 Journal of Cancer Therapy, 2012, 3, 442-451 http://dx.doi.org/10.4236/jct.2012.324057 Published Online September 2012 (http://www.SciRP.org/journal/jct) Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines Gerhard Hamilton, Ernst Ulsperger, Klaus Geissl er, Ulrike Olszewski Ludwig Boltzmann Cluster of Translational Oncology, Vienna, Austria. Email: gerhard.hamilton@toc.lbg.ac.at Received July 31st, 2012; revised September 5th, 2012; accepted September 23rd, 2012 ABSTRACT Extended stage small cell lung cancer (SCLC) responds to platinum/vepeside-based first-line chemotherapy but relapses early as drug-resistant tumor associated with a dismal prognosis. A pair of SCLC cell lines obtained from a single pa- tient at different time points during treatment allows for the investigation of the changes in gene expression before (GLC14) and following cycles of chemotherapy and irradiation (GLC19). GLC19 cells were reported to reveal an in- creased doubling time and exhibit increased chemoresistance to doxorubicin, etoposide, melphalan and vinblastine. Upregulated transcripts in GLC19, as assessed by microarray analysis, comprised genes involved in regulation of cellu- lar growth (NGFRAP1/BEX3), adhesion, glutathione metabolism and, in particular, WNT/Notch pathways and the pu- tative cancer stem cell phenotype (CD44, ALDH1A1, and AKR1C1/13). Metallothioneins, tubulins TUBA3/4 and tu- mor protein p53 inducible protein 11 (TP53IP11) were downregulated in this cell line compared to GLC14. Except in- creased expression of glutathione transferases no classical markers of chemoresistance were found, pointing to a role of altered growth control/differentiation and reduced accessibility of these SCLC cells that grow as multicellular spheroids. In conclusion, treatment of this single SCLC patient with cyclophosphamide, doxorubicin and etoposide (CDE) fol- lowed by radiotherapy ultimately enriched tumor cells that display the typical signature of tumor-initiating cells/cancer stem cells (CICs/CSCs). Keywords: Small Cell Lung Cancer; GLC14; GLC19; Chemotherapy; Chemoresistance; Gene Expression; Cancer-Initiating Cells 1. Introduction Due to over one million cases diagnosed every year and corresponding low survival rates, lung cancer is still the leading cause of cancer death despite a lower incidence compared to other tumor entities [1]. Small cell lung cancer (SCLC) represents approximately 13% of all lung cancer diagnoses and its incidence has reduced over the last 20 years [2]. These highly malignant neuroendo- crine tumors of the lung originate from neuroendocrine cells (Amine Precursor Uptake and Decarboxyla- tion/APUD cells) in the bronchus called Feyrter cells. Treatment of SCLC has remained challenging because of rapid growth, early dissemination and development of drug resistance during the course of the disease [3]. The predominant risk factor for SCLC is cigarette smoking with smokers facing a 20 - 30-fold higher incidence of SCLC than non-smokers [2]. Although the number of cases of SCLC is declining in man the frequency is rising in women due to increased use of tobacco. Within the context of the next 20 years, the incidence of lung cancer will grow with an increasing number of cases unrelated to smoking [4]. Without treatment SCLC has the most aggressive clinical course of any type of pulmonary tumors, with median survival from diagnosis of only 2 - 4 months [2]. Compared with other types of lung cancer, SCLC has a greater tendency to be widely disseminated at time of diagnosis while being much more responsive to chemo- therapy and radiation therapy first-line. Because pa- tients with SCLC tend to develop distant metastases, lo- calized forms of treatment, such as surgical resection or radiation therapy, rarely produce long-term survival. With current chemotherapy regimens survival is un- equivocally prolonged; however, the overall survival at 5 years is only 5% - 10% [2,5]. At time of diagnosis ap- proximately 30% of patients with SCLC have a tumor confined to either the hemithorax of origin, the media- stinum, or the supraclavicular lymph nodes. These pa- tients are classified as having limited-stage disease and Copyright © 2012 SciRes. JCT  Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines 443 hence a median survival of 16 - 24 months with current forms of treatment can reasonably be expected [1,5]. Patients with tumors that have spread beyond the supra- clavicular areas are categorized as having extensive-stage disease and reveal a worse prognosis than patients with limited-stage disease. In these cases a median survival of 6 - 12 months is reported with currently available therapy, whereas long-term disease-free survival is rare. The majority of patients with SCLC die of their tumor despite the best available treatment [5,6]. Chemotherapy improves the survival of patients with limited-stage or extensive-stage SCLC, but is curative in only a mino- rity of patients. In patients with limited-stage SCLC combination chemotherapy produces results that are clearly superior to single-agent treatment. Current regi- mens yield overall objective response rates of 65% - 90% and complete response rates of 45% - 75%. The combi- nation of etoposide and cisplatin chemotherapy with con- current chest radiation therapy has now been used in various single institutional studies and achieved median survival rates of 18 - 24 months and a 40% - 50% 2-year survival with less than 3% treatment-related mortality [1,5,7]. The prognosis for patients with SCLC that has progressed despite chemotherapy is exceptionally poor regardless of the stage, with expected median survival of 2 - 3 months. These patients should be considered for palliative therapy or clinical trials. Though no single che- motherapy regimen should be regarded standard, those that have