 Neuroscience & Medicine, 2012, 3, 287-293 http://dx.doi.org/10.4236/nm.2012.33033 Published Online September 2012 (http://www.SciRP.org/journal/nm) 287 Growth Hormone Prevents the Memory Def ici t C aus ed by Oxidative Stress in Early Neurodegenerative Stage in Rats Diana Verónica Castillo-Padilla1, Gabino Borgornio-Pérez1, Alejandro Zentella-Dehesa2,3, Adrian Sandoval-Montiel3, José Luis Ventura Gallegos3, Selva Rivas-Arancibia1 1Facultad de Medicina, Departamento de Fisiología, Universidad Nacional Autónoma de México, Ciudad Universitaria, México; 2Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Ciudad Universitaria, México; 3Unidad de Bioquímica, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Tlalpan, México. Email: srivas@unam.mx. Received May 3rd, 2012; revised June 5th, 2012; accepted June 12th, 2012 ABSTRACT Oxidative stress has been involved in neurodegenerative diseases. The growth hormone (GH) counteracts the levels of reactive oxygen species. Previously, we showed that the prolonged exposure to ozone causes oxidative stress in the hippocampus and memory deficits. In this work, we analyzed the effects of the growth hormone on the memory deficit generated by ozone exposure, growth hormone effects on the Insulin-like growth factor I (IGF-I), and the serine- threonine protein kinase (Akt) activation in the dentate gyrus. Our results show that GH prevents memory deficits in early stages of the neurodegenerative process. Keywords: Oxidative Stress; Growth Hormone; Insulin Growth Factor; Ozone, Serine-Threonine Protein Kinase; Water Maze; Passive Avoidance 1. Introduction Oxidative stress in cells is generated because of an im- balance between the reactive oxygen species (ROS) and antioxidants. It has been suggested that oxidative stress is a mechanism for the pathogenesis of neurodegenerative diseases. Previously, it has been demonstrated that ozone inhalation generates oxidative damage through the for- mation of the ROS [1,2]. Oxidative stress produced by ozone inhalation causes damage in the central nervous system [3-5], increases lipid peroxidation (LPO) levels in brain structures [5,6], and causes memory deficits [5-7]. Oxidative stress and the decrease in the growth hor- mone-insulin-like growth factor I (GH-IGF-I) axis are well-characterized markers during aging and the cogni- tive dysfunction that occurs with age [8,9]. It has been reported that GH attenuates the increase in oxidative- stress levels [9,10], and improves the cognitive function deteriorated by aging [11]. The expression of GH recap- tors has been described in the central nervous system in rodents [12]. Furthermore, it has been demonstrated that the mutation of the GH gene causes deficiencies in hip- pocampus-dependent spatial memory in rats [13]. In ad- dition, it has been reported that the GH-IGF-I axis is in- volved in cognitive functions [12,14,15]. Moreover, it has been demonstrated that IGF-I via Akt is a neuropro- tective pathway in neurons [16,17]. In previous studies we demonstrated that oxidative stress generated by ex- posure to ozone causes memory deficit [5], and neuro- genesis alterations in rats [6]. In this work we evaluated the effects of the growth- hormone treatment on the memory deficit generated by the oxidative stress, produced by low doses of ozone, in the water maze and passive-avoidance tasks and on the IGF-I-Akt signaling in the dentate gyrus. 