 Journal of Cancer Therapy, 2012, 3, 412-423 http://dx.doi.org/10.4236/jct.2012.324054 Published Online September 2012 (http://www.SciRP.org/journal/jct) Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) Yixia Li1, Yea-Jyh Chen2, Li-Jung Chang3, Michael Hendryx1,4, Juhua Luo1,4,5* 1Department of Community Medicine, School of Medicine, West Virginia University, Morgantown, USA; 2School of Nursing, West Virginia University, Morgantown, USA; 3Department of Nursing, Tzu Chi College of Technology, Hualien, Taiwan; 4West Virginia Rural Health Research Center, Morgantown, USA; 5Mary Babb Randolph Cancer Center, West Virginia University, Morgantown, USA. Email: *jiluo@hsc.wvu.edu Received May 9th, 2012; revised June 11th, 2012; accepted June 30th, 2012 ABSTRACT Introduction: Lung cancer is the leading cause of cancer death worldwide with poor survival rates. However, the prog- nostic factors for survival of patients with lung cancer are not well-established. In this study, we examined the impact of routine laboratory biomarkers and traditional factors on survival of patients with non-small cell lung cancer (NSCLC). Method: Secondary data analysis was conducted from a retrospective study of 404 patients with newly diagnosed lung cancer in 2005-2007 in Taiwan. There were eight routine laboratory biomarkers and eight traditional factors investi- gated in the analyses. Cox proportional hazards model was used to assess the hazard ratios for the association between risk factors and patient overall survival. The Kaplan-Meier method was used to compare survival curves for each prog- nostic indicator. Results: High WBC counts (HR = 1.798, 95%CI: 1.225 - 2.639), low Hgb level (HR = 1.437, 95%CI: 1.085 - 1.903), and low serum albumin level (HR = 2.049, 95%CI: 1.376 - 3.052) were significant laboratory prognostic biomarkers for poor NSCLC survival. Additionally we confirmed the traditional prognostic factors for poor overall sur- vival among NSCLC patients, including older age, comorbidity conditions, advanced cancer stage, and non-surgical treatment. Conclusions: This study identified three available laboratory biomarkers, high WBC counts, low Hgb level, and low serum albumin level, to be significant prognostic factors for poorer overall survival in NSCLC patients. Further prognostic evaluation studies are warranted to compare different ethnic groups on the prognostic values of these clinical parameters in NSCLC survival outcomes. These identified prognostic biomarkers should be included in early risk screening of hospitalized lung cancer patient population. Keywords: Clinical Biomarkers; Non-Small Cell Lung Cancer (NSCLC); Prognostic Factors 1. Introduction Lung cancer is the leading cause of cancer death world- wide [1]. An estimated 1.6 million new cases of lung cancer occurred worldwide in 2008, accounting for about 13% of total cancer diagnoses [2]; and an estimated 951,000 men and 427,400 women died from the disease worldwide in 2008. Histologically, lung cancer can be classified into two types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC ac- counts for 85% of lung cancer cases, and approximately 65% of patients with NSCLC present with advanced- stage (III or IV) disease [3]. In addition, lung cancer is predominantly a disease of elderly people: the median age of newly diagnosed lung cancer patients is approxi- mately 68 years and as many as 40% of patients are older than 70 years [4]. The five-year survival rates among patients with NSCLC after complete resection are 46% - 81% with stage IA to IIB, compared to 21% - 24% with stage IIIA to IV [5]. Furthermore, overall survival among patients receiving chemotherapy also remains poor with a median 4 to 15 monthssurvival time [6]. Therefore, study- ing prognostic biomarkersas survival predictors may con- tribute toimproveddisease prognosis and treatment guide- lines for lung cancer. Prognostic predictors for lung cancer outcomes are inconsistent in previous literature. The most widely ac- cepted prognostic factors for