shown activity as second-line treatment include oral etoposide, etoposide/cisplatin, cyclophosphamide/ doxorubicin/vincristine (CAV), lomustine/methotrexate, paclitaxel and topotecan [3,5]. Pharmacological inhibit- tors of receptor tyrosine kinases, including c-Kit, epi- dermal growth factor receptor (EGFR), insulin-like growth factor-I receptor (IGFR1) and vascular endothe- lial growth factor receptor (VEGFR), have been investi- gated as potential antitumor agents in SCLC with dis- appointing results [5,8]. Thus, the task of identifying targeted agents with clinically meaningful activity has so far proven to be less successful than in other tumors. In summary, the outcome of treatment of SCLC is characterized by a lack of progress and extremely short survival rates in advanced disease exhibiting little im- provement in the past decades. Despite high initial re- sponse rates the majority of patients relapse and die of their disease. Many suggestions discussed the reasons for this failure, including the presence of resistant stem cells, development of acquired resistance, redundancy in sig- naling pathway etc., while the definite underlying cause has remained unclear. Translational research to correlate cellular target pathway activation/inhibition with clinical endpoints is essential to maximize the knowledge gained from trials and to identify potential predictive markers. Longitudinal biopsies, viable cells or cell lines that re- flect the course of chemosensitivity towards resistant tumors are rarely available for SCLC patients. However, a series of three tumor biopsies with corresponding cell lines derived thereof, namely GLC14, GLC16 and GLC19, was described for a single SCLC patient by Berendsen et al. in 1988 [9]. There was a good accor- dance in the morphological, biochemical, and immuno- histological findings between the cell lines compared to those obtained in the biopsies This tumor progressed from an initially responsive state (GLC14) to increased drug resistance for doxorubicin, etoposide, melphalan and vinblastine (GLC19), allowing for a gene expression analysis of the molecular changes acquired during the relapse and complete treatment failure. In the present work we describe a comparative analysis of the overex- pressed gene transcripts and molecular pathways ex- pected to be linked to tumor progression and chemore- sistance in this case of SCLC. 2. Materials and Methods 2.1. Cells and Reagents GLC14 and GLC19 cell lines were obtained from Dr. Nina Pedersen from the Department of Radiation Bio- logy, The Finsen Centre, National University Hospital, Copenhagen, Denmark. Cells were grown in RPMI-1640 bicarbonate medium (Seromed, Berlin, Germany) supple- mented with 10% fetal bovine serum (Seromed), 4 mM glutamine and antibiotics (10× stock formulated to con- tain ~5000 units penicillin, 5 mg streptomycin and 10 mg neomycin/ml) under tissue culture conditions (37˚C, 5% CO2, 95% humidity) and checked for mycoplasma con- tamination (Mycoplasma PCR ELISA, Roche Diagnos- tics, Vienna, Austria). Cells grow as spheroids in sus- pension and were subcultures two times a week. Except where otherwise stated all reagents were from Sig- ma-Aldrich, St. Louis, MO, USA. 2.2. Chemosensitivity Assay 1 × 104 cells in 100 µl medium per well were distrib- uted in 96-well microtiter plates (Greiner, Kremsmuen- ster, Austria) and the test compound added in another 100 µl. Cisplatin (initial concentration 20 µg/ml) and solute con- trols were serially diluted in twofold steps in triplicate. The microtiter plates were incubated under tissue culture conditions for four days and cell viability was measured using a modified MTT (3-(4,5-dimethyl- thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) assay (EZ4U, Biomedica, Vienna, Austria). Optical density was measured at 450 nm using a microplate reader with an empty well as reference. Values obtained from con- trol wells containing cells and media alone were set to 100% proliferation. Copyright © 2012 SciRes. JCT  Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines 444 2.3. Gene Expression and Bioinformatic Analysis Cells were cultivated in 150 cm2 flasks in triplicate under tissue culture conditions, harvested and pellets of appro- ximately 35 × 106 cells stored frozen at –80˚C. Briefly, lysis with extraction buffer (4 M guanidine isothiocy- anate, 0.5% sodium N-lauroylsarcosinate, 10 mM EDTA, 5 mM sodium citrate, 100 µM β-mercaptoethanol) was performed at 4˚C, and DNA and RNA of the lysates were separated by cesium trifluoroacetate ultracentrifugation. RNA was washed with ice-cold 96% ethanol and dis- solved in water. Measurements of the optical density at 260/280 nm proved content and purity of the RNA. Gene expression analysis was performed in duplicate using the Applied Biosystems Human Genome Survey Microarray V2.0 (Applied Biosystems, Foster City, CA, USA). Therefore, 2 - 5 µg mRNA (20 - 50 µg total RNA) were reverse transcribed to first-strand cDNA and labeled with digoxigenin-UTP according to the Applied Biosystems Chemiluminescent Reverse Transcription protocol. Hy- bridization of cDNA and microarray analysis was per- formed pursuant to the Applied Biosystems Chemilumi- nescence Detection Kit protocol and by use of the Ap- plied Biosystems 1700 Chemiluminescent Microarray Analyzer. Data were processed by filtering and quantile normalization. Microarray probe identities were allocated to the respective gene designations using the microarray data information provided by Applied Biosystems. Sig- nificance Analysis of Microarrays (SAM) was used to identify differentially expressed genes in GLC19 and GLC14 cells with a false discovery rate of 12% [10]. Pathway analysis was carried out by help of the Reac- tome database available at http://www.reactome.org [11]. Statistical analysis was performed using two-tailed Stu- dent’s t-test for normally distributed samples (*p < 0.05 was regarded as statistically significant). 