2. Materials and Methods 2.1. Animals Seventy-nine male Wistar rats weighing 250 to 300 g were used for this experiment. They were housed indi- vidually under a 12-h: 12-h light:dark cycle, with food and water ad libitum. Adequate measures were taken to minimize pain or discomfort, as outlined in the NIH Guide for the Care and Use of Laboratory Animals. Animals were distributed into the groups: 1) CTRL, con- trol group exposed to air free of ozone during 30 days, 2) GH groups, which received an injection (sc) of 0.8 mL of recombinant human growth hormone (r-hGH) (Saizen) daily, 3) O3 groups, exposed to ozone and received a injection (sc) of 0.8 mL of saline daily, and 4) O3 + GH groups, which received GH after ozone exposure. Groups 2, 3, and 4 were divided into 3 subgroups for 7, Copyright © 2012 SciRes. NM  Growth Hormone Prevents the Memory Deficit Caused by Oxidative Stress in Early Neurodegenerative Stage in Rats 288 15, and 30 days of each treatment. The GH was injected sc at doses of 0.08 UI, alone or after each ozone exposure. The groups exposed to ozone received 0.25 ppm for 4 hours daily. All groups were evaluated in a water maze and passive-avoidance tasks. After the last training, three animals in each group were randomly chosen, killed, and the dentate gyrus dissected, and the corresponding sam- ples used for immune-western-blot analyses for IGF-I and phospho-Akt (a downstream product of phospho- inositide-3 kinase (PI-3K)). 2.2. Ozone Exposure Animals were put daily for 4 h into a chamber with a diffuser connected to a variable flux ozone generator (5 L/s). The procedure has previously been described 4,5. Ozone was generated from a tube through which a high- voltage current circulated. Ozone production levels were proportional to the current intensity and airflow. A PCI Ozone & Control System Monitor was used to measure the ozone concentration inside the chamber during the experiment and to keep the ozone concentration constant. 2.3. Water Maze Two types of tests were used, training trials and a reten- tion trial at the end of the experiment. Rats were trained to find a hidden 10-cm platform in a metallic, dark, and circular pool (1.5-m diameter and 60-cm height) with spatial clues surrounding it, and animals were allowed to swim for 100 seconds to locate the platform in each trial. Each rat had five trials (100 seconds each) per day, dur- ing the last three days of each treatment. In the training sessions all rats were released at different points of the pool and were taken to a dry cage after finding the plat- form. In the last trial, the platform was removed, the tank was divided into four quadrants and the time spent by rats in the correct quadrant was measured during 60 s. The time spent by rats in the correct quadrant was used as a direct measurement of memory retention. 2.4. Passive Avoidance Two hours after the last treatment, all groups were trained in a one-trial passive-avoidance conditioning and tested for short-term and long-term memory (10 min and 24 h after training). Training was done in a conditioning chamber with two compartments of the same size (30 × 30 × 30 cm) separated by a guillotine-type door. The floor of the safe compartment was a grid made of alumi- num bars (0.5-cm diameter) separated 1.5-cm center to center. The floor and the walls of the shocking compart- ment were made of stainless steel. Each wall was con- tinuous with half the floor, and each half floor was sepa- rated by a 1-cm slot. The floor was connected to a con- stant current unit (Mod, PSIU6; Grass) fed by a Grass stimulator (Mod. S48, Grass technologies), which deliv- ered 50 square pulses per second at 1.5-mA intensity and 5-ms duration for 5 s. The duration of the stimulus was automatically monitored, and latencies were manually measured with chronometers. During training (acquisi- tion), each animal was placed in the safe compartment for 10 s. Then, the sliding door was lifted, and the time required for the animals to cross the threshold of the shocking compartment was recorded (acquisition la- tency). If an animal required more than 100 s to cross to the other side, it was dropped from the experiment and substituted with another rat exposed to the same condi- tions. Once