patients with NSCLC in- clude tumor stage, performance status, and weight loss [7-12]. Besides these factors, biochemical or hematologic markers, such as white blood cell (WBC) counts, neu- trophil counts and hemoglobin (Hgb) level, are also as- sociated with NSCLC survival. A previous study per- formed by the North Central Cancer Treatment Group (NCCTG), which included 1053 lung cancer patients, *Corresponding author. Copyright © 2012 SciRes. JCT  Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 413 revealed that patients who had high WBC counts and low Hgb levels had significantly worse survival than their counterparts [13]. However, the prognostic importance of complete blood count findings were inconsistent with other studies [14-18] where no significant association was found for WBC counts or Hgb level. In addition, neutrophil counts in a study by Ferrigno et al. were found to independently predict the survival of NSCLC patients [19]. Similar to Ferrigno’s result, the European Lung Cancer Working Group found that a high-neutrophil count was an independent prognostic factor for poor sur- vival in patients with unresectable advanced NSCLC [20]. Paddisonalso reported that high level neutrophil counts and low Hgblevel predicted poor survival of NSCLC patients [21]. Furthermore, another studyfound that plate- let countswere associated with poor overall survival in aunivariate model but werenot significant in multivari- ate analysis [22]. There may be additional biochemical markers related to lung cancer survival, such as serum albumin level and serum sodium level. Serum albumin level, a nutritional indicator, has been examined as a prognostic lung cancer marker among both SCLC and NSCLC patients in se- veral previous studies [12,23,24]. Maeda et al. observed that serum albumin level was one of the most significant prognostic factors for advanced NSCLC in both univari- ate and multivariate analysis [12]. In Win’s study, serum albumin level was identified to be a significant prognos- tic factor in univariatemodelbut not inmultivariable ana- lysis for lung cancer patients [23]. A similar association between serum albumin level and lung cancer survival was observed by Tas et al. [24]. In addition, abnormal serum sodium level has been studied asa poor survival predictor for lung cancer. Previous research has reported thathyponatremia predicts poor survival of SCLC pa- tients [15,25-27], but there appears to be no previous research onhyponatremiain relation to NSCLC survival outcome. Because of incomplete and in some cases inconsistent evidence on prognostic biomarkers for NSCLC survival, the aim of this study was to examine the relationships between routine laboratory parameters including WBC counts, neutrophil percentage, lymphocyte percentage, Hgblevel, platelet counts, serum albumin level, and se- rum sodium level on overall survival in NSCLC patients. 2. Materials and Methods 2.1. Study Design and Measures This study is a secondary data analysis derived froma retrospective study of patients with newly diagnosed lung cancer in the years 2005-2007 in Taiwan. Detailed de- scription of this study sample design and methods are available in Luo et al. [28]. In brief, the retrospective study screened potential patients through hospital medi- cal records. Eligible patients were selected based on ini- tial hospitalization with lung cancer as the primary diag- nosis, who were discharged to home and were older than 40 years old. There were 404 patients eligible in the study for final analysis. Patients’ characteristics, extracted from hospital re- cords, include age, gender, the Eastern Cooperative On- cology Group (ECOG) performance (PS) (0 - 5 with 0 denoting perfect health and 5 denoting death) [29], smok- ing status (never, former, current), body mass index (BMI; kg/m2), Charlson comorbidity Index (CCI; ex- cluded lung cancer as the primary disease), tumor stage (less advanced—stage I, II and IIIA and advanced—stage IIIB and IV), cancer treatment (surgery with/without