 Advances in Microbiology, 2012, 2, 208-215 http://dx.doi.org/10.4236/aim.2012.23025 Published Online September 2012 (http://www.SciRP.org/journal/aim) Biofilm Foptococcus mutans and 2, icultural Science, ai, Japan 2Laboratory ofnce, Tohoku University, Sendai, Japan 3Department of Oral Surgery, Health Sciences University of Hokkaido, Hokkaido, Japan Email: *emiko@bios.tohoku.ac.jp h; it is an infectious sease of the oral cavity in which biofilms play a causative role. Control of biofilms has traditionally relied on lt to remove biofilms by sobrinus are important causative a. They produce a homologous exocellular polysaccharide called glucan, which strongly ad- amel surface. This is a review of oral microbial biofilm formation by S. mutans and other related bacte- to can; c nd cc ica of for t i S. re highly associated with caries in hu o f ent ur at are toxic to most other bacter s are exemplary and serve as a m system for bacterial adhesion [3]. In this review, we describe biofilm formation by S. mutans and related bacteria. 1.1. Dental Plaque and Decalcification in Caries Dental plaque is caused by a complex and dynamic proc- esses that involves the progressive destruction of tooth cteria. Acid forma- mutans drives the n the hydroxyapa- by acid from the ctors including the concentration of the carbon source, the amount and ac- e microflora, the flow rate of saliva, he tooth surface. The major species mutans and S. so- eral mutans strep- ]. The mutans l agents in dental The etiology of caries disease is well established and bacterial colonization appears to be an important step for oral diseases, leading to biofilm formation [6-8]. Oral biofilms mostly consist of multiple bacterial strains. It has been recently shown that more than 700 bacterial strains are present in dental plaque [9]. Specific patho- gens, including Gram-negative bacteria, can cause seri- ous clinical diseases. The microflora in healthy individu- als is dominated by the presence of Gram-positive bacte- *Corresponding author. rmation by Stre Related Bacteria Junko Nishimura1, Tadao Saito1, Hiroshi Yoneyama2, Lan Lan Bai Kazuhiko Okumura3, Emiko Isogai2* 1Laboratory of raduate School of AgrAnimal Products Chemistry, G Tohoku University, Send Animal Microbiology, Graduate School of Agricultural Scie Received April 22, 2012; revised May 29, 2012; accepted June 8, 2012 ABSTRACT Caries is a disease of human dentition characterized by the loss of mineralized surfaces of the toot di non-specific removal of plaque by mechanical means such as brushing, although it is difficu this method. Caries is also a widespread infection in children. Streptococcus mutans and S. gents of caries heres to the en ria. Keywords: Caries; Streptococcus mutans; Strep Glucosyltransferase 1. Introduction Caries has been characterized as an ecological in the mouth, involving infectious bacteria a available sugar in drinks and foods. Streptoco tans has been reported as the principal etiolog of dental cavities and a normal inhabitant plaque [1,2]. Oral microbial biofilms cause to plex bacterial communities based on co-agglu Specific important pathogens in the oral biofilm mutans streptococci, particularly S. mutans and nus, which a coccus sobrinus; Biofilm; Exocellular Polysaccharide; Glu ollision readily us mu- l agent dental soluble polymers of glucose that aid in persist zation of solid surfaces. These bacteria can s enamel, dentine and cementum by ba tion by cariogenic bacteria such as S. dissolution of calcium and phosphate i tite crystal structure. Decalcification bacteria is dependent on a variety of fa m com-tivity of the plaqu and the nature of t ination. nclude sobri- mans. S. orm in- coloni- vive at ial spe- odel found in human dental plaque are S. brinus, S. rattus, S. cricetus and sev tococci are cariogenic in animals [4,5 streptococci are important etiologica caries. 