J. Yang et al. / Advances in Bioscience and Biotechnology 3 (2012) 603-608 607
0
0.2
0.4
0.6
0.8
1
1.2
1.4
0‐10 10‐20 20‐30 30‐40 40‐50 50‐60 60‐70 70‐80 90‐100
Time(min)
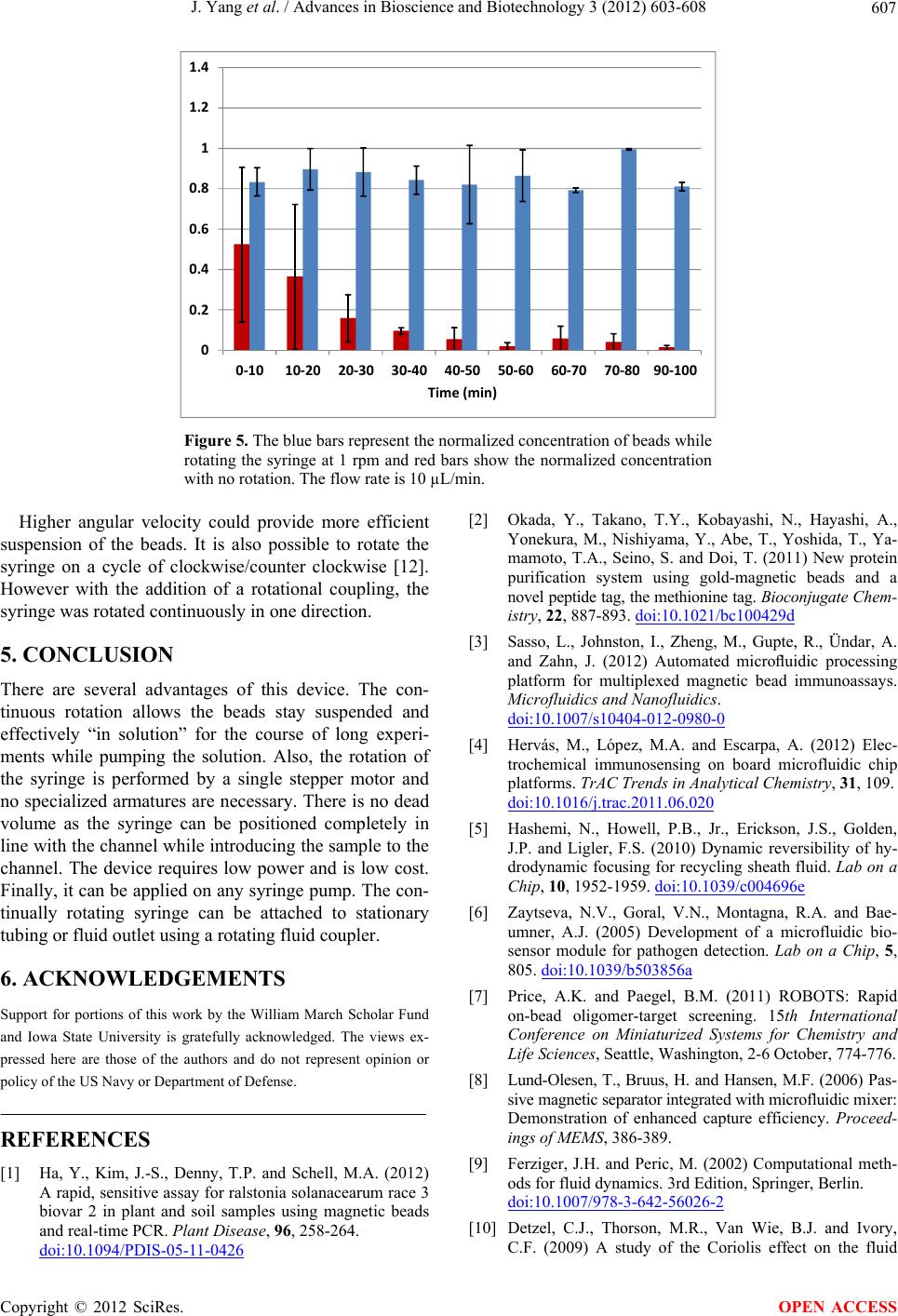
Figure 5. The blue bars represent the normalized concentration of beads while
rotating the syringe at 1 rpm and red bars show the normalized concentration
with no rotation. The flow rate is 10 µL/min.