3. Results During the course of treatment the primary SCLC, rep- resented by the chemosensitive GLC14 cell line, ac- quired a resistant phenotype that is retained in form of the GLC19 SCLC cell line. Since for this patient therapy had not included platinum-based drugs, we tested both cell lines in an MTT assay for their sensitivity to cis- platin in the present study. Dose-response curves re- vealed IC50 values of 1.3 ± 0.2 and 0.5 ± 0.09 µg/ml for GLC14 and GLC19, respectively. Gene transcripts overexpressed in GLC19 in compari- son to GLC14 cells are listed in Table 1. 440 transcripts were found to be upregulated more than 2.5-fold in GLC19 vs. GLC14 SCLC cells. They include a number of proteins involved in regulation of cell growth, differ- entiation and cell death/apoptosis. The most prominent adaptor proteins affected were nerve growth factor re- ceptor-associated protein (NGFRAP1/BEX3) and CDK5 regulatory subunit associated protein 1 (CDK5RAP1). Increased expression was detected for the protooncogens myc and fos, as well as for the growth factors neuro- tensin (NTS), midkine (MDK; neurite growth-promoting factor 2), platelet-derived growth factor D (PDGFD), ser- ine/threonine protein kinase MST4 and Mps one binder kinase activators (MOBs). Transcripts for vasoactive in- testinal peptide receptor 1 (VIPR1), fibroblast growth factor receptor 1 (FGFR1) and ERBB4 exhibited relative overexpression in GLC19 cells. A second group of upregulated messengers comprised proteins involved in cell adhesion/cell membrane signal transduction including cell surface associated mucin 15 (MUC15), epithelial membrane protein 2 (EMP2), Pleck- strin homology domain-containing family C member (PLEKHC1), receptor for hyaluronic acid CD44, cal- cium-dependent cell-adhesion protein protocadherin 18 (PCDH18), claudin 1 (CLDN1), cwcv and kazal-like domains proteoglycan sparc/osteonectin (SPOCK) and the neural cell adhesion molecule 2 (NCAM2). In- creased transcription of glutathione S-transferases glu- tathione S-transferase theta 1 (GSTT1), glutathione S- transferase alpha 4 (GSTA4), microsomal glutathione S-transferase 2 (MGST2), and thioredoxin interacting protein (TXNIP) function in elevated conjugation of re- duced glutathione to a wide number of exogenous and endogenous hydrophobic electrophiles for elimination and in balancing the redox state, respectively. Several mediators upregulated in GLC19 cells belong to the wingless-type MMTV integration site family (Wnt) and to Notch signaling pathways. They comprise se- creted frizzled-related protein 1 (SFRP1), WNT5B, coac- tivator mastermind-like 2 (MAML2), transducin-like enhancer of split 2 (E(sp1)) homolog (TLE2) and the Notch receptor ligands jagged 1/2 (JAG1 and JAG2). Putative markers of CSCs overexpressed in GLC19 cells moreover include CD44, aldehyde dehydrogenase 1 fam- ily, member A1 (ALDH1A1) and the aldo-keto reductase family 1 members C1/13 (AKR1C1 and AKR1C13). Furthermore, several members of the MAGE family that may play a role in embryonal development, tumor trans- formation or aspects of tumor progression were elevated in GLC19 cells. 60 gene transcripts were downregulated more than 3-fold in GLC19 vs. GLC14 cells and include several metallothioneins (MTs; 1A, 1F, 1X, 3 and 4), tumor protein 53 inducible protein 11 (TP53I11), as well as members of the tubulin family (TUBA3, TUBB4) and tubulin folding cofactor A (TBCA; data not shown). The expression data was further analyzed for overrep- resented pathways with help of the Reactome software (www.reactome.org) and the significant results are listed in Table 2. Increases in sphingolipid metabolism, expre- ssion of mediators of neuronl, Notch, nitric oxide and a Copyright © 2012 SciRes. JCT 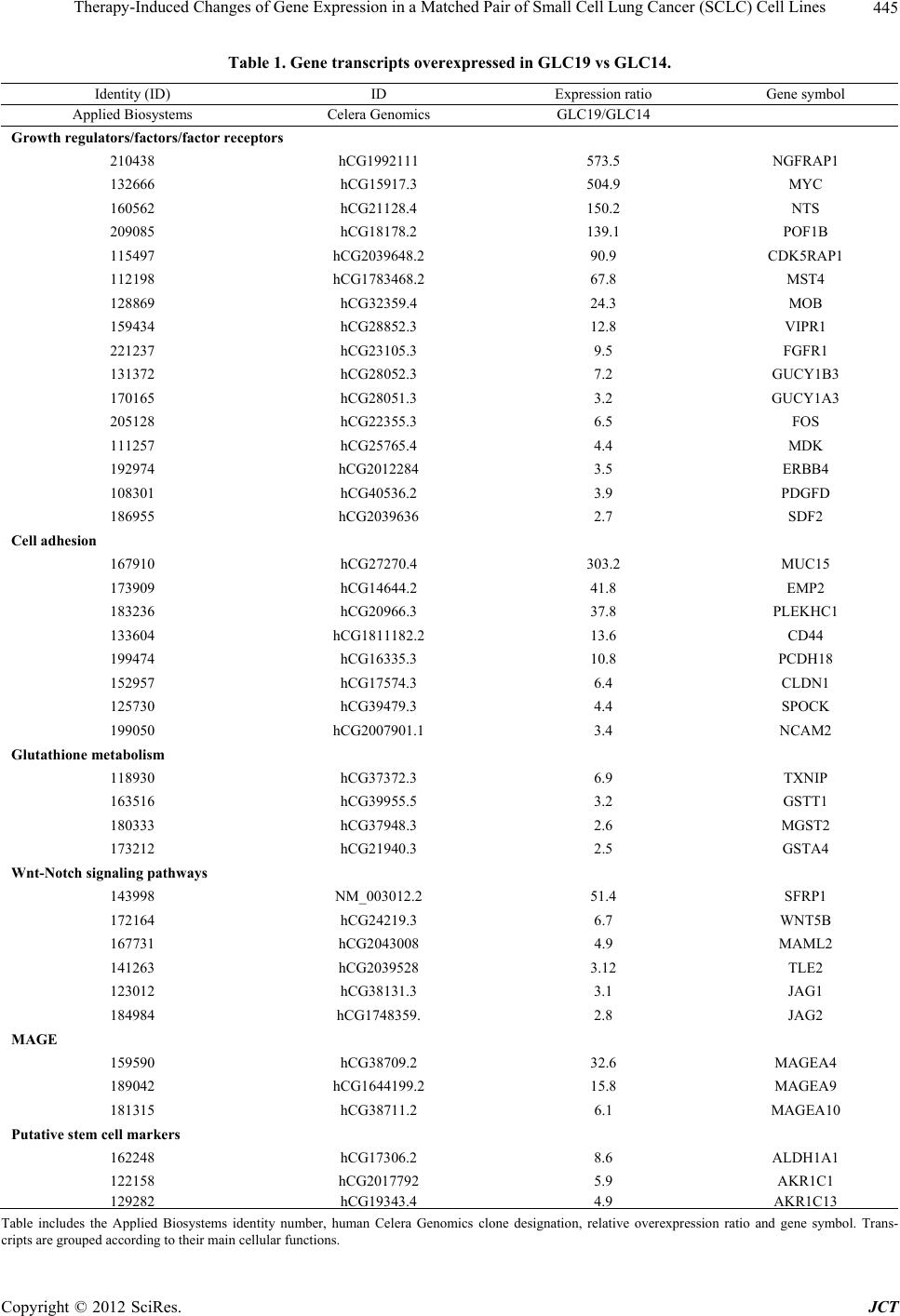 Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines Copyright © 2012 SciRes. JCT 445 Table 1. Gene transcripts overexpressed in GLC19 vs GLC14. Identity (ID) ID Expression ratio Gene symbol Applied Biosystems Celera Genomics GLC19/GLC14 Growth regulators/factors/factor receptors 210438 hCG1992111 573.5 NGFRAP1 132666 hCG15917.3 504.9 MYC 160562 hCG21128.4 150.2 NTS 209085 hCG18178.2 139.1 POF1B 115497 hCG2039648.2 90.9 CDK5RAP1 112198 hCG1783468.2 67.8 MST4 128869 hCG32359.4 24.3 MOB 159434 hCG28852.3 12.8 VIPR1 221237 hCG23105.3 9.5 FGFR1 131372 hCG28052.3 7.2 GUCY1B3 170165 hCG28051.3 3.2 GUCY1A3 205128 hCG22355.3 6.5 FOS 111257 hCG25765.4 4.4 MDK 192974 hCG2012284 3.5 ERBB4 108301 hCG40536.2 3.9 PDGFD 186955 hCG2039636 2.7 SDF2 Cell adhesion 167910 hCG27270.4 303.2 MUC15 173909 hCG14644.2 41.8 EMP2 183236 hCG20966.3 37.8 PLEKHC1 133604 hCG1811182.2 13.6 CD44 199474 hCG16335.3 10.8 PCDH18 152957 hCG17574.3 6.4 CLDN1 125730 hCG39479.3 4.4 SPOCK 199050 hCG2007901.1 3.4 NCAM2 Glutathione metabolism 118930 hCG37372.3 6.9 TXNIP 163516 hCG39955.5 3.2 GSTT1 180333 hCG37948.3 2.6 MGST2 173212 hCG21940.3 2.5 GSTA4 Wnt-Notch signaling pathways 143998 NM_003012.2 51.4 SFRP1 172164 hCG24219.3 6.7 WNT5B 167731 hCG2043008 4.9 MAML2 141263 hCG2039528 3.12 TLE2 123012 hCG38131.3 3.1 JAG1 184984 hCG1748359. 2.8 JAG2 MAGE 159590 hCG38709.2 32.6 MAGEA4 189042 hCG1644199.2 15.8 MAGEA9 181315 hCG38711.2 6.1 MAGEA10 Putative stem cell markers 162248 hCG17306.2 8.6 ALDH1A1 122158 hCG2017792 5.9 AKR1C1 129282 hCG19343.44.9AKR1C13 Table includes the Applied Biosystems identity number, human Celera Genomics clone designation, relative overexpression ratio and gene symbol. Trans- cripts are grouped according to their main cellular functions.  Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines 446 Table 2. Cellular pathways overrepresented in GLC19 vs GLC14 SCLC cells. p-value Identifier Name of event 0.001 REACT_19323 Sphingolipid metabolism 0.004 REACT_13685 Neuronal system 0.005 REACT_118859 Signaling by Notch 0.008 REACT_23862 Nitric oxide stimulates guanylate cyclase 0.018 REACT_24989 ATPase type IV transporters - phospholipid transfer 0.027 REACT_75770 Voltage gated potassium channels 0.03 REACT_15518 Transmembrane transport of small molecules 0.048 REACT_21310 Phospholipase C-mediated cascade The list of overexpressed gene transcripts in GLC19 vs GLC14 was used to identify overrepresented cellular pathways. phospolipase C signaling, as well as of ion/small mole- cules/phospolipid membrane transporters were observed. 4. Discussion The GLC14 cell line was established using a biopsy from a right supraclavicular node of a 55-year-old woman with extended SCLC. Treatment was started with cyclophos- phamide, doxorubicin and etoposide (CDE), which re- sulted in a complete response [9]. After seven months tumor recurrence was detected in chest X-ray and rein- duction chemotherapy with CDE led to a partial response. Radiotherapy was given to the left hilar region and me- diastinum and led to a normal chest X-ray, before SCLC reappeared in the left upper lobe of the lung. At this stage another cell line (GLC19) was established from a biopsy. The tumor progressed despite two additional cycles of CDE and the patient died after discontinuation of therapy, 17 months after first presentation. The electron micro- scopic appearance of all cell lines was compatible with SCLC of the “classic type”. Additionally, dense core vesicles were present in moderate amounts in all cell lines, whereas desmosomes with associated tonofila- ments were identified in GLC19 cells only. The IC50 for doxorubicin and etoposide increased 1.7-fold from 0.44 for GLC14 to 0.75 mM for GLC19 and 5.5-fold from 4.5 for GLC14 to 24.8 ng/ml for GLC19, respectively. Simi- larly, IC50 values for GLC14 and GLC19 for vinblastine were 445 and 1936 ng/ml, for vincristine, 145.7 and 179.9 ng/ml and for actinomycin, 0.084 and 0.337 µg/ml, revealing an increased drug resistance for GLC19 cells for doxorubicin, etoposide, Vinca alkaloids and actino- mycin. Whereas in RPMI-1640 with 10% heat-inactivated fetal calf serum GLC14 cells grew almost completely attached to the culture flask, GLC19 cells formed float- ing aggregates and exhibited a prolonged doubling time of 44 hrs compared to 26 hrs for GLC14 cells [12]. Cis- platin was not used for treatment of this patient and we found a slight decrease of the chemosensitivity for this drug in GLC19 vs GLC14 cells in good agreement with a previous report [12]. In order to detect global changes in gene expression linked to SCLC tumor progression and chemoresistance, we compared gene transcripts of GLC14 and GLC19 cells using analysis by the Applied Biosystems Human Genome Survey Microarrays V2.0. Approximately 440 genes were more than 2.5-fold overexpressed in GLC19 cells and 100 genes were more than 3-fold downregu- lated in this cell line compared to GLC14 cells. Of the 440 upregulated transcripts, approximately 300 could be assigned to annotated genes. 40 of these with strongest elevations and highest relevance for the SCLC phenotype under investigation are listed in Table 1. 37/60 down- regulated transcripts could be traced to annotated genes. A panel of genes upregulated in GLC19 vs. GLC14 cells are involved in regulation of cell growth, differenti- ation and cell death/apoptosis. Overexpression of NGFR- AP1/BEX3, a p75NTR (low-affinity neurotrophin recep- tor p75)-associated cell death executor initially cloned from a human ovarian granulosa cell cDNA library as an unknown protein termed pHGR74, was likewise reported by Lawson et al. and constituted the most upregulated gene in our data set [13,14]. BEX3 belongs to a family of genes, including BEX1, NGFRAP1 (alias BEX3), BEXL1 (alias BEX4), and NGFRAP1L1 (alias BEX5) [15]. Both BEX1 and NGFRAP1 interact with p75NTR and modulate NGF signaling through NF-κB to regulate cell cycle, apoptosis and differentiation in neural tissues [16]. Enforced expression of NGFRAP1 in Chinese ham- ster ovary cells and MDA-MB-231 human breast cancer cells had little effect on the growth of the cells in vitro, while cellular growth was dramatically suppressed in vivo [17]. Furthermore, the cells stably transfected with NGFRAP1 did not respond neither to NGF nor TNF. These findings may be linked to the prolonged doubling time of GLC19 vs. GLC14 cells. In breast cancer cells, NGF inhibits ceramide-homolog-induced apoptosis through binding of p75NTR and NF-κB activation via BEX2. Additionally, overexpression of NGFRAP1 enhances the antiproliferative effect of tamoxifen at pharmacological dose. Furthermore, NGFR-AP1 was reported to consti- Copyright © 2012 SciRes. JCT  Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines 447 tute a marker of androgen-dependent primary prostate cancer [18]. A second cell cycle regulator, namely CDK5RAP1, specifically inhibits CDK5 activation by CDK5R1, which is the catalytic subunit of neuronal Cdc2-like kinase (Nclk) involved inneuronal cell differ- entiation, and apoptosis [19]. Increased expression was detected for the protoonco- gens myc and fos, as well for the growth factors neuro- tensin (NTS), MDK, PDGFD, serine/threonine protein kinase MST4 and Mps one binder kinase activators (MOBs). Upregulation of the expression of myc in GLC19 was previously described by Kok et al. [20]. Amplification and overexpression of the MYC family of oncogenes, MYC (c-Myc), MYCN (N-Myc) or MYCL1 (L-Myc), occurs in 18% - 31% of SCLCs, and is more common in chemorefractory disease [21]. Transcripts for VIPR1, FGFR1 and ERBB4 exhibited relative overex- pression in GLC19 cells, which indicates cellular re- sponses to their cognate growth factors VIP, FGF and EGF, respectively. VIP