one or more types of supportive care [e.g. chemotherapy, radiation, tyrosin kinase inhibitor targeted therapy], che- motherapy with/without other supportive care, or no can- cer treatment). The CCI, a weighted index score with a possible range of 0 - 35, wasused to evaluate patients’ comorbid conditions according to the influence of co- morbidity on overall mortality risk [30,31]. The patients’ BMI was calculated and classified into four categories: underweight (<18.5 kg/m2), normal (18.5 - <25 kg/m2), overweight (25 - <30 kg/m2) and obese (>=30 kg/m2). Laboratory data including WBC counts, Hgb level, neu- trophil percentage, lymphocyte percentage, platelet counts, monocyte percentage, serum albumin level, and serum sodium level were measured at the index visit. These variables were considered as the main prognostic factors of interest for the study. We used the admission or first-time measure if more than one set of laboratory bio- markers were examined during the index visit. In order not to lose study power, we kept patients with missing data in the final analysis and categorized those patients as an additional group. The outcome of interest in this study was survival duration, which was defined as the interval in months from the discharge date of the index visit to death from any cause. Patients’ survival data were follow- ed through cancer registry records until the end of 2010. 2.2. Statistical Analysis We categorized all potential prognostic variables includ- ing WBC counts, Hgb level, neutrophil percentage, lym- phocyte percentage, platelet counts, monocyte percentage, albumin level, and sodium level into normal and abnor- mal values according to standard laboratory norms (cut- points are listed in Table 1) [32]. For sodium level, we divided it into two groups since therewas only one pa- tient with sodium level higher than 145 (mmol/l). For univariate analyses, we estimated survival curves using the Kaplan-Meier method and compared these curves by the log-rank test. Survival duration was also estimated by fitting the data with a Cox regression model [33]. All variables reaching statistical significance (p < Copyright © 2012 SciRes. JCT 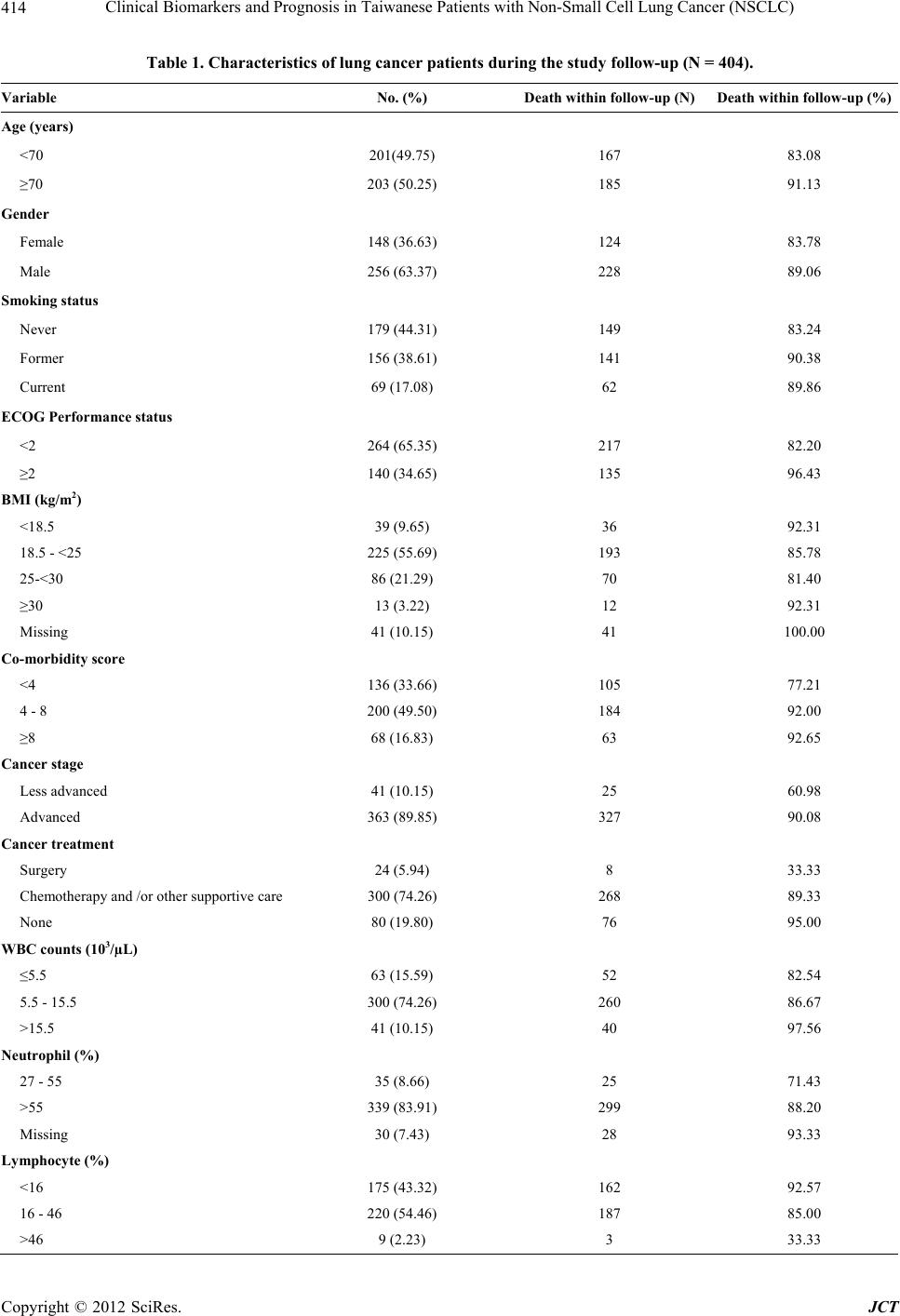 Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) Copyright © 2012 SciRes. JCT 414 Table 1. Characteristics of lung cancer patients during the study follow-up (N = 404). Variable No. (%) Death within follow-up (N) Death within follow-up (%) Age (years) <70 201(49.75) 167 83.08 ≥70 203 (50.25) 185 91.13 Gender Female 148 (36.63) 124 83.78 Male 256 (63.37) 228 89.06 Smoking status Never 179 (44.31) 149 83.24 Former 156 (38.61) 141 90.38 Current 69 (17.08) 62 89.86 ECOG Performance status <2 264 (65.35) 217 82.20 ≥2 140 (34.65) 135 96.43 BMI (kg/m2) <18.5 39 (9.65) 36 92.31 18.5 - <25 225 (55.69) 193 85.78 25-<30 86 (21.29) 70 81.40 ≥30 13 (3.22) 12 92.31 Missing 41 (10.15) 41 100.00 Co-morbidity score <4 136 (33.66) 105 77.21 4 - 8 200 (49.50) 184 92.00 ≥8 68 (16.83) 63 92.65 Cancer stage Less advanced 41 (10.15) 25 60.98 Advanced 363 (89.85) 327 90.08 Cancer treatment Surgery 24 (5.94) 8 33.33 Chemotherapy and /or other supportive care 300 (74.26) 268 89.33 None 80 (19.80) 76 95.00 WBC counts (103/µL) ≤5.5 63 (15.59) 52 82.54 5.5 - 15.5 300 (74.26) 260 86.67 >15.5 41 (10.15) 40 97.56 Neutrophil (%) 27 - 55 35 (8.66) 25 71.43 >55 339 (83.91) 299 88.20 Missing 30 (7.43) 28 93.33 Lymphocyte (%) <16 175 (43.32) 162 92.57 16 - 46 220 (54.46) 187 85.00 >46 9 (2.23) 3 33.33  Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 415 Continued Hemoglobin (g/dL) <11.5 122 (30.20) 118 96.72 11.5 - 13.5 152 (37.62) 129 84.87 >13.5 130 (32.18) 105 80.77 Platelet counts (Thou/μl) <150 157(38.86) 133 84.71 150 - 400 164 (40.59) 144 87.80 >400 51 (12.62) 48 94.12 Missing 32 (7.92) 27 84.38 Monocyte (%) <4 99 (24.50) 85 85.86 4 - 11 280 (69.31) 243 86.79 >11 25 (6.19) 24 96.00 Albumin (g/dl) <3.1 40 (9.90) 40 100.00 3.1 - 4.3 175 (43.32) 151 86.29 >4.3 28 (6.93) 20 71.43 missing 161 (39.85) 141 