1.2. Role of Biofilms in Caries mutans readily metabolizes dietary sucrose t low pH values th cies. Oral biofilm C opyright © 2012 SciRes. AiM 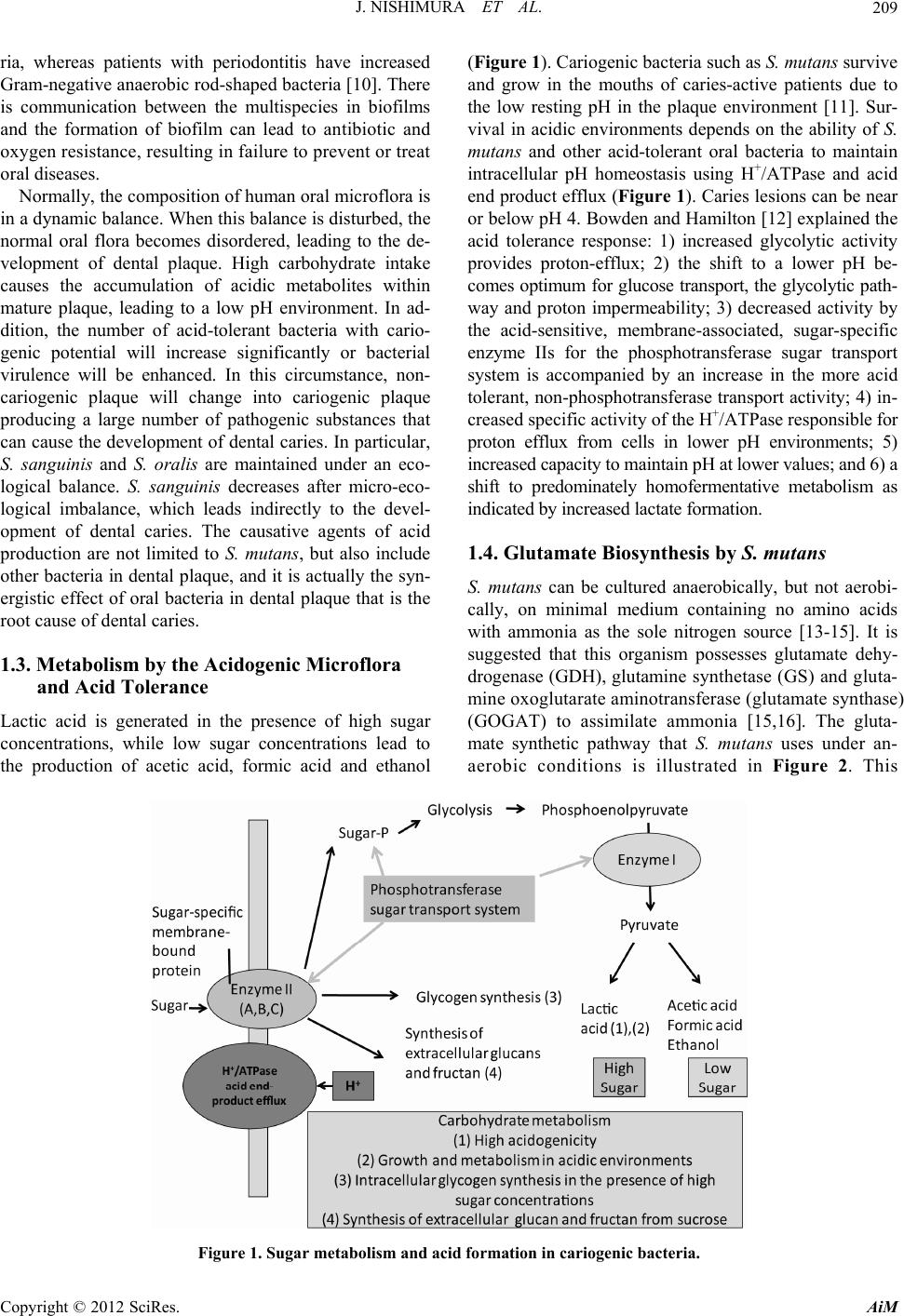 J. NISHIMURA ET AL. 209 ria, whereas patients with periodontitis have in Gram-negative anaerobic rod-shaped bacteria [1 is communication between the multispecies in and the fo creased . There iofilms tic and or treat flora is bed, the the de- intake within . In ad- cario- acterial e, non- plaque ces that ergistic effect of oral bacteria in dental plaque t root cause of dental caries. (Figure 1). Cariogenic bacteria such a and grow in the mouths of caries-ac the low resting pH in the plaque env vival in acidic environments depends mutans and other acid-tolerant oral b intracellular pH homeostasis using H end product efflux (Figure 1). Caries or below pH 4. Bowden and Hamilton acid tolerance response: 1) increased provides proton-efflux; 2) the shift t comes optimum for glucose transport, t way and proton impermeability; 3) de the acid-sensitive, membrane-associ enzyme IIs for the phosphotransfera system is accompanied by an increas tolerant, non-phosphotransferase transpo creased specific activit 0] b rmation of biofilm can lead to antibio nt to ate s nt ith b c ic an a er an ic hich leads indirectly to the ents also ot plaque, and it is actually t ha 1.3. Metabolism by the Acidogenic Microflora and Acid Tolerance Lactic acid is generated in the presence of high sugar concentrations, while low sugar concentrations lead to the production of acetic acid, formic acid and ethanol s S. mutans survive tive patients due to ironment [11]. Sur- on the ability of S. acteria to maintain +/ATPase and acid lesions can be near [12] explained the glycolytic activity o a lower pH be- he glycolytic path- creased activity by ated, sugar-specific se sugar transport e in the more acid rt activity; 4) in- y of the H+/ATPase responsible for ower pH environments; 5) ower values; and 6) a tive metabolism as . lly, but not aerobi- ng no amino acids urce [13-15]. It is suggested that this organism possesses glutamate dehy- drogenase (GDH), glutamine synthetase (GS) and gluta- mine oxoglutarate aminotransferase (glutamate synthase) (GOGAT) to assimilate ammonia [15,16]. The gluta- mate synthetic pathway that S. mu tans uses under an- aerobic conditions is illustrated in Figure 2. This oxygen resistance, resulting in failure to preve oral diseases. Normally, the composition of human oral micro in a dynamic balance. When this balance is distur normal oral flora becomes disordered, leading velopment of dental plaque. High carbohydr causes the accumulation of acidic metabolite mature plaque, leading to a low pH environme dition, the number of acid-tolerant bacteria w genic potential will increase significantly or virulence will be enhanced. In this circumstan cariogenic plaque will change into cariogen producing a large number of pathogenic subst can cause the development of dental caries. In p S. sanguinis and S. oralis are maintained und logical balance. S. sanguinis decreases after m logical imbalance, w rticular, proton efflux from cells in l eco- ro-eco- devel- of acid include he syn- t is the increased capacity to maintain pH at l shift to predominately homofermenta indicated by increased lactate formation 1.4. Glutamate Biosynthesis by S. mutans S. mutans can be cultured anaerobica cally, on minimal medium containi with ammonia as the sole nitrogen so opment of dental caries. The causative ag production are not limited to S. mutans, but her bacteria in dental Figure 1. Sugar metabolism and acid formation in cariogenic bacteria. Copyright © 2012 SciRes. AiM 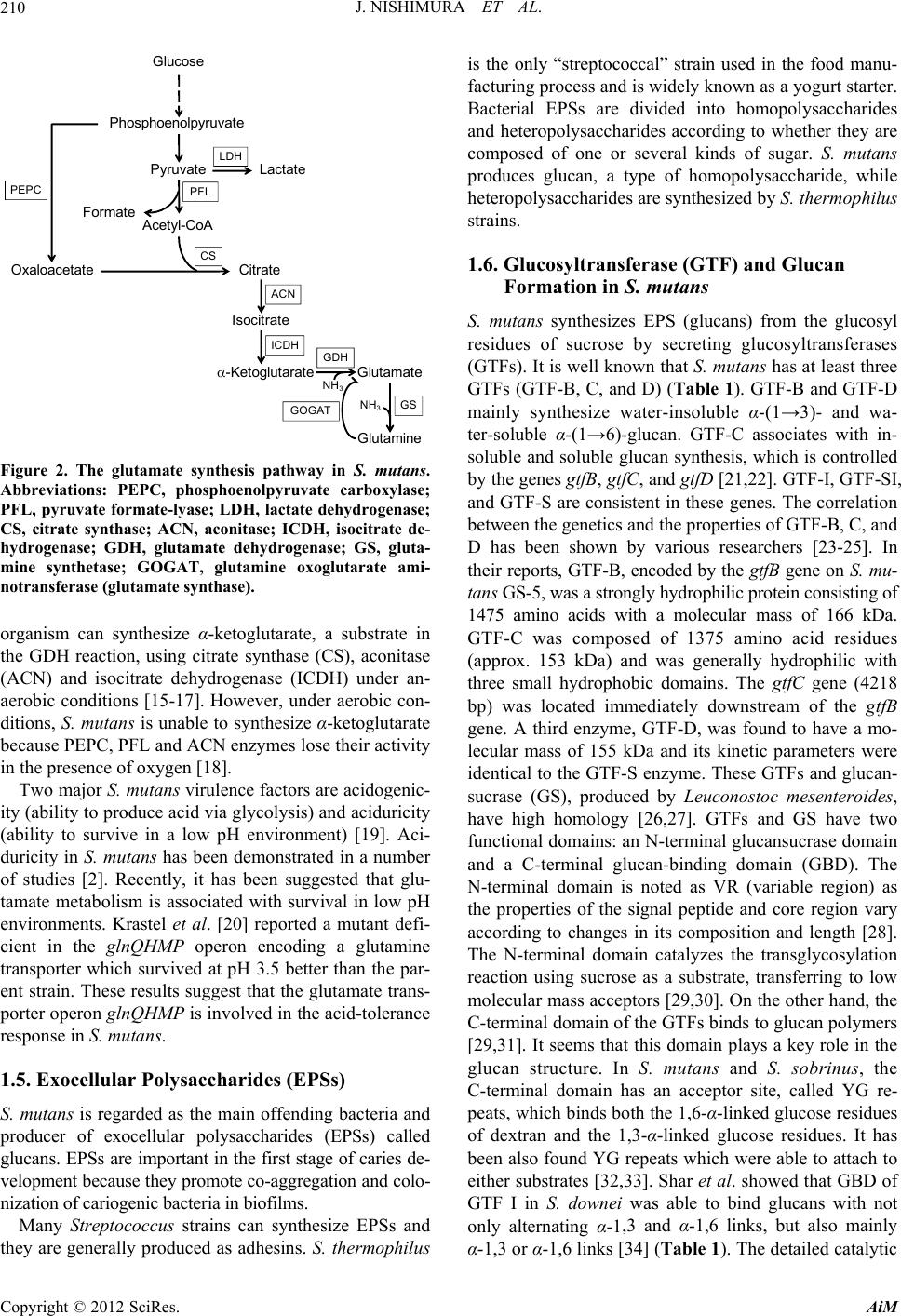 J. NISHIMURA ET AL. 210 Gluc os e Phosphoenolpyr uv ate Pyruvate Lactate Acetyl- CoA Oxaloacetate Citrate Isocitrate LDH -Ketoglutarate Glu Gl t amate utamine PFL PEPC CS ACN ICDH GDH GOGAT GS Formate NH NH 3 3 mutans. oxylase; genase; rate de- gluta- te ami- trate in onitase der an- ic con- lutarate activity ogenic- duricity ]. Aci- number at glu- low pH nt defi- tamine he par- e trans- lerance 1.6. Glucosyltransferase (GTF) a Formation in S. mutans S. mutans synthesizes EPS (glucans) residues of sucrose by secreting g (GTFs). It is well known that S. muta GTFs (GTF-B, C, and D) (Table 1). G mainly synthesize water-insoluble α ter-soluble α-(1→6)-glucan. GTF-C soluble and soluble glucan synthesis, w by the genes gtfB, gtfC, and gtfD [21, and GTF-S are consistent in these genes between the genetics and the propertie D has been shown by various resea their reports, GTF-B, encoded by the tans GS-5, was a strongly hydrophilic 1475 amino acids with a molecular GTF-C was composed of 1375 am (approx. 