in SCLC is involved in upregula- tion of VEGF via fos, and FGFs as well as EGF are sup- port the proliferation of CSCs under serum-depleted conditions [22,23]. In general, neuropeptides can func- tion as autocrine growth factors in cancer cells or modu- late the microenvironment [24,25]. GLC19 cells overexpress WNT5B, a ligand for mem- bers of the frizzled family of seven transmembrane re- ceptors, SFRP1 which functions as modulator of Wnt signaling through direct interaction with Wnts, and TLE2, which inhibits the transcriptional activation mediated by β-catenin (CTNNB1) and TCF/LEF family members in Wnt signaling [26]. The Notch pathway powerfully in- fluences stem cell maintenance, development and cell fate and is increasingly recognized for the key roles it plays in cancer [27]. Notch promotes cell survival, an- giogenesis and resistance in numerous cancers, making it a promising target for cancer therapy. In respect to the Notch signaling pathway, MAML2, which acts as a tran- scriptional coactivator for Notch proteins as well as in- creased expression of JAG1 and JAG2, that are ligands for multiple Notch receptors are distinctive characteris- tics of GLC14 and GLC19 cells. Notch activation in- duces the transcription of both subunits of the soluble guanylyl cyclase (sGC) heterodimer, namely GUCY1A3 and GUCY1B3, which form the nitric oxide receptor [28]. Additionally, the core stem cell signaling networks, such as the Wnt, Notch and Hedgehog pathways, also criti- cally regulate the self-renewal and survival of CSCs [29]. A number of upregulated transcripts in GLC19 cells comprise genes involved in cell adhesion/cell membrane signaling in accordance with the exclusive finding of desmosomes in GLC19 exclusively in this series of SCLC lines [9]. CD44 cell-surface glycoprotein, which represents the receptor for hyaluronic acid (HA), is in- volved in cell-cell interactions, cell adhesion and migra- tion [30]. It mediates cell-cell and cell-matrix interact- tions through its affinity for HA and possibly also for other ligands such as osteopontin, collagens, and matrix metalloproteinases (MMPs). Furthermore, CD44 has been defined as a CIC marker in many tumor entities, promoting epithelial-to-mesenchymal transition (EMT), motility, endothelial cell and niche adhesion, thereby contributing to niche generation and modulation [31]. MUC15 may play a role in cell adhesion to the extracel- lular matrix (ECM) and promotes the oncogenic poten- tial of human colon cancer cells [32]. The upregulated transcripts PLEKHC1, SPOCK, CLDN1 and EMP2 con- nect ECM adhesion sites to the actin cytoskeleton, medi- ate calcium-independent cell-adhesion and regulate the surface display and signaling from selected integrin pairs, respectively [33-35]. NCAM2 is a close homolog of neural cell adhesion molecule NCAM1 (CD56), which is expressed in several types of tumors, constitutes a diag- nostic marker of SCLCs, and is associated with tumori- genesis in salivary gland tumors [36]. Compared with NCAM1, the function of NCAM2 in tumors, such as prostate and breast cancer, is unknown, but may repre- sent a therapeutic target [37]. Many principal antitumor agents, such as platinum complexes and doxorubicin are electrophiles or re- dox-active agents and may preferentially react with sulf- hydryl groups [38]. The elevated amount of glutathione (GSH) and the increased activity of GSTs can explain the resistance to alkylating agents and other drugs that act by releasing free radicals. Our finding of increased tran- scription of the GSTs GSTT1, GSTA4 and MGST2 in GLC19 cells is in good agreement with data published in a previous analysis of its mechanisms of resistance [10]. The GSH level of GLC19 and global GST activity were significantly increased compared to GLC14, whereas the amount of total sulfhydryl groups remained the same and, furthermore, L-buthionine-sulfoximine (BSO), which depletes the cellular GSH pool, increased both doxorubi- cin- and cisplatin-induced cytotoxicity. This seems to constitute an important mechanism of chemoresistance for GLC19 cells, since other effectors like P-gp are ab- sent in GLC19 [10]. ALDH1A1 was expressed at low level in GLC14 cells with the amount rising in the GLC19 cells. This gene is used as a CSC marker and is also associated with cyclo- phosphamide resistance [39-41]. The AKR1C1/13 genes encode members of the aldo/keto reductase super-family, that consists of more than 40 known enzymes, which catalyze the conversion of aldehyde and ketone moieties of metabolites and xenobiotics to corresponding alcohols by utilizing NADH and/or NADPH as cofactors. AKR1C1 and C2 were found as genes linked to poor clinical prognosis in non-small cell lung cancer (NSCLC) Copyright © 2012 SciRes. JCT  Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines 448 following characterization of elevated transcripts of the A549 CSCs Hoechst 33342-positive side population [42]. AKR1C13 transcription was increased in leukemia cells following transcription factor Meis1-triggered induction of a CSC phenotype [43]. In our data set MEIS1 (hCG1818196.1) was upregulated 3.5-fold in GLC19 compared to GLC14 cells. A family of cancer-related molecules are the MAGEs that are in normal tissues ex- clusively found in testis and often highly expressed in cancer cell lines and tumors. They are targets for many studies on immunotherapy for melanoma; however, since tumor xenografts of SCLC cell lines had a markedly lower amount of MAGEs, expression of these genes seems to be activated in tissue culture only and therefore not to represent a promising target for this tumor entity [44]. Lawson et al. analyzed the GLC14/16/19 series of SCLC cell lines using the Illumina Human WGv3.0 Ex- pression BeadChip microarray and identified polymerase B (POLB) and NKX2.2 as transcripts