87.58 Sodium (mmol/l) <136 189 (46.78) 177 93.65 136 - 147 215 (53.22) 175 81.40 0.05) at the univariate level were included in the multi- variate analysis. We performed Pearson correlations among the selected variables for collinearity before the multivariate analysis. If any two variables were highly correlated (r > 0.6), only one was selected in the multi- variate model. We used the Cox proportional hazards model to perform the multivariate analysis to adjust for all included variables. The hazard ratios were calculated to assess the death risk for various prognostic factors. All statistical analyses were done using SAS version 9.2 with a significant p-value criterion of 0.05 or less. 3. Results 3.1. Baseline Patient Characteristics Of the total 404 patients with newly diagnosed NSCLC, the median follow-up duration time was 10.7 months (range, 0 - 70 months). Mean age of the study sample population was 67.6 years (SD = 11.0). Over 60% of the patients (N = 256) were male; approximately 39% of pa- tients were former smokers and 17% were current smok- ers. The majority of patients (90%) were diagnosed at an advanced stage (stage IIIB or stage IV). Overall, 352 patients (87%) died by the end of 2010. Table 1 summa- rizes the study patient characteristics. 3.2. Univariate Analysis Univariate analysis revealed the following patient char- acteristics to be significant prognostic factors for poor survival: older age (≥70 years), male, current smoker, poor performance status (PS ≥ 2), low BMI (≤18.5 kg/m2), high CCI score (≥4), advanced cancer stage (IIIB and IV), cancer treatment (chemotherapy with/without other supportive cancer treatment or no cancer treatment), high WBC (>15.5 × 103/µL), high neutrophil percentage (>55%), lower (<16%)/ higher (>46%) lymphocyte per- centage, low Hgblevel (<11.5 g/dl), lower (<3.1 g/dl)/ higher (>4.3 g/dl) albumin level, and low sodium level (<136 mmol/l). Other variables, including platelet counts and monocyte percentage were not observed to be sig- nificantly associated with overall NSCLC survival (p > 0.05; see Table 2). In patients with advanced cancer stage, the median survival time was 10.1 (95%CI: 8.6 - 12.0) months com- pared with 26.2 survival months among those patients with less advanced stage (p < 0.0001). In patients with high WBC counts, the median survival was 1.93 (95%CI: 1.1 - 4.1) months, compared with the patients with nor- mal and low WBC counts (11.9 vs 14.3 survival months, respectively; p < 0.0001). Similarly, patients with low Copyright © 2012 SciRes. JCT 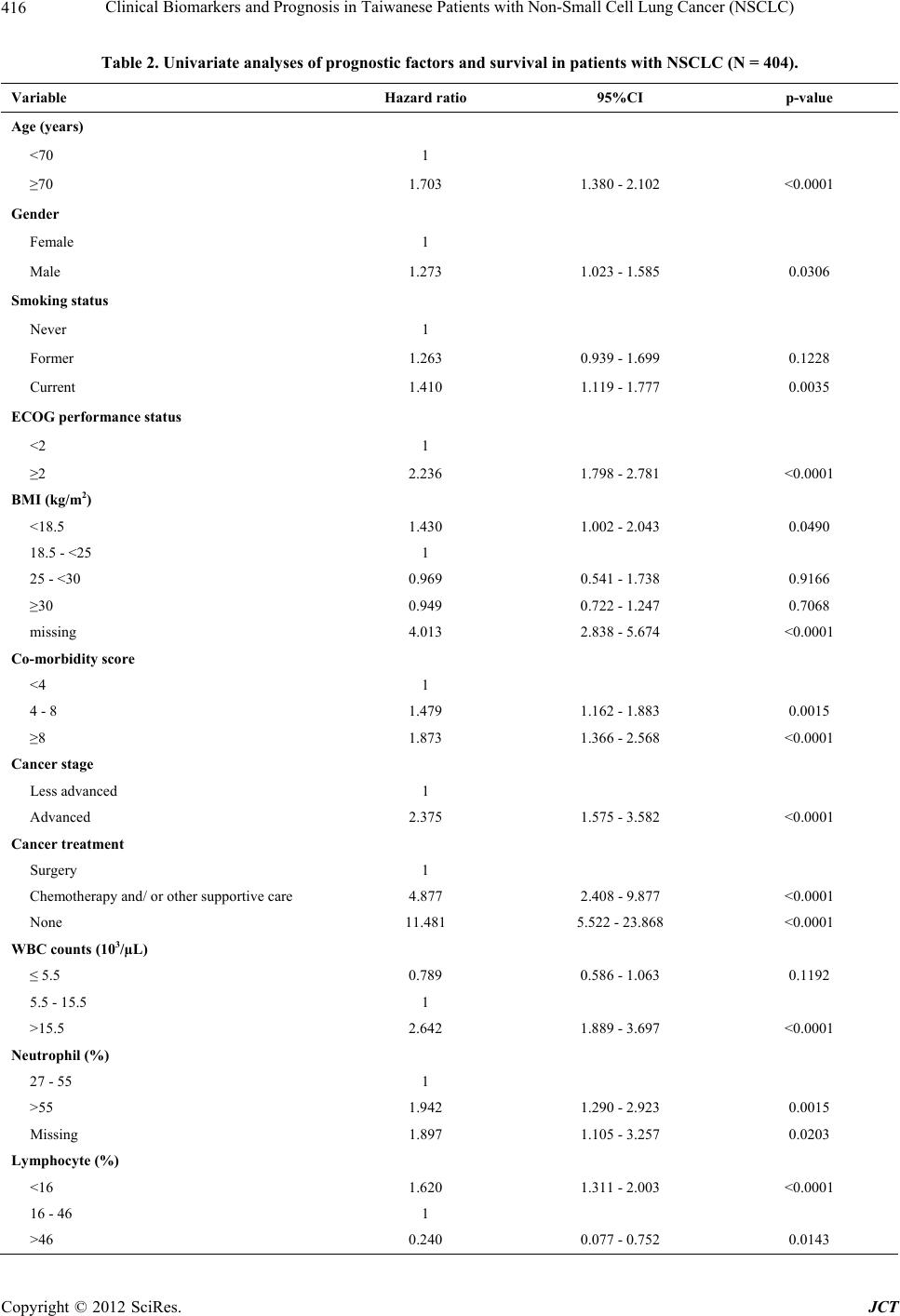 Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 416 Table 2. Univariate analyses of prognostic factors and survival in patients with NSCLC (N = 404). Variable Hazard ratio 95%CI p-value Age (years) <70 1 ≥70 1.703 1.380 - 2.102 <0.0001 Gender Female 1 Male 1.273 1.023 - 1.585 0.0306 Smoking status Never 1 Former 1.263 0.939 - 1.699 0.1228 Current 1.410 1.119 - 1.777 0.0035 ECOG performance status <2 1 ≥2 2.236 1.798 - 2.781 <0.0001 BMI (kg/m2) <18.5 1.430 1.002 - 2.043 0.0490 18.5 - <25 1 25 - <30 0.969 0.541 - 1.738 0.9166 ≥30 0.949 0.722 - 1.247 0.7068 missing 4.013 2.838 - 5.674 <0.0001 Co-morbidity score <4 1 4 - 8 1.479 1.162 - 1.883 0.0015 ≥8 1.873 1.366 - 2.568 <0.0001 Cancer stage Less advanced 1 Advanced 2.375 1.575 - 3.582 <0.0001 Cancer treatment Surgery 1 Chemotherapy and/ or other supportive care 4.877 2.408 - 9.877 <0.0001 None 11.481 5.522 - 23.868 <0.0001 WBC counts (103/µL) ≤ 5.5 0.789 0.586 - 1.063 0.1192 5.5 - 15.5 1 >15.5 2.642 1.889 - 3.697 <0.0001 Neutrophil (%) 27 - 55 1 >55 1.942 1.290 - 2.923 0.0015 Missing 1.897 1.105 - 3.257 0.0203 Lymphocyte (%) <16 1.620 1.311 - 2.003 <0.0001 16 - 46 1 >46 0.240 0.077 - 0.752 0.0143 Copyright © 2012 SciRes. JCT 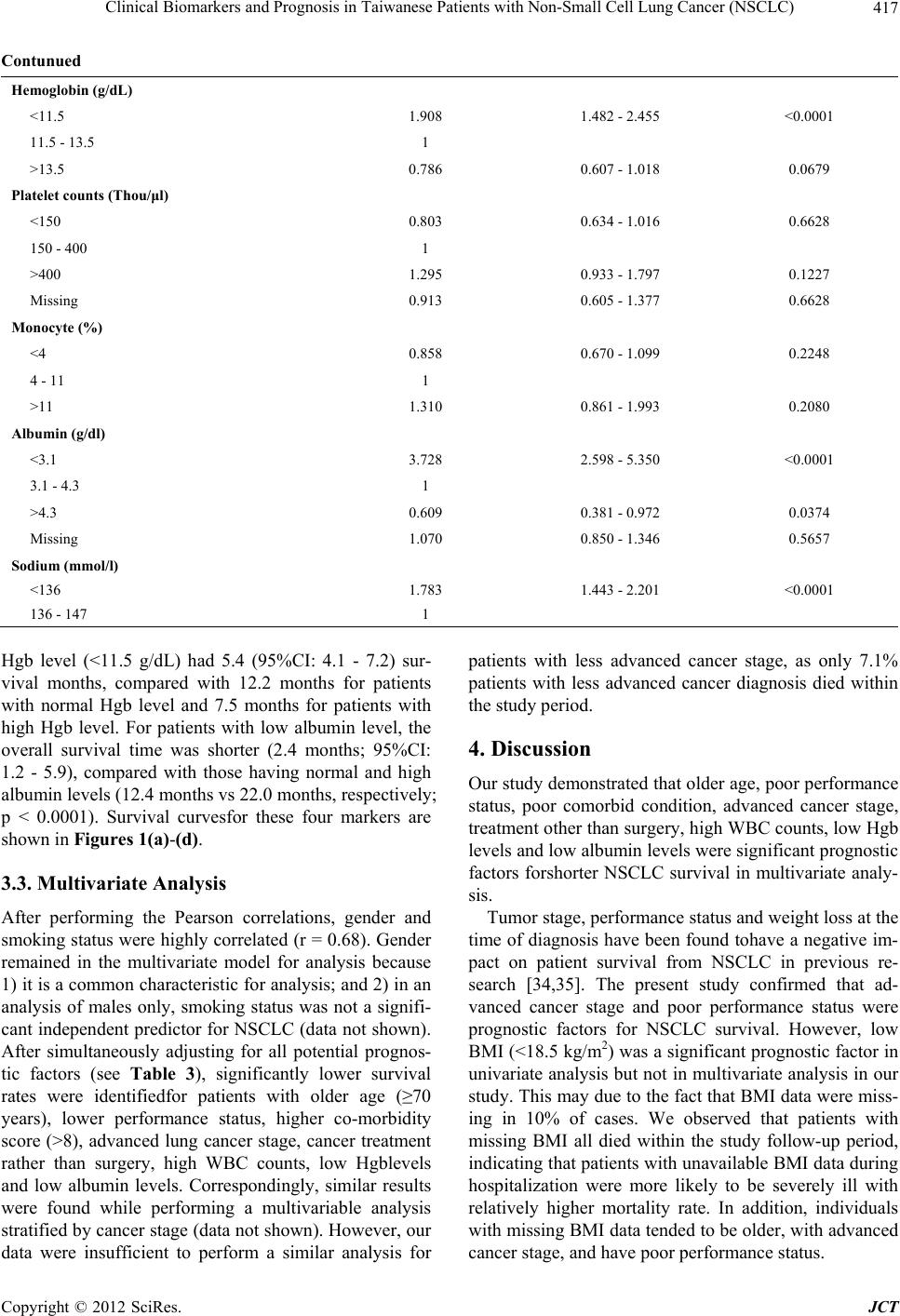 Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 417 Contunued Hemoglobin (g/dL) <11.5 1.908 1.482 - 2.455 <0.0001 11.5 - 13.5 1 >13.5 0.786 0.607 - 1.018 0.0679 Platelet counts (Thou/μl) <150 0.803 