the animals crossed with all four paws into next compartment, the door was closed and a 3-mA foot shock was delivered for 5 s. Then, the door was opened, and the time required for the animal to return to the safe compartment was measured (escape latency). The animal was again placed in the safety compartment for 10 s, the door was opened, and the time that the animal remained was recorded (retention latency). The test session ended when the animal either entered the shock compartment or remained in the safe compartment for 600 s. 2.5. Western Blot The dentate gyrus was microdissected [18]. The test was done in triplicate on three animals from each group. The tissue was homogenized and centrifuged. The Bradford method was used to quantify the protein, and 100 μg of protein from each sample were used. The protein was separated with SDS-polyacrylamide gel electrophoresis (10%) (Sigma-Aldrich) and transferred to nitrocellulose membranes (Sigma-Aldrich). The membranes containing the samples of different rat groups were blocked with 5% skimmed milk in TRIS buffer solution with 0.01% Tween 20 (TBS-T) (Sigma-Aldrich) for 2 h at 37 ˚C and incubated overnight individually with IGF-I (1:500 Ab- cam), phospho-Akt (1:1000 Chemicon), total Akt (1:1000, Chemicon), and actin (1:10,000) Santa Cruz Antibodies) antibodies under gentle shaking at 4˚C (BrinkmannOrbiMix 110, Brinkmann, Germany). The membranes were rinsed three times with TBS-T, incu- bated with goat anti-rabbit IgG (Vector, Burlingame, CA) and anti-mouse conjugated to horseradish peroxidase (1:10,000) (Biotechnology, Santa Cruz, CA) for 1 h, and then rinsed three times with TBS-T. The recognized bands were visualized by chemiluminescence (ECL; General Electric, Santa Clara, CA). The band densities were first normalized to the untreated control (100%). The IGF-I was normalized to actin and the phospho-Akt was then normalized to total Akt with the values ex- pressed as a percentage. We did the densitometry using the software off-line ImageJ (NIH, USA). Copyright © 2012 SciRes. NM 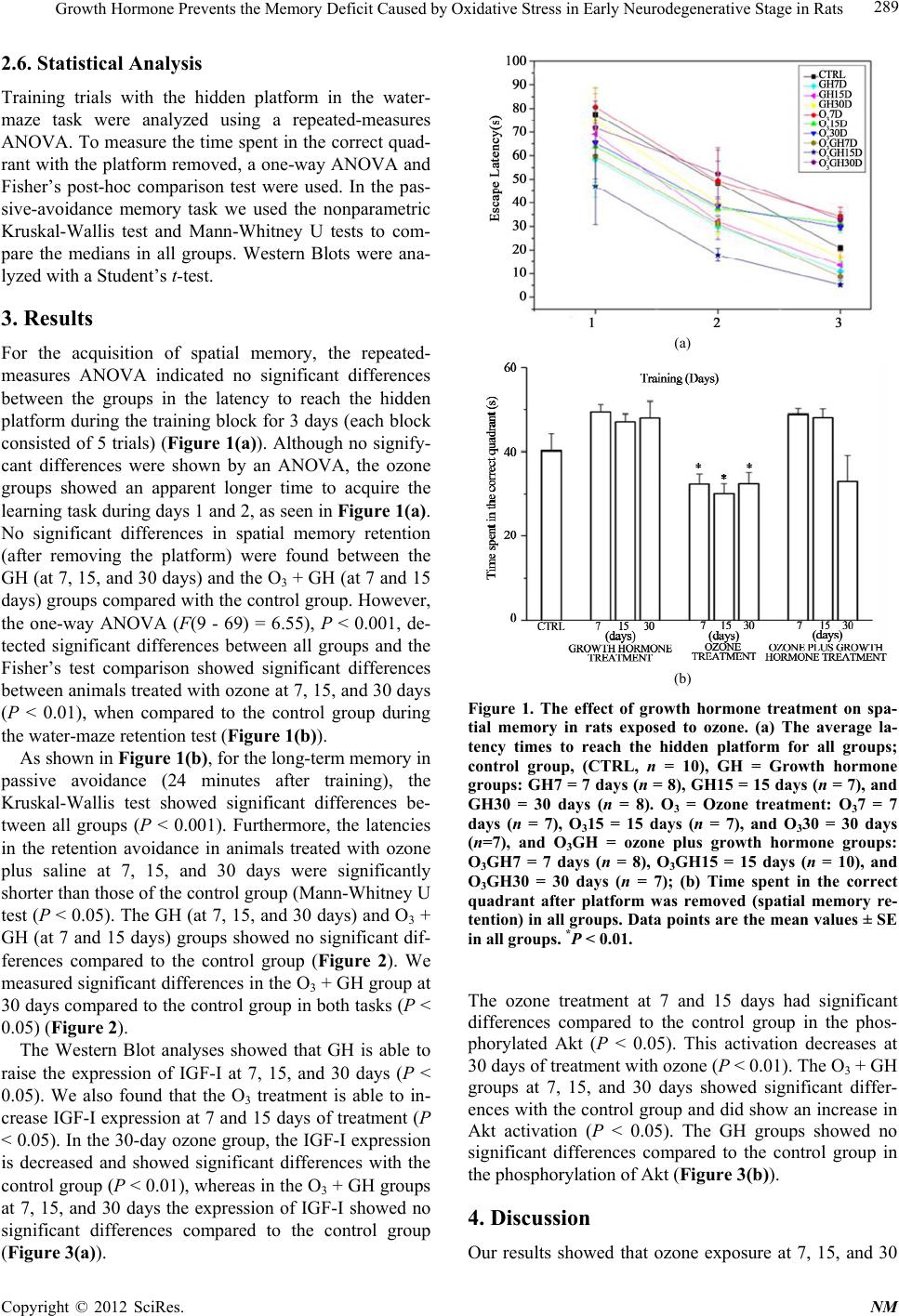 Growth Hormone Prevents the Memory Deficit Caused by Oxidative Stress in Early Neurodegenerative Stage in Rats 289 2.6. Statistical Analysis Training trials with the hidden platform in the water- maze task were analyzed using a repeated-measures ANOVA. To measure the time spent in the correct quad- rant with the platform removed, a one-way ANOVA and Fisher’s post-hoc comparison test were used. In the pas- sive-avoidance memory task we used the nonparametric Kruskal-Wallis test and Mann-Whitney U tests to com- pare the medians in all groups. Western Blots were ana- lyzed with a Student’s t-test. 3. Results For the acquisition of spatial memory, the repeated- measures ANOVA indicated no significant differences between the groups in the latency to reach the hidden platform during the training block for 3 days (each block consisted of 5 trials) (Figure 1(a)). Although no signify- cant differences were shown by an ANOVA, the ozone groups showed an apparent longer time to acquire the learning task during days 1 and 2, as seen in Figure 1(a). No significant differences in spatial memory retention (after removing the platform) were found between the GH (at 7, 15, and 30 days) and the O3 + GH (at 7 and 15 days) groups compared with the control group. However, the one-way ANOVA (F(9 - 69) = 6.55), P < 0.001, de- tected significant differences between all groups and the Fisher’s test comparison showed significant differences between animals treated with ozone at 7, 15, and 30 days (P < 0.01), when compared to the control group during the water-maze retention test (Figure 1(b)). As shown in Figure 1(b), for the long-term memory in passive avoidance (24 minutes after training), the Kruskal-Wallis test showed