153 kDa) and was general three small hydrophobic domains. Th bp) was located immediately downs gene. A third enzyme, GTF-D, was fo lecular mass of 155 kDa and its kinetic identical to the GTF-S enzyme. These sucrase (GS), produced by Leucono have high homology [26,27]. GTFs a functional domains: an N-terminal glu and a C-terminal glucan-binding do N-terminal domain is noted as VR (v the properties of the signal peptide a according to changes in its compositi The N-terminal domain catalyzes the tran reaction using sucrose as a substrate, molecular mass acceptors [29,30]. On C-terminal domain of the GTFs binds [29,31]. It seems that this domain pla Figure 2. The glutamate synthesis pathway in Abbreviations: PEPC, phoS. sphoenolpyruvate carb ro it ara α-ketoglutarate, a subs ac n ob tog ir id 19 ans has been demonstrated in a suggested th survival in ta lu t mat to 1.5. Exocellular Polysaccharides (EPSs) S. mutans is regarded as the main offending bacteria and producer of exocellular polysaccharides (EPSs) called glucans. EPSs are important in the first stage of caries de- velopment because they promote co-aggregation and colo- nization of cariogenic bacteria in biofilms. Many Streptococcus strains can synthesize EPSs and they are generally produced as adhesins. S. thermophilus in the food manu- ogurt starter. mopolysaccharides o whether they are f sugar. S. mutans produces glucan, a type of homopolysaccharide, while esized by S. thermophilus nd Glucan from the glucosyl lucosyltransferases ns has at least three TF-B and GTF-D -(1→3)- and wa- associates with in- hich is controlled 22]. GTF-I, GTF-SI, . The correlation s of GTF-B, C, and rchers [23-25]. In gtfB gene on S. mu- protein consisting of mass of 166 kDa. ino acid residues ly hydrophilic with e gtfC gene (4218 tream of the gtfB und to have a mo- parameters were GTFs and glucan- stoc mesenteroides, nd GS have two cansucrase domain main (GBD). The ariable region) as nd core region vary on and length [28]. sglycosylation transferring to low the other hand, the to glucan polymers ys a key role in the glucan structure. In S. mutans and S. sobrinus, the C-terminal domain has an acceptor site, called YG re- peats, which binds both the 1,6-α-linked glucose residues of dextran and the 1,3-α-linked glucose residues. It has been also found YG repeats which were able to attach to either substrates [32,33]. Shar et al. showed that GBD of GTF I in S. downei was able to bind glucans with not only alternating α-1,3 and α-1,6 links, but also mainly α-1,3 or α-1,6 links [34] (Table 1 ). The detailed catalytic PFL, pyruvate formate-lyase; LDH, lactate dehyd CS, citrate synthase; ACN, aconitase; ICDH, isoc hydrogenase; GDH, glutamate dehydrogenase; GS, mine synthetase; GOGAT, glutamine oxoglut notransferase (glutamate synthase). organism can synthesize the GDH reaction, using citrate synthase (CS), (ACN) and isocitrate dehydrogenase (ICDH) u aerobic conditions [15-17]. However, under aer ditions, S. mutans is unable to synthesize α-ke because PEPC, PFL and ACN enzymes lose the in the presence of oxygen [18]. Two major S. mutans virulence factors are ac ity (ability to produce acid via glycolysis) and aci (ability to survive in a low pH environment) [ duricity in S. mut of studies [2]. Recently, it has been tamate metabolism is associated with environments. Krastel et al. [20] reported a mu cient in the glnQHMP operon encoding a g transporter which survived at pH 3.5 better than ent strain. These results suggest that the gluta porter operon glnQHMP is involved in the acid- response in S. mutans. is the only “streptococcal” strain used facturing process and is widely known as a y Bacterial EPSs are divided into ho and heteropolysaccharides according t composed of one or several kinds o