0.634 - 1.016 0.6628 150 - 400 1 >400 1.295 0.933 - 1.797 0.1227 Missing 0.913 0.605 - 1.377 0.6628 Monocyte (%) <4 0.858 0.670 - 1.099 0.2248 4 - 11 1 >11 1.310 0.861 - 1.993 0.2080 Albumin (g/dl) <3.1 3.728 2.598 - 5.350 <0.0001 3.1 - 4.3 1 >4.3 0.609 0.381 - 0.972 0.0374 Missing 1.070 0.850 - 1.346 0.5657 Sodium (mmol/l) <136 1.783 1.443 - 2.201 <0.0001 136 - 147 1 Hgb level (<11.5 g/dL) had 5.4 (95%CI: 4.1 - 7.2) sur- vival months, compared with 12.2 months for patients with normal Hgb level and 7.5 months for patients with high Hgb level. For patients with low albumin level, the overall survival time was shorter (2.4 months; 95%CI: 1.2 - 5.9), compared with those having normal and high albumin levels (12.4 months vs 22.0 months, respectively; p < 0.0001). Survival curvesfor these four markers are shown in Figures 1(a)-(d). 3.3. Multivariate Analysis After performing the Pearson correlations, gender and smoking status were highly correlated (r = 0.68). Gender remained in the multivariate model for analysis because 1) it is a common characteristic for analysis; and 2) in an analysis of males only, smoking status was not a signifi- cant independent predictor for NSCLC (data not shown). After simultaneously adjusting for all potential prognos- tic factors (see Table 3), significantly lower survival rates were identifiedfor patients with older age (≥70 years), lower performance status, higher co-morbidity score (>8), advanced lung cancer stage, cancer treatment rather than surgery, high WBC counts, low Hgblevels and low albumin levels. Correspondingly, similar results were found while performing a multivariable analysis stratified by cancer stage (data not shown). However, our data were insufficient to perform a similar analysis for patients with less advanced cancer stage, as only 7.1% patients with less advanced cancer diagnosis died within the study period. 4. Discussion Our study demonstrated that older age, poor performance status, poor comorbid condition, advanced cancer stage, treatment other than surgery, high WBC counts, low Hgb levels and low albumin levels were significant prognostic factors forshorter NSCLC survival in multivariate analy- sis. Tumor stage, performance status and weight loss at the time of diagnosis have been found tohave a negative im- pact on patient survival from NSCLC in previous re- search [34,35]. The present study confirmed that ad- vanced cancer stage and poor performance status were prognostic factors for NSCLC survival. However, low BMI (<18.5 kg/m2) was a significant prognostic factor in univariate analysis but not in multivariate analysis in our study. This may due to the fact that BMI data were miss- ing in 10% of cases. We observed that patients with missing BMI all died within the study follow-up period, indicating that patients with unavailable BMI data during hospitalization were more likely to be severely ill with relatively higher mortality rate. In addition, individuals with missing BMI data tended to be older, with advanced cancer stage, and have poor performance status. Copyright © 2012 SciRes. JCT 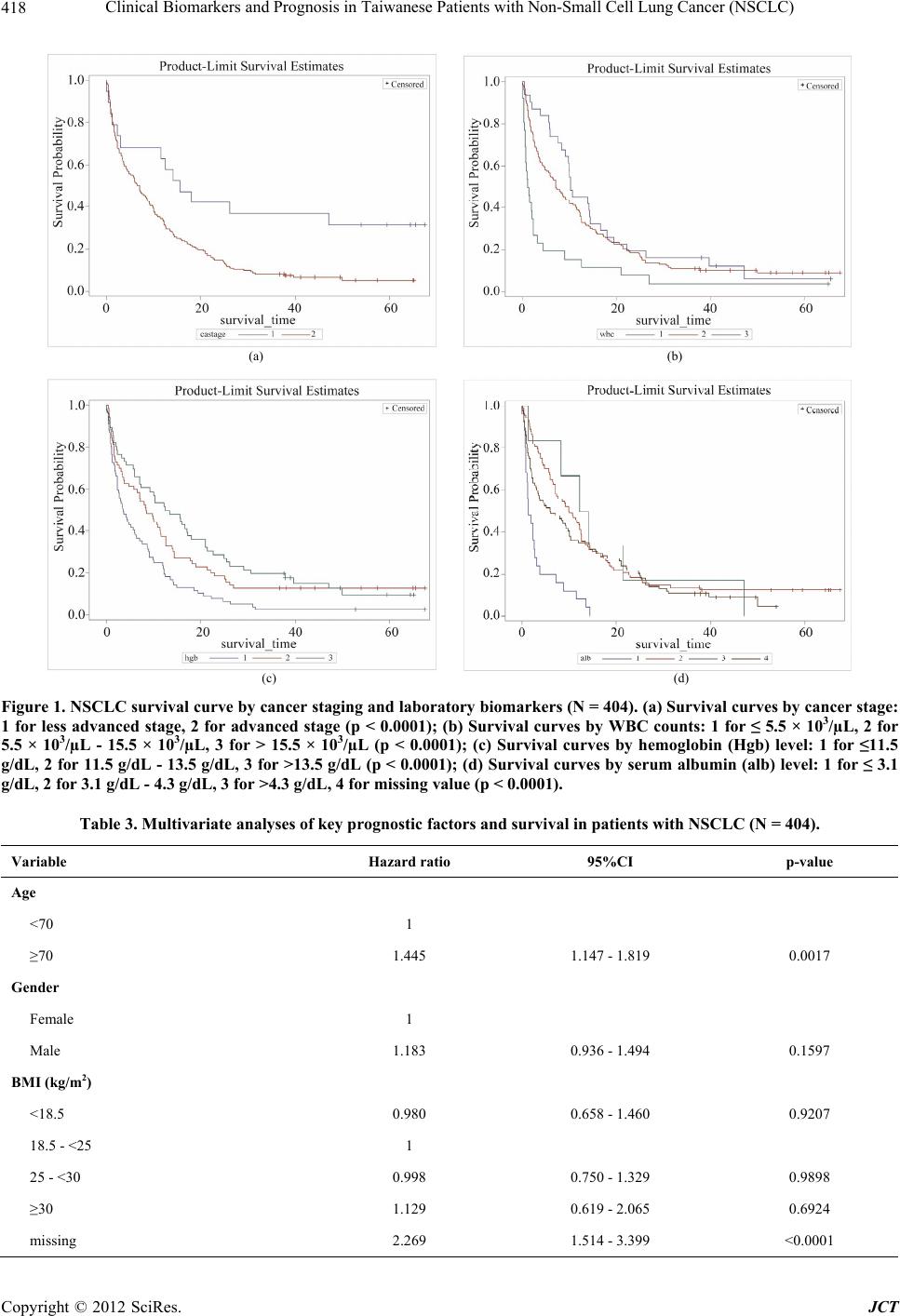 Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 418 (a) (b) (c) (d) Figure 1. NSCLC survival curve by cancer staging and laboratory biomarkers (N = 404). (a) Survival curves by cancer stage: 1 for less advanced stage, 2 for advanced stage (p < 0.0001); (b) Survival curves by WBC counts: 1 for ≤ 5.5 × 103/µL, 2 for 5.5 × 103/µL - 15.5 × 103/µL, 3 for > 15.5 × 103/µL (p < 0.0001); (c) Survival curves by hemoglobin (Hgb) level: 1 for ≤11.5 g/dL, 2 for 11.5 g/dL - 13.5 g/dL, 3 for >13.5 g/dL (p < 0.0001); (d) Survival curves by serum albumin (alb) level: 1 for ≤ 3.1 g/dL, 2 for 3.1 g/dL - 4.3 g/dL, 3 for >4.3 g/dL, 4 for missing value (p < 0.0001). Table 3. Multivariate analyses of key prognostic factors and survival in patients with NSCLC (N = 404). Variable Hazard ratio 95%CI p-value Age <70 1 ≥70 1.445 1.147 - 1.819 0.0017 Gender Female 1 Male 1.183 0.936 - 1.494 0.1597 BMI (kg/m2) <18.5 0.980 0.658 - 1.460 0.9207 18.5 - <25 1 25 - <30 0.998 0.750 - 1.329 0.9898 ≥30 1.129 0.619 - 2.065 0.6924 missing 2.269 1.514 - 3.399 <0.0001 Copyright © 2012 SciRes. JCT  Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 419 Contunued ECOG Performance status <2 1 ≥2 1.506 1.167 - 1.943 0.0017 Co-morbidity score <4 1 4 - 8 1.354 1.023 - 1.791 0.0340 ≥8 1.557 1.103 - 2.199 0.0118 Cancer stage Less advanced 1 Advanced 1.818 1.153 - 2.867 0.0101 Cancer treatment Surgery 1 Chemotherapy and/ or other supportive care 2.513 1.202 - 5.257 0.0144 None 5.704 2.665 - 12.211 <0.0001 WBC counts (103/µL) ≤5.5 0.798 0.577 - 1.104 0.1724 5.5 - 15.5 1 >15.5 1.798 1.225 - 2.639 0.0027 Neutrophil (%) 27 - 55 1 >55 1.308 0.826 - 2.070 0.2521 Missing 1.650 0.891 - 3.055 0.1112 Lymphocyte (%) <16 0.931 0.705 - 1.230 0.6157 16 - 46 1 >46 0.475 0.139 - 1.622 0.2350 Hemoglobin (g/dL) <11.5 1.437 1.085 - 1.903 0.0115 11.5-13.5 1 >13.5 0.901 0.686 - 1.183 0.4522 Albumin (g/dl) <3.1 2.149 1.376 - 3.052 0.0004 3.1 - 4.3 1 >4.3 0.943 0.580 - 1.534 0.8131 Missing 1.279 1.006 - 1.625 0.0443 Sodium (mmol/l) <136 1.141 0.895 - 1.454 0.2875 136 - 147 1 Comorbidity condition was also observed as a predic- tor of lung cancer survival. Previous findings on comor- bidity are inconsistent. Janssen et al. reported no inde- pendent prognostic effect of comorbidity condition for patients with non-small cell lung cancer [36]. However, in line with our study, Wang and colleagues revealed that patients with CCI score ≥ 2 had higher perioperative mortality and death from NSCLC compared with patients with CCI score < 2 [37]. Firat et al. and Moro-Sibilot et al. also studied the significance of comorbidity scores in stage I NSCLC patients and found it to be a significant prognostic impact [38,39]. Our study confirmed that elevated WBC counts were significantly associated with poor prognosis among Copyright © 2012 SciRes. JCT  Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 420 NSCLC patients, which aligned with most previous ob- servations [13]. In a nationally representative adult co- hort study, WBC counts werefound to be positively and independently associated with cancer mortality after ad- justing for age, gender and race [40]. In that study, in- flammation was associated with WBC counts as well. WBC as a marker of inflammatory level reflects either a greater burden of tumor cells within the bone marrow, a possible concomitant subclinical infection, or the effect of a yet undescribed chemokine or cytokine secreted by the tumor into the circulation. Stromal tissues of tumors contain large numbers of WBC counts, and the inflam- matory cell number and their cytokine production corre- late with tumor severity and prognosis [41-44]. We also identified the relationship of both low Hgb and serum albumin levels with poor survival as reported in other studies [12,13,23,24,45,46]. Takugawa et al. stated low Hgb level correlated with overall survival among patients with NSCLC [47]. Similar findings were reported in Albain’s study [48]. For serum albumin level, Phillips reported a marked increase in mortality rate with decreasing serum albumin concentrations among cancer and cardiovascular patients [49]. Another study found an approximate 25% reduction in cancer mortality among middle aged men with a one standard deviation increase in serum albumin [50]. Similar to the elevated WBC counts, low Hgb and serum albumin levels also play an influential role in body inflammation. Serum albumin is a negative acute phase protein; its concentration in the blood is reduced in response to inflammation [51]. Hy- poalbuminemia is the result of the combined effects of inflammation and inadequate protein and caloric intake in patients with chronic disease and cancer. Inflammation and malnutrition both reduce albumin concentration by decreasing its rate of synthesis, while inflammation alone is associated with a greater fractional catabolic rate (FCR) and, when extreme, increased transfer of albumin out of the vascular