significant differences be- tween all groups (P < 0.001). Furthermore, the latencies in the retention avoidance in animals treated with ozone plus saline at 7, 15, and 30 days were significantly shorter than those of the control group (Mann-Whitney U test (P < 0.05). The GH (at 7, 15, and 30 days) and O3 + GH (at 7 and 15 days) groups showed no significant dif- ferences compared to the control group (Figure 2). We measured significant differences in the O3 + GH group at 30 days compared to the control group in both tasks (P < 0.05) (Figure 2). The Western Blot analyses showed that GH is able to raise the expression of IGF-I at 7, 15, and 30 days (P < 0.05). We also found that the O3 treatment is able to in- crease IGF-I expression at 7 and 15 days of treatment (P < 0.05). In the 30-day ozone group, the IGF-I expression is decreased and showed significant differences with the control group (P < 0.01), whereas in the O3 + GH groups at 7, 15, and 30 days the expression of IGF-I showed no significant differences compared to the control group (Figure 3(a)). (a) (b) Figure 1. The effect of growth hormone treatment on spa- tial memory in rats exposed to ozone. (a) The average la- tency times to reach the hidden platform for all groups; control group, (CTRL, n = 10), GH = Growth hormone groups: GH7 = 7 days (n = 8), GH15 = 15 days (n = 7), and GH30 = 30 days (n = 8). O3 = Ozone treatment: O37 = 7 days (n = 7), O315 = 15 days (n = 7), and O330 = 30 days (n=7), and O3GH = ozone plus growth hormone groups: O3GH7 = 7 days (n = 8), O3GH15 = 15 days (n = 10), and O3GH30 = 30 days (n = 7); (b) Time spent in the correct quadrant after platform was removed (spatial memory re- tention) in all groups. Data points are the mean values ± SE in all groups. *P < 0.01. The ozone treatment at 7 and 15 days had significant differences compared to the control group in the phos- phorylated Akt (P < 0.05). This activation decreases at 30 days of treatment with ozone (P < 0.01). The O3 + GH groups at 7, 15, and 30 days showed significant differ- ences with the control group and did show an increase in Akt activation (P < 0.05). The GH groups showed no significant differences compared to the control group in the phosphorylation of Akt (Figure 3(b)). 4. Discussion Our results showed that ozone exposure at 7, 15, and 30 Copyright © 2012 SciRes. NM  Growth Hormone Prevents the Memory Deficit Caused by Oxidative Stress in Early Neurodegenerative Stage in Rats 290 Figure 2. The effect of growth hormone treatment on the passive-avoidance task in rats exposed to ozone. Long-term memory retention latency, 24 hours after acquisition of passive-avoidance task. Data points are median values (in seconds) in all groups. (CTRL, n = 10), GH7 = 7 days (n = 8), GH15 = 15 days (n = 7), GH30 = 30 days (n = 8), O37 = 7 days (n = 7), O315 = days (n = 7), O330 = 30 days (n = 7), O3GH7 = 7 days (n = 8), O3GH15 = 15 days (n = 10), and O3GH30 = 30 days (n = 7). *P < 0.05). (a) (b) *P < 0.05, **P < 0.01. Figure 3. The effects of growth hormone on IGF-expression and Akt activation in rats exposed to ozone. Representative western blots show normalized immunoreactivity for IGF-I expression and Akt activation. (a) IGF-I expression in dif- ferent treatments; (b) Akt phosphorylation in different treatments. Bars are the mean ± SE in all groups (n = 3). days generates memory—retention impairments in the water maze and the passive-avoidance learning (Figures 1(b) and 2(b)). These data indicate that ozone is able to cause long-term memory deficits. We found that treat- ment with GH alone shows a tendency to facilitate the execution of two tasks in 7, 15, and 30 days (Figures 1 (b) and 2(b)) and activates the expression of IGF-I (Figure 3(a)). This is in agreement with previous studies showing that GH participates in the improvement