro- teins Using Inclusions Produced from Cloned Genes of Bacillus thuringiensis subsp. israelensis,” Molecular Mi- crobiology, Vol. 13, No. 6, 1994, pp. 965-972. doi:10.1111/j.1365-2958.1994.tb00488.x [13] L. Allwin, J. S. Kennedy and V. Radhakrishnan, “Char- acterization of Different Geographical Strains of Bacillus thuringiensis from Tamil Nadu,” Research Journal of Agriculture and Biological Sciences, Vol. 3, No. 5, 2007, pp. 362-366. [14] J. Das and T. K. Dangar, “Diversity of Bacillus thur- ingiensis in the Rice Field Soils of Different Ecologies in India,” Indian Journal of Microbiology, Vol. 47, No. 4, 2007, pp. 364-368. doi:10.1007/s12088-007-0065-z [15] R. Lezama-Gutiérrez, J. J. Hamm, J. Molina-Ochoa, M. López-Edwards, A. Pescador-Rubio, M. González-Ra- mirez and L. E. Styer, “Occurrence of Entomopathogens of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Mexican States of Michoacán, Colima, Jalisco and Tamaulipas,” Florida Entomologist, Vol. 84, No. 1, 2001, pp. 23-30. [16] S. B. Alves, “Isolamento de patógenos,” In: S. B. Alves, Ed., Controle Microbiano de Insetos, Manole, São Paulo, 1986. [17] S. Kalman, K. L. Kiehne, J. L. Libs and T. Yamamoto, “Cloning of a Novel cryIC Type Gene from a Strain of Bacillus thuringiensis subsp. galleriae,” Applied and En- vironmental Microbiology, Vol. 59, No. 4, 1993, pp. 1131-1137. [18] J. E. Ibarra and B. A. Federici , “Parasporal Bodies of Bacillus thuringiensis subsp. morrisoni (PG-14) and Ba- cillus thuringiensis subsp. israelensis are Similar in Pro- tein Composition and Toxicity,” FEMS Microbiology Letters, Vol. 34, No. 1, 2006, pp. 79-84. [19] P. H. Johnson and L. I. Grossman, “Electrophoresis of DNA in Agarose gel. Optimizing Separations of Confor- mational Isomers of Double and Single-Stranded DNAs,” Biochemistry, Vol. 16, No. 19, 1977, pp. 4217-4225. [20] J. Spizizen, “Transformation of Biochemically Deficient Strains of Bacillus subtilis by Deoxyribonucleate,” Pro- ceedings of the National Academy of Sciences USA, Vol. 44, No. 10, 1958, pp. 1072-1078. doi:10.1073/pnas.44.10.1072 [21] M. Meadows, D. Ellis, J. Butt, P. Jarrett and H. Burges, “Distribution, Frequency and Diversity of Bacillus thur- ingiensis in an Animal Feed Mill,” Applied and Environ- mental Microbiology, Vol. 58, No. 4, 1992, pp. 1344- 1350. [22] H. De Barjac and M. M. Lecadet, “Biochemical Deter- mination of Bacillusthuringiensis Thermostabile, Exo- toxin, Using the Inhibition of Bacterial RNA Poly- merases,” Comptes Rendus Hebdomadaires des Seances de l Academie des Sciences. D: Sciences Naturelles, Vol. 282, No. 23, 1976, pp. 2119-2122. [23] W. A. Smirnoff and D. C. O’Connell, “Detection of Poly- hedra from Insect Virus Diseases on Filter Membranes,” Stain Technology, Vol. 37, No. 4, 1962, pp. 207-210. [24] C. Johnson, A. H. Bishop and C. L. Turner, “Isolation and Activity of Strains of Bacillus thuringiensis Toxic to Larvae of the Housefly (Diptera: Muscidae) and Tropical Blowflies,” Journal of Invertebrate Pathology, Vol. 71, No. 2, 1998, pp. 138-144. doi:10.1006/jipa.1997.4720 [25] K. Higuchi, H. Saitoh, E. Mizuki, T. Ichimatsu and M. Ohba, “Larval Susceptibility of the Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae), to Bacillus thuringiensis H Serovars Isolated in Japan,” Microbi- ological Research, Vol. 155, No. 1, 2000, pp. 23-29. doi:10.1016/S0944-5013(00)80018-X [26] D. Finney, “Probit Analysis,” 3rd Edition, Cambridge University