compartment [50]. This study is the first to assess clinical prognostic factors in a Taiwanese popula- tion, although several other Asian research articles havei- dentified the traditional factors for lung cancer survival including old age, no surgery treatment, performance status, and advanced lung cancer stage [28,52,53]. The findings of this study should be considered in the context of its strengths and limitations. Study strengths include the fact that 1) all lung cancer cases were newly diagnosed which ruled out impact on patients’ outcomes by possible cancer pretreatment that patients may have received. In addition, 2) the outcome measure (death yes/no) was tracked up to 3 - 5 years in order to predict long term prognosis for NSCLC; and 3) we were able to collect and adjust for most potential prognostic factors such as smoking status, BMI, and performance status. However, the present study was also limited in several respects. Using data derived from retrospective hospital records review, we found that routinely examined labo- ratory item such as albumin level was not available for every individual during the hospital stay. These missing data limited the ability of our study to conduct a com- plete data analysis. In addition, the study uses a sample from a Taiwanese patient populationmost of whom reside in a rural area, which may limit the generalizability of study findings of NSCLC survival outcomes to other populations. 5. Conclusion This study contributes to research on overall NSCLC survival by concurrently identifying significant labora- tory biomarkers for survival, including WBC counts, Hgb level, and serum albumin level. Additionally we confirmed the widely accepted prognostic factors of lung cancer survival such as old age, advanced cancer, severe comorbidity, performance status, and lack of surgery treatment. Because these identified biomarkers are rou- tinelychecked during hospital admissions, the findings of this study could help in the early identification of patients atrisk of shorter NSCLC survival to provide better clini- cal patients care. Moreover, to extend and confirm the current study findings, future studies shouldapply more comprehensive prognostic assessments, conduct longer follow-ups, and study additional ethnic populations. 6. Acknowledgements This study was supported by the Faculty Research Grant (TCCT-981A11), Buddhist Tzu Chi College of Tech- nology, to L. J. Chang. We would like to express our gratitude to Tzu Chi General Hospital Dalin Branch and Cancer Tumor Center for their efforts and assistance for data collection. REFERENCES [1] A. Jemal, R. Siegel, E. Ward, Y. Hao, J. Xu and M. J. Thun, “Cancer Statistics, 2009,” CA—A Cancer Journal for Clinicians, Vol. 59, No. 4, 2009, pp. 225-249. doi:10.3322/caac.20006 [2] A. Jemal, F. Bray, M. M. Center, J. Ferlay, E. Ward and D. Forman, “Global Cancer Statistics,” CA—A Cancer Journal for Clinicians, Vol. 61, No. 6, 2011, pp. 69-90. doi:10.3322/caac.20107 [3] C. L. B. Ott, N. Ratna, R. Prayag, Z. Nugent, K. Badiani and S. Navaratnam, “Survival and Treatment Patterns in Elderly Patients with Advanced Non-Small-Cell Lung Cancer in Manitoba,” Current Oncology, Vol. 18, No. 5, 2011, pp. e238-e242. [4] E. Quoix, V. Westeel, G. Zalcman and B. Milleron, “Chemotherapy in Elderly Patients with Advanced Non- Small Cell Lung Cancer,” Lung Cancer, Vol. 74, No. 3, Copyright © 2012 SciRes. JCT  Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 421 2011, pp. 364-368. doi:10.1016/j.lungcan.2011.06.006 [5] W. D. Wei, Z. S. Wen, X. D. Su, P. Lin, T. H. Rong and L. K. Chen, “Multivariate Survival Analysis of 899 Pa- tients with Non-Small Cell Lung Cancer after Complete Resection,” Cancer, Vol. 26, No. 11, 2007, pp. 1231- 1236. [6] K. Hotta, Y. Fujiwara, K. Kiura, N. Takigawa, M. Tabata, H. Ueoka and M. Tanimoto, “Relationship between Re- sponse and Survival in More than 50,000 Patients with Advanced Non-Small Cell Lung Cancer Treated with Systemic Chemotherapy in 143 Phase III Trials,” Journal of