of the memory function [6,19]. In addition, GH has been reported to reduce oxidative stress in the central nervous system [10,11]. Our results show that GH treatment at 7 and 15 days is able to prevent the long-term memory deficit caused by oxidative stress generated by exposure to ozone (Figures 1(b) and 2(b)). However, at 30 days of exposure the GH injections do not prevent the long-term memory deficits, demonstrating that ozone causes irre- versible oxidative damage at 30 days. Our results suggest that the GH has a neuroprotective effect on memory al- terations caused by oxidative stress in an early stage of the neurodegenerative process. Ramsey [9] reported that GH treatment attenuated the alterations generated by age in hippocampal plasticity and spatial memory in rats. We had also previously reported that the hippocampus is a region susceptible to oxidative stress caused by ozone [5,7]. Moreover, the memory deficits caused by ozone could be caused by morphologic damage in nerve tissue [3,4] and by neurogenesis alterations in the hippocampus [5]. Much evidence has shown that GH through IGF-I is involved in structural and cognitive functions [12,14,15]. The GH is directly related to the mRNA IGF-I expres- sion in the brain [12]. Furthermore, it has been demon- strated that IGF-I has neuroprotective effects against oxidative damage through the PI-3K-Akt pathway in neurons [16,22,23]. In addition, the hippocampal function and adult neu- rogenesis appears to be related to both the passive- avoidance and water-maze memory retention, as has been reported [20,21,24]. We found that GH is able to increase the expression of IGF-I at 7, 15, and 30 days in the dentate gyrus. Ozone inhalation itself caused in- creases in the IGF-I expression and Akt phosphoryla- tion at 7 and 15 days, probably to prevent cell death caused by oxidative stress in dentate gyrus. Previous studies that shown ROS are capable to activate IGF-I and Akt [22,25,26]. In this sense, the IGF and Akt activation might modulate the cell survival or death in an oxidative- stress in a dependent manner [26-29]. Moreover, a pre- vious study showed that ROS, growt factors, and PI3K- Akt signaling has been involved as modulators in the neurogenesis process [30]. In this sense IGF/Akt signal- ing pathway might promote neurogenesis in early states Copyright © 2012 SciRes. NM  Growth Hormone Prevents the Memory Deficit Caused by Oxidative Stress in Early Neurodegenerative Stage in Rats 291 of neurodegenerative process caused by ROS increases produced by ozone chronic inhalation [5]. Previously a study found in CaMKIIαh KO model that is high neurogenesis levels and an increase in the expression of genes involved in oxidative stress [31]. In addition, CaMKIIαh KO animals show increased neurogenesis, neuron immaturity and memory deficits [32], as we shown in our results (in ozone exposure groups), in this and a previous study [5]. These results suggest that ex- acerbated oxidative stress could dysregulate the normal neurogenesis process in rats and can cause memory deficits. We also found that at 30 days of ozone exposure, IGF-I expression and Akt activation are completely inhibited (Figures 3(a) and 3(b)). This is in concordance with a previous study that showed that the Akt-activity status is dependent on oxidative-stress levels in neuronal cells [17] and with the theory that IGF-I supression induced by DNA damage might be a protective effect of the cell against oxidative stress [33]. These results suggest that IGF-I-Akt signaling pathway might parti- cipate in cell survival during the early processes of neurodegeneration in an oxidative stress state caused by ozone exposure; however, after the chronic oxidative stress, the cell trigger mechanisms to downregulate these proteins. On the other hand, in the O3 + GH