linked to resistance to etoposide [14]. Of the selected unique gene transcripts that were upregulated in GLC19 compared to GLC14 as published by Lawson et al. 18/23 probes were as well found. Additionally, we found overexpression of growth factors and their cognate receptors that was not reported before. Downregulationed gene expression in GLC19 in com- parison to GLC14 cells was detected for a compara- tively low number of approximately 60 transcripts. Sur- prisingly, GLC14 cells expressed significant amounts of MTs (1A, 1F, 1X, 3 and 4), which showed lower levels of transcripts after several cycles of therapy in GLC19 cells. Presence of these MTs in the chemonaive GLC14 cells is could be explained by a smoking history of the affected patient, which is known to be associated with increased expression of these heavy metal-binding pro- teins [45]. The tumor suppressor p53 regulates the ex- pression of various genes that promote apoptosis. TP53I11 is a direct target gene of p53 and was markedly upregulated in arsenic trioxide-induced apoptosis and, thus, downregulation of this protein may impede drug- induced apoptosis [46]. Downregulation of the transcrip- tion of TUBA3, TUBB4 and tubulin folding cofactor A TBCA seems to be a consequence of the crossresistance of GLC19 cells to Vinca alkaloids [47]. Sphingolipid metabolism constituted the most over- represented pathway in GLC19 vs. GLC14 cells. Sphin- golipids are known to play important roles in the regula- tion of cell proliferation, response to chemo-therapeutic agents, and/or prevention of cancer. [48,49]. Bioactive sphingolipids like ceramide, sphingosine 1-phosphate and globotriaosylceramide initiate and process cellular signaling to alter cell behaviour to respond immediately to oncogenic stress or treatment challenges. More than ten sphingolipids and glycosphingolipids selectively me- diate the expression of approximately 50 genes encoding c-myc, p21, c-fos, telomerase reverse transcriptase, cas- pase-9, Bcl-x, cyclooxygenase-2, MMPs, integrins, Oct-4, glucosylceramide synthase and P-gp [50]. Through di- verse functions of these genes, sphingolipids enduringly affect cellular processes of mitosis, apoptosis, migration, stemness of CSCs and cellular resistance to therapies. Mechanistic studies indicate that sphingolipids regulate gene expression by modulation of phosphorylation and acetylation of proteins that serve as transcription factors (β-catenin, Sp1), repressor of transcription (histone H3) and regulator (SRp30a) of RNA splicing. In conclusion, GLC19 cells are distinguished from GLC14 by a reduced growth rate, formation of multicel- lular spheroids, expression of CD44, increased expres- sion of components of the Wnt and Notch signaling pathways, as well as receptors of the growth factors FGFs and EGF and the elevated transcription of the CSC markers ALDH1A1 and AKR1C1/13. Although the mi- croarray results need to be confirmed by PCR, our find- ings are compatible with the typical signature estab- lished for CICs or CSCs and different tumor entities [51,52]. Characterization of CICs in SCLC is being on- going and increased expression of ABCG2, FGF1, IGF1, MYC, SOX1/2, WNT1, as well as genes involved in an- giogenesis, Notch and Hedgehog pathways was reported for this type of tumor cells that comprise a fraction of less than one percent in SCLC cell lines [53]. The gene expression profile of GLC19 cells indicates a ther- apy-induced increase of typical stem cell markers in vivo and enhanced cell adhesion and formation of spheroids may constitute an important part in the acquisition of chemoresistance through a reduced access of chemo- therapeutic drugs [54]. 5. Acknowledgements This work was funded by the “Medical Scientific Fund of the Mayor of the City of Vienna“, project number 11016. We wish to thank the team of the “Oncology Lab” of the University Clinic of Gynecology, Medical University of Vienna, Austria, for help with the microarry experiments. REFERENCES [1] D. R. Youlden, S. M. Cramb and P. D. Baade, “The In- ternational Epidemiology of Lung Cancer: Geographical Distribution and Secular Trends,” Journal of Thoracic Oncology, Vol. 3, No. 8, 2008, pp. 819-831. doi:10.1097/JTO.0b013e31818020eb [2] S. G. Spiro and G. A. Silvestri, “One Hundred Years of Lung Cancer,” American Journal of Respiratory Critical Care Medicine, Vol. 172, No. 5, 2005, pp. 523-529. doi:10.1164/rccm.200504-531OE [3] R. Califano, A. Z. Abidin, R. Peck, C. Faivre-Finn and P. Copyright © 2012 SciRes. JCT  Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines 449 Lorigan, “Management of Small Cell Lung Cancer: Re- cent Developments for Optimal Care,” Drugs, Vol. 72, No. 4, 2012, pp. 471-490. doi:10.2165/11597640-000000000-00000 [4] R. Govindan, N. Page, D. Morgensztern, et al., “Chang- ing Epidemiology of Small-Cell Lung Cancer in the United States over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database,” Journal of Clinical Oncology, Vol. 24, 2006, pp. 4539- 4544. doi:10.1200/JCO.2005.04.4859 [5] W. N. William and B. S. Glisson, “Novel Strategies for the Treatment of Small-Cell Lung Carcinoma, “Nature Reviews Clinical Oncology, Vol. 8, No. 10, 2011, pp. 611-619. doi:10.1038/nrclinonc.2011.90 [6] Y. Agra, M. Pelayo, M. Sacristan, et al., “Chemotherapy versus Best Supportive Care for Extensive Small Cell Lung Cancer,” Cochrane Database Systematic Review, Vol. 4, 2003, Article ID: CD001990. [7] M. Takada, M. Fukuoka, M. Kawahara, et al., “Phase III Study of Concurrent versus Sequential Thoracic Radio- therapy in Combination with Cisplatin and Etoposide for Limited-Stage Small-Cell Lung Cancer,” Journal of Cli- nical Oncology, Vol. 20, 2002, pp. 3054-3060. doi:10.1200/JCO.2002.12.071 [8] B. Fischer and A. Arcaro, “Current Status of Clinical Trials for Small Cell Lung Cancer,” Review of