Higher angular velocity could provide more efficient
suspension of the beads. It is also possible to rotate the
syringe on a cycle of clockwise/counter clockwise [12].
However with the addition of a rotational coupling, the
syringe was rotated continuously in one direction.
5. CONCLUSION
There are several advantages of this device. The con-
tinuous rotation allows the beads stay suspended and
effectively “in solution” for the course of long experi-
ments while pumping the solution. Also, the rotation of
the syringe is performed by a single stepper motor and
no specialized armatures are necessary. There is no dead
volume as the syringe can be positioned completely in
line with the channel while introducing the sample to the
channel. The device requires low power and is low cost.
Finally, it can be applied on any syringe pump. The con-
tinually rotating syringe can be attached to stationary
tubing or fluid outlet using a rotating fluid coupler.
6. ACKNOWLEDGEMENTS
Support for portions of this work by the William March Scholar Fund
and Iowa State University is gratefully acknowledged. The views ex-
pressed here are those of the authors and do not represent opinion or
policy of the US Navy or Department of Defense.
REFERENCES
[1] Ha, Y., Kim, J.-S., Denny, T.P. and Schell, M.A. (2012)
A rapid, sensitive assay for ralstonia solanacearum race 3
biovar 2 in plant and soil samples using magnetic beads
and real-time PCR. Plant Disease, 96, 258-264.
doi:10.1094/PDIS-05-11-0426
[2] Okada, Y., Takano, T.Y., Kobayashi, N., Hayashi, A.,
Yonekura, M., Nishiyama, Y., Abe, T., Yoshida, T., Ya-
mamoto, T.A., Seino, S. and Doi, T. (2011) New protein
purification system using gold-magnetic beads and a
novel peptide tag, the methionine tag. Bioconjugate Chem-
istry, 22, 887-893. doi:10.1021/bc100429d
[3] Sasso, L., Johnston, I., Zheng, M., Gupte, R., Ündar, A.
and Zahn, J. (2012) Automated microfluidic processing
platform for multiplexed magnetic bead immunoassays.
Microfluidics and Nanofluidics.
doi:10.1007/s10404-012-0980-0
[4] Hervás, M., López, M.A. and Escarpa, A. (2012) Elec-
trochemical immunosensing on board microfluidic chip
platforms. TrAC Trends in Analytical Chemistry, 31, 109.
doi:10.1016/j.trac.2011.06.020
[5] Hashemi, N., Howell, P.B., Jr., Erickson, J.S., Golden,
J.P. and Ligler, F.S. (2010) Dynamic reversibility of hy-
drodynamic focusing for recycling sheath fluid. Lab on a
Chip, 10, 1952-1959. doi:10.1039/c004696e
[6] Zaytseva, N.V., Goral, V.N., Montagna, R.A. and Bae-
umner, A.J. (2005) Development of a microfluidic bio-
sensor module for pathogen detection. Lab on a Chip, 5,
805. doi:10.1039/b503856a
[7] Price, A.K. and Paegel, B.M. (2011) ROBOTS: Rapid
on-bead oligomer-target screening. 15th International
Conference on Miniaturized Systems for Chemistry and
Life Sciences, Seattle, Washington, 2-6 October, 774-776.
[8] Lund-Olesen, T., Bruus, H. and Hansen, M.F. (2006) Pas-
sive magnetic separator integrated with microfluidic mixer:
Demonstration of enhanced capture efficiency. Proceed-
ings of MEMS, 386-389.
[9] Ferziger, J.H. and Peric, M. (2002) Computational meth-
ods for fluid dynamics. 3rd Edition, Springer, Berlin.
doi:10.1007/978-3-642-56026-2
[10] Detzel, C.J., Thorson, M.R., Van Wie, B.J. and Ivory,
C.F. (2009) A study of the Coriolis effect on the fluid
Copyright © 2012 SciRes. OPEN ACCESS