groups at 7 and 15 days, the IGF-I expression appears attenuated slightly, but had no effects on ozone activated Akt (Figure 3(b)). These results are in accordance with a previous study where IGF-I expression is caused by oxidative stress, but is decreased by antioxidant agents [34]. In addittion, GH might regulate different signalling pathways [35]. More- over, at 30 days of O3 + GH treatment IGF-I-Akt acti- vation was maintained, but the GH treatment is unable to prevent memory deficit, probably because at 30 days the oxidative stress levels are high and there is a deregulation in the oxidation-reduction balance and the GH activates Akt independently of the IGF-I signaling pathway [36]. 5. Conclusion In summary, our results suggest that the GH may prevent memory deficit caused by ozone in the adult brain only in early stages of the neurodegenerative process. Also, GH probably activates different signaling pathways de- pending on the oxidation-reduction balance. 6. Acknowledgements This study was supported by DGAPA-UNAM Postdoc- toral scholarship (2009-2010) and DGAPA IN215408D and IN219511-3 to S.R-A. Thanks to E. Jalpa-Hernandez for her review of this manuscript and Dr. Ellis Glazier for editing this English-language text. REFERENCES [1] M. A. Mehlman and C. Borek, “Toxicity and Biochemi- cal Mechanism of Ozone,” Enviromenmental Research”, Vol. 42, No 1, 1987, pp. 36-53. doi:10.1016/S0013-9351(87)80005-1 [2] M. G. Mustafa, “Biochemical Basis of Ozone Toxicity,” Free Radicals Biology and Medicine, Vol. 9, No. 3, 1990, pp. 245-265. doi:10.1016/0891-5849(90)90035-H [3] M. R. Ávila-Costa, L. Colín-Barranque, T. I. Fortoul, J. P. Machado-Salas, J. Espinosa-Villanueva, C. Rugerio-Var- gas, G. Borgornio, C. Dorado and S. Rivas-Arancibia, “Motor Impairments in an Oxidative Stress Model and Its Correlation with Cytological Changes on Rat Striatum and Prefrontal Cortex,” International Neuroscience, Vol. 108, No. 3-4, 2001, pp. 193-200. [4] N. Pereyra-Muñoz, C. Rugerio-Vargas, M. Angoa-Pérez, G. Borgonio-Pérez and S. Rivas-Arancibia, “Oxidative Damage in Substantia Nigra and Striatum of Rats Chro- nically Exposed to Ozone,” Journal of Chemical Neuro- anatomy, Vol. 31, No. 2, 2006, pp. 114-123. doi:10.1016/j.jchemneu.2005.09.006 [5] S. Rivas-Arancibia, R. Guevara-Guzmán, Y. López-Vidal, E. Rodríguez-Martínez, M. Zanardo-Gomes, M. Angoa- Pérez and R. Raisman-Vozari, “Oxidative Stress Caused by Ozone Exposure Induces Loss of Brain Repair in the Hippocampus of Adult Rats,” Toxicological Science, Vol. 113, No. 1, 2010, pp. 187-197. doi:10.1093/toxsci/kfp252 [6] S. Rivas-Arancibia, R. Vázquez-Sandoval, D. González- Kladiano, S. Schneider-Rivas and A. Lechuga-Guerrero, “Effects of Ozone Exposure in Rats on Memory and Lev- els of Brain and Pulmonary Superoxide Dismutase,” En- viromental Research, Vol. 76, No. 1, 1998, pp. 33-39. doi:10.1006/enrs.1997.3784 [7] M. R. Ávila-Costa, L. Colin-Barenque, T. I. Fortoul, J. P. Machado-Salas, J. Espinosa-Villanueva, C. Rugerio-Var- gas and S. Rivas-Arancibia, “Memory Deterioration in an Oxidative Stress Model and Its Correlation with Cyto- logical Changes on Rat Hippocampus CA1,” Neurosci- ence Letters, Vol. 270, No. 2, 1999, pp. 107-109. doi:10.1016/S0304-3940(99)00458-9 [8] M. J. Forster, A. Dubey and K. M. Dawson, “Age Related Losses of Cognitive Function and Motor Skills in Mice Are Associated with Oxidative Protein Damage in the Brain,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 93, No. 10, 1996, pp. 4765-4769. doi:10.1073/pnas.93.10.4765 [9] M. M. Ramsey, J. L. Weiner, T. P. Moore, C. S. Carter and W. E. Sonntag, “Growth Hormone Treatment Atten- uates Age-Related Changes in Hippocampal Short-Term Plasticity and Spatial Learning,” Neuroscience, Vol. 129, No. 1, 2004, pp. 119-127. doi:10.1016/j.neuroscience.2004.08.001 [10] A. Martínez-Canabal, M. Angoa-Pérez, C. Rugerio- Vargas, G. Borgonio-Pérez and S. Rivas-Arancibia, “Ef- fect of Growth Hormone on Cyclooxygenase-2 Expres- sion in the Hippocampus of Rats Chronically Exposed to Ozone,” International Journal of Neuroscience, Vol. 118, No. 3, 2008, pp. 455-469. doi:10.1080/00207450701593160 Copyright © 2012 SciRes. NM  Growth Hormone Prevents the Memory Deficit Caused by Oxidative Stress in Early Neurodegenerative Stage in Rats 292 [11] A. N. Donahue, M. Aschner, L. H. Lash, T. Syversen and W. E. Sonntag, “Growth Hormone Administration to Aged Animals Reduces Disulfide Glutathione Levels in Hippocampus,” Mechanims of Ageing and Development, Vol. 127, No. 1, 2006, pp. 57-63. [12] P. E. Lobie, J. García-Aragón, D. T. Lincoln, R. Barnard, J. N. Wilcox and M. J. Waters, “Localization and Ontog- eny of Growth Hormone Receptor Gene Expression in the Central Nervous System,” Brain Research, Develop- mental Brain Research, Vol. 74, No. 2, 1993, pp. 225- 233. doi:10.1016/0165-3806(93)90008-X [13] E. Li, K. D. Hyun, M. Cai, S. Lee, Y. Kim, E. Lim, J. H. Ryu, T. G. Unterman and S. Park, “Hippocampus-De- pendent Spatial Learning and Memory Are Impaired in Growth Hormone-Deficient Spontaneous Dwarf Rats,” Endocrine Journal, Vol. 58, No. 4, 2011, pp. 257-267. doi:10.1507/endocrj.K11E-006 [14] C. Bondy and E. Chin, “IGF-I mRNA Localization in Trigeminal and Sympathetic Nerve Target Zones during Rat Embryonic development,” Advance in Experimental Medicine and Biology, Vol. 293, 1991, pp. 431-437. [15] K. L. Stenvers, P. K. Lund and M. Gallagher, “Increased Expression of Type 1 Insulin-Like Growth Factor Recep- tor Messenger RNA in Rat Hippocampal Formation Is Associated with Aging and Behavioral Impairment,” Neuroscience, Vol. 72, No. 2, 1996, pp. 505-518. doi:10.1016/0306-4522(95)00524-2 [16] H. Dudek, S. R. Datta, T. F. Franke, M. J. Birnbaum, R. Yao, G. M. Cooper, R. A. Segal, D. R. Kaplan and M. E. Greenberg, “Regulation of Neuronal Survival by the Ser- ine-Threonine Protein Kinase Akt,” Science, Vol. 275, No. 5300, 1997, pp. 661-665. doi:10.1126/science.275.5300.661 [17] D. Dávila and I. Torres-Aleman, “Neuronal Death by Oxidative Stress Involves Activation of FOXO3 through a Two-Arm Pathway That Activates Stress Kinases and Attenuates Insulin-Like Growth Factor I Signaling,” Mo- lecular Biology of the Cell, Vol. 19, No. 5. 2008, pp. 2014-2025. doi:10.1091/mbc.E07-08-0811 [18] E. S. Lein, X. Zhao and F. H. Gage, “Defining a Molecu- lar Atlas of the Hippocampus Using DNA Microarrays and High-Throughput in Situ Hybridization,” Journal of Neuroscience, Vol. 24, No. 15, 2004, pp. 3879-3889. doi:10.1523/JNEUROSCI.4710-03.2004 [19] I. C. van Nieuwpoort and M. L. Drent, “Cognition in the Adult with Childhood-Onset GH Deficiency,” European Journal Endocrinology, Vol. 159. No. 1, 2008, pp. S53- S57. doi:10.1530/EJE-08-0279 [20] G. Mereu, M. Fà, L. Ferraro, R. Cagiano, T. Antonelli, M. Tattoli, G. L.Gessa and V. Cuomo, “Prenatal Exposure to Cannabinoid Agonist Produces Memory Deficits Linked to Dysfunction in Hippocampal Log-Term Potentiation and Glutamate Release,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 100, No. 8, 2003, pp. 4915-4920. doi:10.1073/pnas.0537849100 [21] R. G. M. Morris, P. Garrud, J. N. P. Rawlins and J. O’Keefe, “Place Navigation Impaired in Rats with Hip- pocampal Lesions,” Nature, Vol. 297, No. 5868, pp. 681- 683. doi:10.1038/297681a0 [22] A. I. Duarte, P. Santos, C. R. Oliveira, M. S. Santosa and A. C. Rego, “Insulin Neuroprotection against Oxidative Stress Is Mediated by Akt and GSK-3Beta Signaling Pathways and Changes in Protein Expression,” Biochi- mica et Biophysica Acta, Vol. 1783, No. 6, 2008, pp. 994- 1002. doi:10.1016/j.bbamcr.2008.02.016 [23] J. Piriz, A. Muller, J. L. Trejo and I. Torres-Alemán, “IGF-I and the Aging