Recent Clinical Trials, Vol. 3, 2008, pp. 40-61. doi:10.2174/157488708783330503 [9] H. H. Berendsen, L. de Leij, E. G. de Vries, G. Mesander, N. H. Mulder, B. de Jong, C. H. Buys, P. E. Postmus, S. Poppema, H. J. Sluiter, et al., “Characterization of Three Small Cell Lung Cancer Cell Lines Established from One Patient during Longitudinal Follow-Up,” Cancer Re- search, Vol. 48, No. 23, 1988, pp. 6891-6899. [10] V. G. Tusher, R. Tibshirani and G. Chu, “Significance Analysis of Microarrays Applied to the Ionizing Radia- tion Response,” Proceedings of the National Academy of Science of the USA, Vol. 98, No. 9, 2001, pp. 5116-5121. doi:10.1073/pnas.091062498 [11] D. Croft, G. O’Kelly, G. Wu, R. Haw, M. Gillespie, L. Matthews, M. Caudy, P. Garapati, G. Gopinath, B. Jassal, S. Jupe, I. Kalatskaya, S. Mahajan, B. May, N. Ndegwa, E. Schmidt, V. Shamovsky, C. Yung, E. Birney, H. Hermjakob, P. D’Eustachio and L. Stein, “Reactome: A Database of Reactions, Pathways and Biological Proc- esses,” Nucleic Acids Research, Vol. 39, 2011, pp. D691- D697. doi:10.1093/nar/gkq1018 [12] E. G. de Vries, C. Meijer, H. Timmer-Bosscha, H. H. Berendsen, L. de Leij, R. J. Scheper and N. H. Mulder, “Resistance Mechanisms in Three Human Small Cell Lung Cancer Cell Lines Established from One Patient during Clinical Follow-Up,” Cancer Research, Vol. 49, No. 15, 1989, pp. 4175-4178. [13] G. Rapp, J. Freudenstein, J. Klaudiny, J. Mucha, F. Wempe, M. Zimmer and K. H. Scheit, “Characterization of Three Abundant mRNAs from Human Ovarian Gran- ulosa Cells,” DNA Cell Biology, Vol. 9, No. 7, 1990, pp. 479-485.doi:10.1089/dna.1990.9.479 [14] M. H. Lawson, N. M. Cummings, D. M. Rassl, R. Russell, J. D. Brenton, R. C. Rintoul and G. Murphy, “Two Novel Determinants of Etoposide Resistance in Small Cell Lung Cancer,” Cancer Research, Vol. 71, No. 14, 2011, pp. 4877-4887. doi:10.1158/0008-5472.CAN-11-0080 [15] A. Naderi, A. E. Teschendorff, J. Beigel, M. Cariati, I. O. Ellis, J. D. Brenton and C. Caldas, “BEX2 Is Overex- pressed in a Subset of Primary Breast Cancers and Medi- ates Nerve Growth Factor/Nuclear Factor-KappaB Inhibi- tion of Apoptosis in Breast Cancer Cell Lines,” Cancer Research, Vol. 67, No. 14, 2007, pp. 6725-6736. doi:10.1158/0008-5472.CAN-06-4394 [16] N. H. Molloy, D. E. Read and A. M. Gorman, “Nerve Growth Factor in Cancer Cell Death and Survival,” Can- cers, Vol. 3, 2011, pp. 510-530. doi:10.3390/cancers3010510 [17] X. Tong, D. Xie, W. Roth, J. Reed, H. P. Koeffler, “NADE (p75NTR-Associated Cell Death Executor) Sup- press Cellular Growth in Vivo,” International Journal of Oncology, Vol. 22, 2003, pp. 1357-1362. [18] T. L. Romanuik, T. Ueda, N. Le, S. Haile, T. M. Yong, T. Thomson, R. L. Vessella and M. D. Sadar, “NGFRAP1 Novel Biomarkers for Prostate Cancer Including Non- coding Transcripts,” American Journal of Pathology, Vol. 175, No. 6, 2006, pp. 2264-2276. doi:10.2353/ajpath.2009.080868 [19] Y. P. Ching, A. S. Pang, W. H. Lam, R. Z. Qi and J. H. Wang, “Identification of a Neuronal Cdk5 Activator- Binding Protein as Cdk5 Inhibitor,” Journal of Biological Chemistry, Vol. 277, No. 18, 2002, pp. 15237-15240. doi:10.1074/jbc.C200032200 [20] K. Kok, J. Osinga, D. C. Schotanus, H. H. Berendsen, L. F. de Leij and C. H. Buys, “Amplification and Expression of Different Myc-Family Genes in a Tumor Specimen and 3 Cell Lines Derived from One Small-Cell Lung Cancer Patient during Longitudinal Follow-Up,” International Journal of Cancer, Vol. 44, No. 1, 1989, pp. 75-78. doi:10.1002/ijc.2910440114 [21] I. I. Wistuba, A. F. Gazdar and J. D. Minna, “Molecular Genetics of Small Cell Lung Carcinoma,” Seminars in Oncology, Vol. 28, No. 2-4, 2001, pp. 3-13. [22] Z. Zhao, Q. Cheng, X. Li, X.Wang and K. Liu, “c-fos Antisense Oligodeoxynucleotide Reduces VIP-Induced Upregulation of VEGF Expression in Small Cell Lung Cancer Cells,” Chinese Journal of Lung Cancer, Vol. 9, No. 4, 2006, pp. 312-315. [23] C. A. Gilbert and A. H. Ross, “Cancer Stem Cells: Cell Culture, Markers and Targets for New Therapies,” Jour- nal of Cellular Biochemistry, Vol. 108, No. 5, 2009, pp. 1031-1038. doi:10.1002/jcb.22350 [24] T. W. Moody, D. Chan, J. Fahrenkrug and R. T. Jensen, “Neuropeptides as Autocrine Growth Factors in Cancer Cells,” Current Pharmaceutical Design, Vol. 9, No. 6, 2003, pp. 495-509. doi:10.2174/1381612033391621 [25] U. Olszewski and G. Hamilton, “Neurotensin Signaling Induces Intracellular Alkalinization and Interleukin-8 Ex- pression in Human Pancreatic Cancer Cells,” Molecular Oncology, Vol. 3, 2009, pp. 204-213. doi:10.1016/j.molonc.2009.01.006 [26] H. Yao, E. Ashihara and T. Maekawa, “Targeting the Copyright © 2012 SciRes. JCT  Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines 450 Wnt/β-Catenin Signaling Pathway in Human Cancers,” Expert Opinion on Therapeutic Targets, Vol. 15, No. 7, 2011, pp. 873-887. doi:10.1517/14728222.2011.577418 [27] B. Purow, “Notch Inhibition as a Promising New Ap- proach to Cancer Therapy,” Advances in Experimental Medicine and Biology, Vol. 727, 2012, pp. 305-319. doi:10.1007/978-1-4614-0899-4_23 [28] A. C. Chang, Y. Fu, V. C. Garside, K. Niessen, L. Chang, M. Fuller, A. Setiadi, J. Smrz, A. Kyle, A. Minchinton, M. Marra, P. A. Hoodless and A. Karsan, “Notch Initiates the Endothelial-to-Mesenchymal Transition in the Atrioven- tricular Canal through Autocrine Activation of Soluble Guanylyl Cyclase,” Developmental Cell, Vol. 21, No. 2, 2011, pp. 288-300. doi:10.1016/j.devcel.2011.06.022 [29] J. Wang, B. A. Sullenger and J. N. Rich, “Notch Signal- ing in Cancer Stem Cells,” Advances in Experimental Medicine and Biology, Vol. 727, 2012, pp. 174-185. doi:10.1007/978-1-4614-0899-4_13 [30] J. Lesley, R. Hyman and P. W. Kincade, “CD44 and Its Interaction with Extracellular Matrix,” Advances in Im- munology, Vol. 54, 1993, pp. 271-335. doi:10.1016/S0065-2776(08)60537-4 [31] M. Zöller, “CD44: Can a Cancer-Initiating Cell Profit from an Abundantly Expressed Molecule?” Nature Re- views Cancer, Vol. 11, No. 4, 2011, pp. 254-267. doi:10.1038/nrc3023 [32] J. Huang, M. I. Che, Y. T. Huang, M. K. Shyu, Y. M. Huang, Y. M. Wu, W. C. Lin, P. H. Huang, J. T. Liang, P. H. Lee and M. C. Huang, “Overexpression of MUC15 Activates Extracellular Signal-Regulated Kinase 1/2 and Promotes the Oncogenic Potential of Human Colon Can- cer Cells,” Carcinogenesis, Vol. 30, No. 8, 2009, pp. 1452-1458. doi:10.1093/carcin/bgp137 [33] F. Siddiq, F. H. Sarkar, A. Wali, H. I. Pass and F. Lonardo, “Increased Osteonectin Expression Is Associ- ated with Malignant Transformation and Tumor Associ- ated Fibrosis in the Lung,” Lung Cancer, Vol. 45, No. 2, 2004, pp. 197-205.doi:10.1016/j.lungcan.2004.01.020 [34] M. Fu, E. L. Maresh, R. A. Soslow, M. Alavi, V. Mah, Q. Zhou, A. Iasonos, L. Goodglick, L. K. Gordon, J. Braun and M. Wadehra, “Epithelial Membrane Protein-2 Is a Novel Therapeutic Target in Ovarian Cancer,” Clinical Cancer Research, Vol. 16, No. 15, 2010, pp. 3954-3963. doi:10.1158/1078-0432.CCR-10-0368 [35] R. O. Hynes, “The Extracellular Matrix: Not Just Pretty Fibrils,” Science, Vol. 326, No. 5957, 2009, pp. 1216- 1219. doi:10.1126/science.1176009 [36] M. Jensen and F. Berthold, “Targeting the Neural Cell Adhesion Molecule in Cancer,” Cancer Letters, Vol. 258, 2007, pp. 9-21. doi:10.1016/j.canlet.2007.09.004 [37] S. Takahashi, K. Kato, K. Nakamura, R. Nakano, K. Ku- bota and H. Hamada, “Neural Cell Adhesion Molecule 2 as a Target Molecule for Prostate and Breast Cancer Gene Therapy,” Cancer Science, Vol. 102, No. 4, 2011, pp. 808-814. doi:10.1111/j.1349-7006.2011.01855.x [38] M. Namdarghanbari, W. Wobig, S. Krezoski, N. M. Ta- batabai and D. H. Petering, “Mammalian Metallothionein in Toxicology, Cancer, and Cancer Chemotherapy,” Journal of Biologic Inorganic Chemistry, Vol. 16, No. 7, 2011, pp. 1087-1101. [39] J. S. Moreb, “Aldehyde Dehydrogenase as a Marker for Stem Cells,” Current Stem Cell Research and Therapy, Vol. 3, No. 4, 2008, pp. 237-246. doi:10.2174/157488808786734006 [40] J. Hilton, “Role of Aldehyde Dehydrogenase in Cyclo- phosphamide-Resistant L1210 Leukemia,” Cancer Re- search, Vol. 44, No. 11, 1984, pp. 5156-5160. [41] X. Li, L. Wan, J. Geng, C. L. W and X. Bai, “Aldehyde Dehydrogenase 1A1 Possesses Stem-Like Properties and Predicts Lung Cancer Patient Outcome,” Journal of Tho- racic Oncology, 2012, in Press. doi:10.1097/JTO.0b013e318257cc6d [42] D. C. Seo, J. M. Sung, H. J. Cho, H. Yi, K. H. Seo, I. S. Choi, D. K. Kim, J. S. Kim, A. El-Aty and H. C. Shin, “Gene Expression Profiling of Cancer Stem Cell in Hu- man Lung Adenocarcinoma A549 Cells,” Molecular Cancer, Vol. 6, No. 75, 2007. doi:10.1186/1476-4598-6-75 [43] G. G. Wang, M. P. Pasillas and M. P. Kamps, “Meis1 Programs Transcription of FLT3 and Cancer Stem Cell Character, Using a Mechanism That Requires Interaction with Pbx and a Novel Function of the Meis1 C-Termi- nus,” Blood, Vol. 106, No. 1, 2005, pp. 254-264. doi:10.1182/blood-2004-12-4664 [44] N. Pedersen, S. Mortensen, S. B. Sørensen, M. W. Peder- sen, K. Rieneck, L. F. Bovin and H. S. Poulsen, “Tran- scriptional Gene Expression Profiling of Small Cell Lung Cancer Cells,” Cancer Research, Vol. 63, No. 8, 2003, pp. 1943-1953. [45] Koomägi, G. Stammler, C. Manegold, J. Mattern and M. Volm, “Expression of Resistance-Related Proteins in Tumoral and Peritumoral Tissues of Patients with Lung Cancer,” Cancer Letters, Vol. 110, No. 1-2, 1996, pp. 129-136. doi:10.1016/S0304-3835(96)04471-0 [46] X. Q. Liang, E. H. Cao, Y. Zhang and J. F. Qin, “A P53 Target Gene, PIG11, Contributes to Chemosensitivity of Cells to Arsenic Trioxide,” FEBS Letters, Vol. 569, No. 1-3, 2004, pp. 94-98. doi:10.1016/j.febslet.2004.05.057 [47] A. Cormier, M. Knossow, C. Wang and B. Gigant, “The Binding of Vinca Domain Agents to Tubulin: Structural and Biochemical Studies,” Methods in Cell Biology, Vol. 95, 2010, pp. 373-390. doi:10.1016/S0091-679X(10)95020-6 [48] B. Ogretmen, “Sphingolipids in Cancer: Regulation of Pathogenesis and Therapy,” FEBS Letters, Vol. 580, No. 23, 2006, pp. 5467-5476. doi:10.1016/j.febslet.2006.08.052 [49] G. A. Patwardhan and Y. Y. Liu, “Sphingolipids and Expression Regulation of Genes in Cancer,” Progess in Lipid Research, Vol. 50, No. 1, 2011, pp. 104-114. doi:10.1016/j.plipres.2010.10.003 [50] M. Kohno, M. L. Momoi, J. H. Oo, Y. M. Paik, K. Lee, Venkataraman, et al., “Intracellular Role for Sphingosine Kinase 1 in Intestinal Adenoma Cell Proliferation,” Mo- lecular Cell Biology, Vol. 26, 2006, pp. 7211-7223. doi:10.1128/MCB.02341-05 Copyright © 2012 SciRes. JCT  Therapy-Induced Changes of Gene Expression in a Matched Pair of Small Cell Lung Cancer (SCLC) Cell Lines Copyright © 2012 SciRes. JCT 451 [51] B. Malik and D. Nie, “Cancer Stem Cells and Resistance to Chemo and Radio Therapy,” Frontiers of Bioscience, Vol. 4, 2012, pp. 2142-2149. doi:10.2741/E531 [52] M. R. García Campelo, G. A. Curbera, G. Aparicio Gallego, E. G. Pulido and L. M. A. Aparicio, “Stem Cell and Lung Cancer Development: Blaming the Wnt, Hh and Notch Signalling Pathway,” Clinical and Transla- tional Oncology, Vol. 13, No. 2, 2011, pp. 77-83. doi:10.1007/s12094-011-0622-0 [53] C. D. Salcido, A. Larochelle, B. J. Taylor, C. E. Dunbar and L. Varticovski, “Molecular Characterisation of Side Population Cells with Cancer Stem Cell-Like Character- istics in Small-Cell Lung Cancer,” British Journal of Cancer, Vol. 102, No. 11, 2010, pp. 1636-1644. doi:10.1038/sj.bjc.6605668 [54] R. E. Durand and P. L. Olive, “Resistance of Tumor Cells to Chemo- and Radiotherapy Modulated by the Three- Dimensional Architecture of Solid Tumors and Sphe- roids,” Methods in Cell Biology, Vol. 64, 2001, pp. 211- 233. doi:10.1016/S0091-679X(01)64015-9
|