Press, Cambridge, 1971. [27] U. K. Laemmli, “Cleavage of Structural Proteins during Assembly of Head of Bacteriophage-T4,” Nature, Vol. 227, 1970, pp. 680-685. doi:10.1038/227680a0 Copyright © 2012 SciRes. AiM  A. MAHALAKSHMI ET AL. Copyright © 2012 SciRes. AiM 226 [28] F. Al-Momani, M. Obeidat, I. Saadoun and M. Meqdam, “Serotyping of Bacillus thuringiensis Isolates, Their Dis- tribution in Different Jordanian Habitats and Pathogenic- ity in Drosophila melanogaster,” World Journal of Mi- crobiology and Biotechnology, Vol. 20, No. 7, 2004, pp. 749-753. doi:10.1007/s11274-004-4517-x [29] J. H. Wang, A. Boets, J. Van Rie and G. X. Ren, “Char- acterization of cry1, cry2 and cry9 Genes in Bacillus thur- ingiensis Isolates from China,” Journal of Invertebrate Pathology, Vol. 82, No. 1, 2003, pp. 63-71. doi:10.1016/S0022-2011(02)00202-1 [30] M. A. Hossain, S. Ahmed and S. Hoque, “Abundance and Distribution of Bacillus thuringiensis in the Agricultural Soil of Bangladesh,” Journal of Invertebrate Pathology, Vol. 70, No. 3, 1997, pp. 221-225. doi:10.1006/jipa.1997.4694 [31] S. Hastowo, B. W. Lay and M. Ohba, “Naturally Occur- ring Bacillus thuringiensis in Indonésia,” Journal of Ap- plied Bacteriology, Vol. 73, No. 2, 1992, pp. 108-113. doi:10.1111/j.1365-2672.1992.tb01695.x [32] K. Bernhard, P. Jarrett, M. Meadows, J. Butt, D. J. Ellis, G. M. Roberts, S. Pauli, P. Rodgers and H. G. Burges, “Natural Isolates of Bacillus thuringiensis: World Wide Distribution, Characterization, and Activity against Insect Pests,” Journal of Invertebrate Pathology, Vol. 70, No. 1, 1997, pp. 59-68. doi:10.1006/jipa.1997.4669 [33] M. Obeidat, “Toxicity of Local Bacillus thuringiensis Isolates against Drosophila melanogaster,” World Jour- nal of Agricultural Sciences, Vol. 4, No. 2, 2008, pp. 161- 167. [34] M. Porcar and V. Juarez-Perez, “PCR Based Identifica- tion of Bacillus thuringiensis Pesticidal Crystal Genes,” FEMS Microbiology Reviews, Vol. 26, 2003, pp. 419-432. doi:10.1111/j.1574-6976.2003.tb00624.x [35] S. Ibanez-Bernal, D. Strickman and C. Martínez-Campos “Culidae (Diptera),” In: J. Llorente Bousquets, A. N. García Aldrete and E. González Soriano, Eds., Biodiver- sidad, Taxonomía y Biogeografía de Artrópodos en México, Universidad Nacional Autónoma, México City, 1996, pp. 591-602. [36] A. Bravo, S. Sarabia, L. Lopez, H. Ontiveros, C. Abarca, A. Ortiz, M. Ortiz, L. Lina, J. V. Francisco, P. Guadalupe, N. V. María-Eugenia, S. Mario and Q. Rodolfo, “Charac- terization of cry Genes in a Mexican Bacillus thuringien- sis Strain Collection,” Applied and Environmental Mi- crobiology, Vol. 64, No. 12, 1998, pp. 4965-4972. [37] A. Uemori, M. Maeda, K. Yasutake, A. Ohgushi, K. Ka- goshima and M. Ohba, “Ubiquity of Parasporin-1 Pro- ducers in Bacillus thuringiensis Natural Population of Japan,” Naturwissenschaften, Vol. 94, No. 1, 2007, pp. 34-38. doi:10.1007/s00114-006-0153-7 [38] M. Ohba, “Bacillus thuringiensis in Nature: Ubiquity and Diversity” In: M. Ohba, O. Nakamura, E. Mizuki and E. Akao, Eds., Proceedings of a Centennial Symposium Commemorating Ishiwata’s Discovery 416 of Bacillus thuringiensis, Kurume, Japan, 2001, pp. 141-145. [39] C. Martinez and P. Caballero, “Contents of cry Gene s a n d Insecticidal Toxicity of Bacillus thuringiensis Strains from Terrestrial and Aquatic Habitats,” Journal of Applied Mi- crobiology, Vol. 92, No. 4, 2002, pp. 745-752. doi:10.1046/j.1365-2672.2002.01579.x
|