Mammalian Brain,” Experimental Gerontology, Vol. 46, No. 2-3, 2011, pp. 96-99. doi:10.1016/j.exger.2010.08.022 [24] S. Jessberger, R. L. Clark, N. J. Broadbent, G. D. Clem- enson, A. Consiglio, D. C. Lie, L. Squire and F. H. Gage, “Dentate Gyrus-Specific Knockdown of Adult Neuro- genesis Impairs Spatial and Object Recognition Memory in Adult Rats,” Learning and Memory, Vol. 16, 2009, pp. 147-154. doi:10.1101/lm.1172609 [25] A. E. Handayaningsih, G. Iguchi, H. Fukuoka, H. Nishi- zawa, M. Takahashi, M. Yamamoto, E. H. Herningtiyas, Y. Okimura, H. Kaji, K. Chihara, S. Seino and Y. Taka- hashi, “Recative Oxygen Species Play an Essential Role in IGF-I Induced Myocyte Hypertrophy in C2C12 Myo- cytes,” Endocrinology, Vol. 152, No. 3, 2011, pp. 912- 921. [26] J. Papaconstantinou, “Insulin/IGF-1 and ROS Signaling Pathway Cross-Talk in Aging and Longevity Deter- mination,” Molecular and Cellular Endocrinology, Vol. 299, No. 1, 2009, pp. 89-100. doi:10.1016/j.mce.2008.11.025 [27] D. V. Castillo and S. Rivas-Arancibia, “Interacción Entre los Factores Neurotróficos y Las Especies Reactivas de Oxígeno en los Mecanismos de Muerte y Proliferación Celular,” Archivos en Neurociencias, Vol. 16, No. 1, 2011, pp. 26-32. [28] N. R. Leslie, “The Redox Regulation of PI 3-Kinase- Dependet Signaling,” Antioxidants and Redox Signal, Vol. 8, No. 9-10, 2006, pp. 1765-1774. [29] J. L. Martindale and N. J. Holbrook, “Cellular Response to Oxidative Stress: Signaling for Suicide and Survival,” Journal of Cellular and Comparative Physiology, Vol. 192, No. 1, 2002, pp. 1-15. [30] J. E. Le Belle, N. M. Orozco, A. A. Paucar, J. P. Saxe, J. Mottahedeh, A. D. Pyle, H. Hu and H. I. Kornblum, “Pro- liferative Neural Stem Cells Have High Endogenous ROS Levels That Regulate Self-Renewal and Neurogenesis in a PI3-K/Akt-Dependent Manner,” Cell Stem Cell, Vol. 8, No. 1, 2011, pp. 59-71. doi:10.1016/j.stem.2010.11.028 [31] N. M. Walton, R. Shin, K. Tajinda, C. L. Heusner, J. H. Kogan, S. Miyake, Q. Chen, K. Tamura and M. Matsu- moto, “Adult Neurogenesis Transiently Generates Oxida- tive Stress,” PLoS One, Vol. 7, No. 4, 2012, Article ID: e35264. [32] N. Yamasaki, M. Maekawa, K. Kobayashi, Y. Kajii, J. Maeda, M. Soma, K. Takao, K. Tanda, K. Ohira, K. To- yama, K. Kanzaki, K. Fukunaga, Y, Sudo, H. Ichinose, M. Ikeda, N. Iwata, N. Ozaki, H. Suzuki, M. Higuchi, T. Su- hara, S. Yuasa and T. Miyakawa, “Alpha-CaMKII Defi- ciency Causes Immature Dentate Gyrus, a Novel Candi- date,” Endophenotype of Psychiatry Disrordes, Vol. 1, Copyright © 2012 SciRes. NM  Growth Hormone Prevents the Memory Deficit Caused by Oxidative Stress in Early Neurodegenerative Stage in Rats Copyright © 2012 SciRes. NM 293 No. 6, 2008, pp. 1-21. [33] A. S. Araujo, A. T. Enzveiler, P. Schenkel, M. F. Fer- nandes, W. A. Partata, S. Llesuy and A. Belló-Klein, “Oxi- dative Stress Activates Insulin-Like Growth Factor I Re- ceptor Protein Expression, Mediating Cardiac Hyper- trophy Induced by Thyrosine,” Molecular and Cell Bio- chemistry, Vol. 303, No. 1-2, 2007, pp. 89-95. doi:10.1007/s11010-007-9459-9 [34] G. Hinkal and L. A. Donehower, “How Does Supression of IGF-I Signaling by DNA Damage Affect Aging and Longevity,” Mechanisms of Ageing and Development, Vol. 129, No. 5, 2008, pp. 243-253. [35] M. E. Díaz, L. González, J. G. Miquet, C. S. Martínez, A. I. Bartke and D. Turyn, “Growth Hormone Modulation of EGF-Induced PI3K-Akt Pathway in Mice Liver,” Cell Signal, Vol. 24, No. 2, 2011, pp. 514-523. [36] H. M. Brown-Borg, S. G. Rakoczy, M. A. Romanick and M. A. Kennedy, “Effects of Growth Hormone and Insu- lin-Like Growth Factor-1 on Hepatocyte Antioxidative Enzymes,” Experimental Biology and Medicine, Vol. 227, No. 2, 2002, pp. 94-104. Abbreviations GH (growth hormone), IGF-I (insulin growth factor I), Akt (serine-threonine protein kinase), O3 (ozone).
|