heteropolysaccharides are synth strains. Copyright © 2012 SciRes. AiM  J. NISHIMURA ET AL. 211 Table 1. Composition of GTFs attern ns and bacteria. PrProtein si and glucan ps produced from S. mutad other relate Strain otein ze a.a. Gene atio of lucan t e Re erences S. mutans GS-5 G1475 α-(1→3) α-(1→ [23] TF B gtfB 87% 6) 13% S. mutans GS-5 G1375 α-(1→ α-(1→6) 15% [24] GS-5 GTF D 1430 α-(1→ α-(1→6) 70% [25] L. mesenteriodes NRRL B-1299 1290 α-(1→3) α-(1→6) [26] NRRL B-1299 1508 α-(1→3) 5% α-(1→ [27] fe28 G1556 I α-(1→ α-(1→6) [34] S. downei Mfe28 GTF S 1328 α-(1→ α-(1→6) 90% [34] G1592 α-(1→[29,35] nus B13N GTF-S1 gftU α-(1→ α-(1→3,6) [35,36] 1[35,37] TF C gtfC 3) 85% S. mutansgtfD 3) 30% dsrA 15% 85% L. mesenteriodes dsrB 6) 95% S. downei MTF I gtf 3) 88% 12% gftS 3) 10% S. sobrinus 6715TF-I gtfIa 3) S. sobri6) S. sobrinus OMZ176 GTF-S2 S. sobrinus GTF-S 542 gftT α-(1→3) 16% α-(1→6) 73% α-(1→3,6) 5% [35,36] gftS mechanisms and structures of GTFs and GS are th e g ut , G tfI ). fo 0. an dd ,6 it o ot and α-1,6 linkages. On the other hand, GTF-S3 (155 kDa) hydrolyzes sucrose and synthesizes water-soluble α-1,6 glucan (oligo-isomaltosaccharides). Hanada et al. [37] showed that GTF-S2 has three repeated sequences of 51 to 52 amino acids, a partial repeat of 18 amino acids and gtfS present in the region immediately upstream of the gtfT gene. They identified the gtfU gene and GTF-S1 enzyme from the S. sobrinus strain B13N [36]. C-ter- minal fragments of the GBD of S. sobrinus GTF-I were tightly to a stretch tion of individually ompanied by the nthesis was corre- the time course of sized glucans vary re divided into four ain glucosidic linkages: α(1-3) glucosidic utan); α(1-4) glucosidic bonds (reuteran); and nds (alternan). Glu- pes, the degree of s, and their spatial s very little knowl- lucans to date. ctures of EPS in S. thermophilus The sugar components of EPS synthesized from S. ther- mophilus are mainly galactose, glucose, and rhamnose. Furthermore, N-acetylgalactosamine, fucose, and ribose are also components of EPS (Figure 3) [39-49]. Galac- tose includes not only galactopyranose, but also galacto- furanose. S. thermophilus synthesizes EPS via intracellu- lar sugar nucleotide precursors (Figure 4). Metabolic flux via sugar nucleotide precursors of EPS in S. the r- not yet at both lucans, ans for TF-S1, prepared and shown that GBD bound of dextran chain through the combina weak subsite/glucose interactions acc entropy change. Recently, glucan sy lated with its kinetic properties and saccharide production [38]. The sizes and structures of synthe according to the strain. Glucans a types based on their different m fully understood. Recently, it has been found GTF-B and GTF-C are necessary to synthesiz and it is important to activate GTF-B on S. m formation of microcolonies [21]. 1.7. Glucan from S. sobrinus Four types of GTFs occur in S. sobrinus, GTF-I GTF-S2, and GTF-S3, which are encoded by g gtfT, and gtfS, respectively [35,36] (Table 1 level of homology is recognized between the zymes and all members of GS proteins GH7 (175 kDa) synthesizes water-insoluble gluc glucan). GTF-S1 (GTF-U) is activated by the a water-soluble glucan (α-1,6 glucan with α-1,3 linkages) and GTF-S2 produces α-1,6 glucan w glucan. These two enzymes can hydrolyze sucr synthesize water-soluble glucan containing b , gtfU, A high α(1-6) glucosidic bonds (dextran); bonds (m ur en- GTF-I (α-1,3 ition of branch h α-1,3 se and h α-1,3 both α(1-6) and α(1-3) glucosidic bo cans vary due to branch linkage ty branching, the length of branch chain arrangement [22]. However, there i edge about the detailed structure of g 1.8. Sugar Components and Stru Copyright © 2012 SciRes. AiM  J. NISHIMURA ET AL. 212 →3)-- D -G alp-(1→4)-- D -G lcp-(1→4)-- D -Glcp-(1→6)- - D -G l cp-(1→ - D -G l cp-(1→4) - D -G alf-(1→6)Strain: ST1 [39] →3)-- D -G alp-(1→3)-- D -G alp-(1→3)-- L -Rhap-(1 →2) -- L -R hap-(1→2)-- D -G alp-(1 - D -G al f2Ac 0.4 -(1 →6) → Str ain: S3 [41] →6)-- D -G al p-(1→6)-- D -G al p-(1→3)-- L -Rh ap-(1→4) -- D -G l cp-(1→6)-- D -G alf-(1→6) - - L -Rha p-(1 →2) -D -G lcp-(1→ Strain: EU20 [42] →3)-- D -G alp-(1→4)-- D -G lcp-(1→ - D -G alp-(1→4)-- D -G lcp-(1→ 6)-- D -G lcp-(1→4) Strai n: THS [40] →3)--D-Galp-(1→3) --D-G lcp-(1 →3)--D-G al pNAc-(1→ -D-G alp-(1 →6) →3)--D-Glcp-(1→3)--D-Glcp-(1→3)--D-G al f-(1 → -D-Ga l p-(1→6) Strain: Sfi39[46]; SY89, SY10 2[45 ] →2)--D-G alp-(1 →3) --D-G a lp-(1 →3)--D-G alp-(1→3)--L-Rhap-(1→2) --L -D-Galp-(1 →6)--D-Galp-(1→4) -R ha p-(1→ Strain: MR-1C [47]L-F uc-(1 →3) Str ain: OR901[ 48]; Rs , Sts [49]-D-Galp-(1 →6)--D-Galp-(1→ →2)--D-G alp-(1 →3) --D-G a lp-(1 →3)--D-G alp-(1→3)--L-Rhap-(1→2) --L-Rha p-(1→ 4) Strain: Sfi12 [46] →2)--L-R ha f-(1→2)--D-Galp-(1→3)--D-Glcp-(1→3) --D-Ga l p-(1→3)- -L-R haf-(1→ -D-Galp-(1 →4) Str ain: Sfi6[ 43]; Sfi20[4 4]; IMDO1,2, 3, NCF B859, 21[4 5] Figure 3. Structures of exocellular polysaccharide produced from S. thermophilus. Copyright © 2012 SciRes. AiM 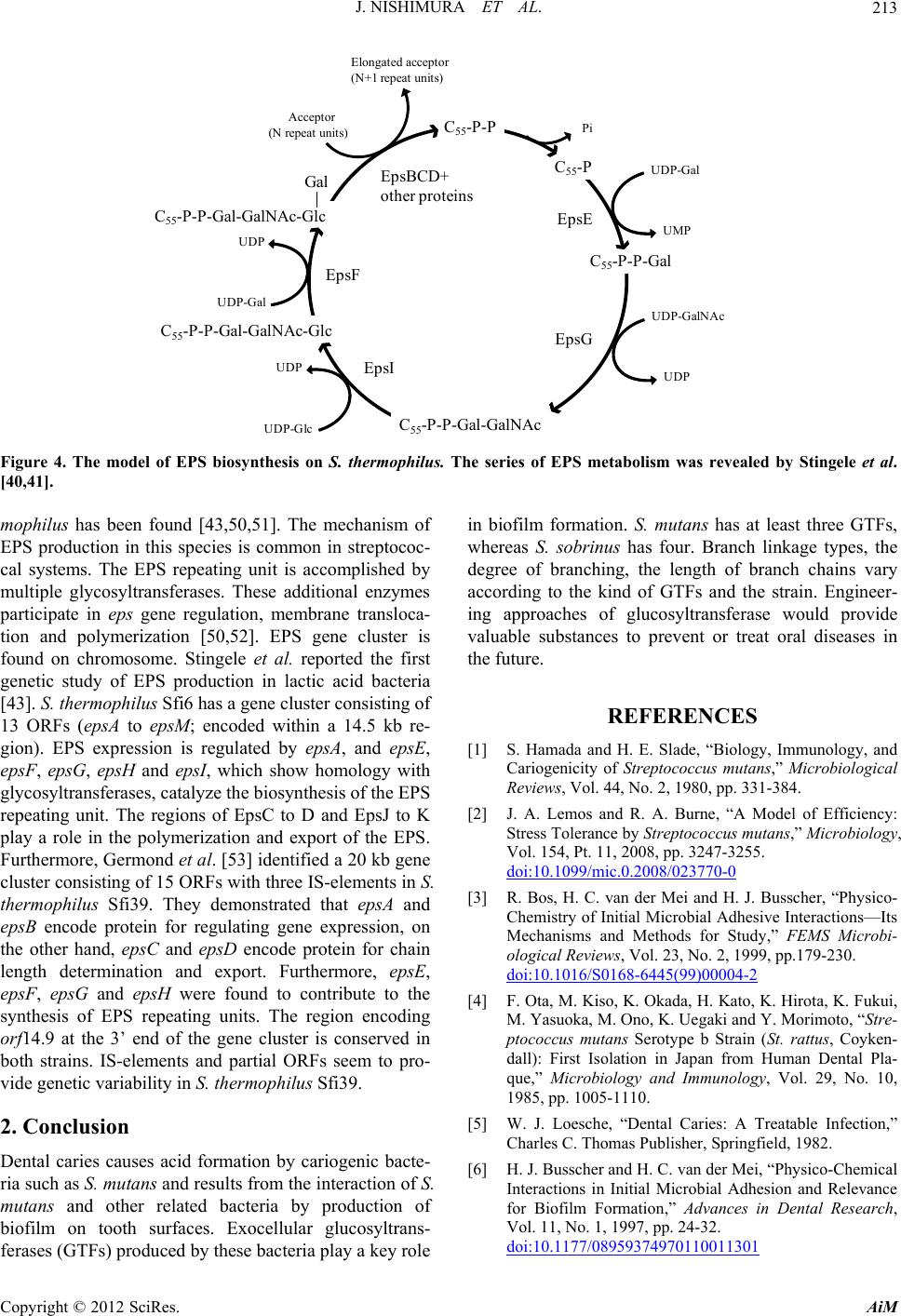 J. NISHIMURA ET AL. 213 C 55 -P-P C 55 -P C 55 -P-P-Gal C 55 -P-P-Gal-GalNAc C 55 -P-P-Gal-GalNAc-Glc C 55 -P-P-Gal-GalNAc-Glc Gal UDP UDP-Gal UDP-GalNAc Pi Elongated acceptor (N+1 repeat units) Acceptor (N repeat units) EpsE EpsG EpsF EpsBCD+ other proteins UDP UDP UDP-Glc UMP UDP-Gal EpsI er ed by Stingele et al. a re l enzymes d th protein for regulating gene d, epsC and epsD encode prot both strains. IS-elements and partial ORFs seem to pro- vide genetic variability in S. thermophilus Sfi39. 2. Conclusion Dental caries causes acid formation by cariogenic bacte- ria such as S. mutans and results from the interaction of S. mutans and other related bacteria by production of biofilm on tooth surfaces. Exocellular glucosyltrans- ferases (GTFs) produced by these bacteria play a key role t least three GTFs, whereas S. sobrinus has four. Branch linkage types, the degree of branching, the length of branch chains vary acc of GTFs and the strain. Engineer- se would provide eat oral diseases in RENCES gy, Immunology, and ans,” Microbiological pp. 331-384. Model of Efficiency: ,” Microbiology, 5. Figure 4. The model of EPS biosynthesis on S. th [40,41]. mophilus has been found [43,50,51]. The mech EPS production in this species is common in st cal systems. The EPS repeating unit is accomplished by multiple glycosyltransferases. These additiona participate in eps gene regulation, membrane tion and polymerization [50,52]. EPS gene found on chromosome. Stingele et al. reporte genetic study of EPS production in lactic acid [43]. S. thermophilus Sfi6 has a gene cluster con 13 ORFs (epsA to epsM ; encoded within a 14 gion). EPS expression is regulated by epsA, epsF, epsG, epsH and epsI, which show homo glycosyltransferases, catalyze the biosynthesis o repeating unit. The regions of EpsC to D and E play a role in the polymerization and export of Furthermore, Germond et al. [53] identified a 2 cluster consisting of 15 ORFs with three IS-ele thermophilus Sfi39. They demonstrate mophilus. The series of EPS metabolism was reveal nism of ptococ- length determination and export. Furthermo epsF, epsG and epsH were found to contribu synthesis of EPS repeating units. The region orf14.9 at the 3’ end of the gene cluster is conse in biofilm formation. S. mutans has a transloca- cluster is first ing approaches of glucosyltransfera valuable substances to prevent or tr the future. d the bacteria sisting of .5 kb re-REFE and epsE, logy with f the EPS o K [1] S. Hamada and H. E. Slade, “Biolo Cariogenicity of Streptococcus mut Reviews, Vol. 44, No. 2, 1980, [2] J. A. Lemos and R psJ t the EPS. 0 kb gene ments in S. at epsA and . A. Burne, “A Stress Tolerance by Streptococcus mutans Vol. 154, Pt. 11, 2008, pp. 3247-325 epsB encode the other han expression, on ein for chain re, epsE, te to the encoding rved in ording to the kind doi:10.1099/mic.0.2008/023770-0 [3] R. Bos, H. C. van der Mei and H. J. Chem Busscher, “Physico- istry of Initial Microbial Adhesive Interactions—Its y,” FEMS Microbi- o. 2, 1999, pp.179-230. 2 Mechanisms and Methods for Stud ological Reviews, Vol. 23, N doi:10.1016/S0168-6445(99)00004- , K. Hirota, K. Fukui, . Ono, K. Uegaki and Y. Morimoto, “Stre- rotype b Strain (St. rattus, Coyken- dall): First Isolation in Japan from Human Dental Pla- que,” Microbiology and Immunology, Vol. 29, No. 10, 1985, pp. 1005-1110. [5] W. J. Loesche, “Dental Caries: A Treatable Infection,” Charles C. Thomas Publisher, Springfield, 1982. [6] H. J. Busscher and H. C. van der Mei, “Physico-Chemical Interactions in Initial Microbial Adhesion and Relevance for Biofilm Formation,” Advances in Dental Research, Vol. 11, No. 1, 1997, pp. 24-32. doi:10.1177/08959374970110011301 [4] F. Ota, M. Kiso, K. Okada, H. Kato M. Yasuoka, M ptococcus mutans Se Copyright © 2012 SciRes. AiM  J. NISHIMURA ET AL. 214 [7] W. J. Loesche, “Role of Streptococcus mutans in Human 50, No. 4, Dental Decay,” Microbiological Reviews, Vol. 1986, pp. 353-380. [8] D. H. Meyer and P. M. Fives-Taylor, from Dental Plaque to Cardia “Oral Pathogens: nt Opinion . c Disease,” Curre in Microbiology, Vol. 1, No. 1, 1998, pp. 88-95 doi:10.1016/S1369-5274(98)80147-1 [9] J. A. Aas, B. J. Paster Dewhirst, “Defining the Norm , L. N. Stokes, I. Olse al Bacteria n and F. E. l Flora of the y, Vol. 43, Oral Cavity,” Journal of Clinical Microbiolog No. 11, 2005, pp. 5721-5732. doi:10.1128/JCM.43.11.5721-5732.2005 [10] K. Hojo, S. Nagaoka, T. Ohshima and N. Maed rial Interactions in Dental Biofilm Developmen of Dental Resea a, “Bacte- t,” Journal . 982-990. rch, Vol. 88, No. 11, 2009, pp doi:10.1177/0022034509346811 [11] J. van Houte, “Bacterial Specificity in the E Dental Caries,” International tiology of , Vol. 30, No. al of Oral and Medicine, Dental Journal iews in Oral Biology 4, 1980, pp. 305-326. [12] G. H. Bowden and I. R. Hamilton, “Surviv Bacteria,” Critical Rev Vol. 9, No. 1, 1998, pp. 54-85. doi:10.1177/10454411980090010401 [13] J. Carisson, “Nutritional Requirements of Streptococcus 0, pp. 305- mutans,” Caries Research, Vol. 4, No. 4, 197 320. doi:10.1159/000259653 [14] B. Terleckj and D. Shockman, “Amino nd J. Carlsson, “Mechanism of Acid Require- ments of Streptococcus mutans and Other O cocci,” Infection and Immunity, Vol. 11, No. 4 656-664. [15] C. J. Griffith a ral Strepto- , 1975, pp. Ammonia ral Micro -Assimilation in Streptococci,” Journal of Gene biology, Vol. 82, No. 2, 1974, pp. 253-260. doi:10.1099/00221287-82-2-253 [16] E. J. St. Martin and C. L. Wittenberger, “Reg Function of Ammonia-Assimilating Enzymes coccus mutans,” Infection and Immunity, Vol. 1980, pp. 220-224. ulation and in Strepto- 28, No. 1, [17] D. G. Cvitkovitch, J. A. Gutierrez and A. S “Role of the Citrate Pathway in Glutamate Bi by Streptococcus mutans,” Journal of Bacteri 179, No . Bleiweis, osynthesis ology, Vol. . 3, 1997, pp. 650-655. us ty, Vol. 55, No. 3, 1987 of Cariogenic Streptococci to Environmental Stresses,” Current Issues in Molecular Biology, Vol. 7, No. 1, 2005, pp. 95-107. [20] K. Krastel, D. B. Senadheera, R. Mair, J. S. Downey, S. D. Goodman and D. G. Cvvitkovitch, “Characterization of a Glutamate Transporter Operon, glnQHMP, in Strep - tococcus mutans and Its Role in Acid Tolerance,” Journal of Bacteriology, Vol. 192, No. 4, 2010, pp. 984-993. doi:10.1128/JB.01169-09 [18] N. Takahashi, K. Abbe, S. Takahashi-Abbe a mada, “Oxygen Sensitivity of Sugar Metabolis terconversion of Pyruvate Formate-Lyase in I of Streptococcus mutans and Streptococc fection and Immuni nd T. Ya- m and In- ntact Cells sanguis,” In- , pp. 652-656. “Responses [19] J. A. Lemos, J. Abranches and R. A. Burne, [21] H. Koo, J. Xiao, M. I. Klein and J. G. Jeon, “Exopoly- cus mutans Glucosyl- ocolonies ultispecies Biofilms,” Journal of Bacteriology, 32. saccharides Produced by Streptococ transferases Modulate the Establishment of Micr within M Vol. 192, No. 12, 2010, pp. 3024-30 doi:10.1128/JB.01649-09 [22] V. Monchois, R. M. Willemot and P. Monsan, “Glucan- d Structure-Function y Reviews, Vol. 23, [23] T. Shiroza, S. Ueda and H. K. Kuramitsu, “Sequence eptococcus mutans,” o. 9, 1987, pp. 4263- uramitsu, “Sequence s mutans pp. 101-109. 4 sucrases: Mechanism of Action an Relationships,” FEMS Microbiolog 1999, pp. 131-151. Analysis of the gtfB Gene from Str Journal of Bacteriology, Vol. 169, N 4270. [24] S. Ueda, T. Shiroza and H. K. K Analysis of the gtfC Gene from Streptococcu GS-5,” Gene, Vol. 69, No. 1, 1988, doi:10.1016/0378-1119(88)90382- solation and Charac- ns gtfD Gene, Coding an Synthesis,” Infec- 89, pp. 2079-2085. Remaud-Simeon, C. nd Sequencing of a sucrase from Leuco- 9 Synthesizing Only , Vol. 182, No. 1-2, 0.1016/S0378-1119(96)00443-X [25] N. Hanada and H. K. Kuramitsu, “I terization of the Streptococcus muta for Primer-Dependent Soluble Gluc tion and Immunity, Vol. 57, No. 7, 19 [26] V. Monchois, R. M. Willemot, M. Croux and P. Monsan, “Cloning a Gene Coding for a Novel Dextran nostoc mesenteroides NRRL B-129 α(1-6) and α(1-3) Linkages,” Gene 1996, pp. 23-32. doi:1 P. Monsan and R. M. of a Gene Coding for SRB) from Leuco- Synthesizing Only a iology Letters, Vol. 159, 76.x [27] V. Monchois, M. Remaud-Simeon, Willemot, “Cloning and Sequencing an Extracellular Dextransucrase (D nostoc mesenteroides NRRL B-1299 α(1-6) Glucan,” FEMS Microb No. 2, 1998, pp. 307-315. doi:10.1111/j.1574-6968.1998.tb128 zimek, L. Dijkhuizen cture-Function Rela- tansucrase Enzymes ology and Molecular , No. 1, 2006, pp. 157-176. 006 [28] S. A. van Hijum, S. Kralj, L. K. O and I. G. van Geel-Schutten, “Stru tionships of Glucansucrase and Fruc from Lactic Acid Bacteria,” Microbi Biology Reviews, Vol. 70 doi:10.1128/MMBR.70.1.157-176.2 , A. Miyagi, H. Ohta, o, H. Matsuno, H. Komatsu, T. e and Enzymatic Pro- rms of the Water-In- osyltransferase from Biochemistry, Vol. 126, No. 2, 1999, pp. 287-295. .a022447 [29] N. Konishi, Y. Torii, T. Yamamoto K. Fukui, S. Hanamot Kodama and E. Katayama, “Structur perties of Genetically Truncated Fo soluble Glucan-Synthesizing Gluc Streptococcus sobrinus,” Journal of doi:10.1093/oxfordjournals.jbchem cosyltransferases,” In: D. Sigman, Ed., The Enzymes, 3rd Edition, Vol. XX, Aca- demic Press, New York, 1992, pp. 187-233. [31] H. Abo, T. Matsumura, T. Kodama, H. Ohta, K. Fukui, K. Kato and H. Kagawa, “Peptide Sequences for Sucrose Splitting and Glucan Binding within Streptococcus so- brinus Glucosyltransferase (Water-Insoluble Glucan Syn- thetase),” Journal of Bacteriology, Vol. 173, No. 3, 1991, pp. 989-996. [32] P. M. Giffard and N. A. Jacques, “Definition of a Funda- mental Repeating Unit in Streptococcal Glucosyltrans- [30] G. Mooser, “Glycosidases and gly Copyright © 2012 SciRes. AiM  J. NISHIMURA ET AL. Copyright © 2012 SciRes. AiM 215 tifs and Glucan ptococci and ferase Glucan-Binding Regions and Related Sequences,” 1994, pp. Journal of Dental Research, Vol. 73, No. 6, 1133-1141. [33] K. B. Kingston, D. M. Allen and N. A. Jacques the C-Terminal YG Repeats of the Primer- Streptoco , “Role of Dependent inding to t. 2, 2002, ccal Glucosyltransferase, GtfJ, in B Dextran and Mutan,” Mic rob iolog y, Vol. 148, P pp. 549-558. [34] D. S. Shah, G. Joucla, M. Remaud-Simeon Russell, “Conserved Repeat Mo by Glucansucrases of Oral Stre and R. R. Binding Leuconostoc . 186, No. mesenteroides,” Journal of Bacteriology, Vol 24, 2004, pp. 8301-8308. doi:10.1128/JB.186.24.8301-8308.2004 [35] A. Nanbu, M. Hayakawa, K. Takada, N. Shi Abiko and K. Fukushima, “Prod nozaki, Y. aracterization, Dis- eptococcus icrobiology, uction, Ch and Application of Monoclonal Antibodies Which tinguish Four Glucosyltransferases from Str sobrinus,” FEMS Immunology and Medical M Vol. 27, No. 1, 2000, pp. 9-15. doi:10.1111/j.1574-695X.2000.tb01405.x [36] N. Hanada, K. Fukushima, Y. Nomura, H. Senpuku, M. ko, “Clon-Hayakawa, H. Mukasa, T. Shiroza and Y. Abi ing and Nucleotide Sequence Analysis of the cus sobrinus gtfU Gene that Produces a Highly Water-Soluble Glucan,” Biochimica et Bioph Streptococ- Branched ysica Acta, Vol. 1570, 2001, pp. 75-79. doi:10.1016/S0304-4165(01)00240-9 [37] N. Hanada, Y. Isobe, Y. Aizawa, T. Katayam and M. Inoue, “Nucleotide Sequence Analys Gene from Streptococcus sob a, S. Sato is of the gtfT Infection 103. rinus OMZ176,” sobrinus Glucosyl and Immunity, Vol. 61, No. 5, 1993, pp. 2096-2 [38] H. Komatsu, Y. Abe, K. Eguchi, H. Matsuno, oka, T. Sadakane, T. Inoue, K. Fukui and T “Kinetics of Dextran-Independent α-(1→3)-Glu thesis by Streptococcus Y. Matsu- . Kodama, can Syn- transferase I,” 31-540. The FEBS Journal, Vol. 278, No. 3, 2011, pp. 5 doi:10.1111/j.1742-4658.2010.07973.x [39] E. Säwén, E. Huttunen, X. Zhang, Z. Yang an malm, “Structural Analysis of d G. Wid- accharide Pro- the Exopolys duced by Streptococcus thermophilus ST1 Sole Spectroscopy,” Journal of Biomolecular NMR No. 2, 2010, pp. 125-134. ly by NMR , Vol. 47, doi:10.1007/s10858-010-9413-0 [40] E. L. Nordmark, Z. Yang, E. Huttunen and G. “Structural Studies of an Exopolysaccharide Pr Streptococcus thermophilus THS,” Biomacro Vol. 6, No. 1, 2005, pp. 105-108. Widmalm, oduced by molecules, doi:10.1021/bm0496514 [41] E. J. Faber, M. J. van den Haak, J. P. Kamerling and J. F. Vliegenthart, “Structure of the Exopolysaccharide Pro- duced by Streptococcus thermophilus S3,” Carbohydrate research, Vol. 331, No. 2, 2001, pp.173-182. doi:10.1016/S0008-6215(01)00013-1 [42] V. M. Marshall, H. Dunn, M. Elvin, N. McLay, Y. Gu and A. P. Laws, “Structural Characterisation of the Exo- polysaccharide Produced by Streptococcus thermophilus EU20,” Carbohydrate Research, Vol. 331, No. 4, 2001, 5(01)00052-0pp. 413-422. doi:10.1016/S0008-621 ollet, “Identification saccharide) Gene philus Sfi6,” Journal 6, pp. 1680-1690. rtocchi, E. Conti, M. ride Produced by Streptococcus ther- of Biological 1, pp. 219-226. 0 [43] F. Stingele, J. R. Neeser and B. M and Characterization of the eps (Exopoly Cluster from Streptococcus thermo of Bacteriology, Vol. 178, No. 6, 199 [44] L. Navarini, A. Abatangelo, C. Be Bosco and F. Picotti, “Isolation and Characterization of the Exopolysaccha mophilus SFi20,” International Journal Macromolecules, Vol. 28, No. 3, 200 doi:10.1016/S0141-8130(01)00118- F. Levander, P. Råd- geest, F. Vaningelgem, H. “Exopolysaccharide-Producing Strains a Cluster into Groups ” Letters in Applied pp. 433-437. 7.x [45] V. M. Marshall, A. P. Laws, Y. Gu, ström, L. De Vuyst, B. De Dunn and M. Elvin, of Thermophilic Lactic Acid Bacteri According to Their EPS Structure, Microbiology, Vol. 32, No. 6, 2001, doi:10.1046/j.1472-765X.2001.0093 [46] J. Lemoine, F. Chirat, J. M. Wieruszeski, G. Strecker, N. l Characterization of duced by Streptococ- ,” Applied and Envi- o. 9, 1997, pp. 3512- . J. McMahon, C. J. f Streptococcus ther- saccharide in Cheese vironmental Micro- 7-2151. ra, T. Shinnai and H. of the Exocellular coccus thermophilus , No. 1-2, 6215(97)00083-9 Favre and J. R. Neeser, “Structura the Exocellular Polysaccharides Pro cus thermophilus SFi39 and SFi12 ronmental Microbiology, Vol. 63, N 3518. [47] D. Low, J. A. Ahlgren, D. Horne, D Oberg and J. R. Broadbent, “Role o mophilus MR-1C Capsular Exopoly Moisture Retention,” Applied and En biology, Vol. 64, No. 6,1998, pp. 214 [48] W. A. Bubb, T. Urashima, R. Fujiwa Ariga, “Structural Characterisation Polysaccharide Produced by Strepto OR 901,” Carbohydrate research, Vol. 301 1997, pp. 41-50. doi:10.1016/S0008- ng and J. F. Vliegen- y Streptococ- nd Sts Have the Same Repeating ir Milk Cultures,” o. 4, 1998, pp. 269- 189-X [49] E. J. Faber, P. Zoon, J. P. Kamerli thart, “The Exopolysaccharides Produced b cus thermophilus Rs a Unit but Differ in Viscosity of The Carbohydrate Research, Vol. 310, N 276. doi:10.1016/S0008-6215(98)00 nt, D. J. McMahon, D. L. Welker, C. J. istry, Genetics, and Production in Strep- iew,” Journal of Dairy 9-4 [50] J. R. Broadbe Oberg and S. Moineau, “Biochem Applications of Exopolysaccharide tococcus thermophilus: A Rev Science, Vol. 86, No. 2, 2003, pp. 407-423. doi:10.3168/jds.S0022-0302(03)7361 . Neeser, “Unraveling es in Streptococcus i6,” Journal of Bacteriology, Vol. 181, No. 20, 1999, pp. 6354-6360. [52] A. D. Welman and I. S. Maddox, “Exopolysaccharides from Lactic Acid Bacteria: Perspectives and Challenges,” Trends in Biotechnology, Vol. 21, No. 6, pp. 269-274. doi:10.1016/S0167-7799(03)00107-0 [51] F. Stingele, J. W. Newell and J. R the Function of Glycosyltransferas thermophilus Sf [53] J. E. Germond, M. Delley, N. D’Amico and S. J. Vincent, “Heterologous Expression and Characterization of the Exopolysaccharide from St re ptococcus thermophilus Sfi39,” European Journal of Biochemistry/FEBS, Vol. 268, No. 19, 2001, pp. 5149-5156.
|