Thoracic Oncology, Vol. 2, 2007, pp. 402-407. doi:10.1097/01.JTO.0000268673.95119.c7 [7] J. L. Port, M. S. Kent, R. J. Korst, D. Libby, M. Pasman- tier and N. K. Altorki, “Tumor Size Predicts Survival within Stage IA Non-Small Cell Lung Cancer,” Chest, Vol. 124, No. 5, 2003, pp. 1828-1833. doi:10.1378/chest.124.5.1828 [8] E. F. Patz Jr., S. Rossi, D. H. Harpole Jr., J. E. Herndon and P. C. Goodman, “Correlation of Tumor Size and Sur- vival in Patients with Stage IA Non-Small Cell Lung Cancer,” Chest, Vol. 117, 2000, pp. 1568-1571. doi:10.1378/chest.117.6.1568 [9] L. M. Forrest, D. C. McMillan, C. S. McArdle, W. J. Angerson, K. Dagg and H. R. Scott, “A Prospective Lon- gitudinal Study of Performance Status, an Inflamma- tion-Based Score (GPS) and Survival in Patients with In- operable Non-Small-Cell Lung Cancer,” British Journal of Cancer, Vol. 92, 2005, pp. 1834-1836. doi:10.1038/sj.bjc.6602591 [10] H. R. Scott, D. C. McMillan, L. M. Forrest, D. J. Brown, C. S. McArdle and R. Milroy, “The Systemic Inflamma- tory Response, Weight Loss, Performance Status and Survival in Patients with Inoperable Non-Small Cell Lung Cancer,” British Journal of Cancer, Vol. 87, No. 3, 2002, pp. 264-267. doi:10.1038/sj.bjc.6600466 [11] M. K. Mohamed, S. Ramalingam, Y. Lin, W. Gooding and C. P. Belani, “Skin Rash and Good Performance Status Predict Improved Survival with Gefitinib in Pa- tients with Advanced Non-Small Cell Lung Cancer,” An- nals of Oncology, Vol. 16, No. 5, 2005, pp. 780-785. doi:10.1093/annonc/mdi157 [12] T. Maeda, H. Ueoka, M. Tabata, K. Kiura, T. Shibayama, K. Gemba, N. Takigawa, A. Hiraki, H. Katayama and M. Harada, “Prognostic Factors in Advanced Non-Small Cell Lung Cancer: Elevated Serum Levels of Neuron Specific Enolase Indicate Poor Prognosis,” Japanese Journal of Clinical Oncology, Vol. 30, No. 12, 2000, pp. 534-541. doi:10.1093/jjco/hyd139 [13] S. J. Mandrekar, S. E. Schild, S. L. Hillman, K. L. Allen, R. S. Marks, J. A. Mailliard, J. E. Krook, A. W. Mak- symiuk, K. Chansky, K. Kelly, A. A. Adjei and J. R. Jett, “A Prognostic Model for Advanced Stage Nonsmall Cell Lung Cancer. Pooled Analysis of North Central Cancer Treatment Group Trials,” Cancer, Vol. 107, No. 4, 2006, pp. 781-792. doi:10.1002/cncr.22049 [14] C. W. Francis, A. Blinc, S. Lee and C. Cox, “Ultrasound Accelerates Transport of Recombinant Tissue Plasmino- gen Activator into Clots,” Ultrasound in Medicine & Bi- ology, Vol. 21, No. 3, 1995, pp. 419-424. doi:10.1016/0301-5629(94)00119-X [15] L. R. Zacharski, M. Z. Wojtukiewicz, V. Costantini, D. L. Ornstein and V. A. Memoli, “Pathways of Coagula- tion/Fibrinolysis Activation in Malignancy,” Semin Thr- omb Hemost, Vol. 18, No. 1, 1992, pp. 104-116. doi:10.1055/s-2007-1002415 [16] K. R. Meehan, L. R. Zacharski, T. E. Moritz and F. R. Rickles, “Pretreatment Fibrinogen Levels Are Associated with Response to Chemotherapy in Patients with Small Cell Carcinoma of the Lung: Department of Veterans Af- fairs Cooperative Study 188,” American Journal of He- matology, Vol. 49, 1995, pp. 143-148. doi:10.1002/ajh.2830490208 [17] O. Taguchi, E. C. Gabazza, H. Yasui, T. Kobayashi, M. Yoshida and H. Kobayashi, “Prognostic Significance of Plasma D-Dimer Levels in Patients with Lung Cancer,” Thorax, Vol. 52, No. 6, 1997, pp. 563-565. doi:10.1136/thx.52.6.563 [18] M. Z. Wojtukiewicz, L. R. Zacharski, T. E. Moritz, K. Hur, R. L. Edwards and F. R. Rickles, “Prognostic Sig- nificance of Blood Coagulation Tests in Carcinoma of the Lung and Colon,” Blood Coagul Fibrinolysis, Vol. 3, No. 4, 1992, pp. 429-437. [19] D. Ferrigno, G. Buccheri and I. Ricca, “Prognostic Sig- nificance of Blood Coagulation Tests in Lung Cancer,” European Respiratory Journal, Vol. 17, No. 4, 2001, pp. 667-673. doi:10.1183/09031936.01.17406670 [20] M. Paesmans, J. P. Sculier, P. Libert, G. Bureau, G. Dabouis, J. Thiriaux, J. Michel, O. Van Cutsem, R. Ser- gysels, P. Momme, et al., “Prognostic Factors for Sur- vival in Advanced Non-Small-Cell Lung Cancer: Uni- variate and Multivariate Analyses Including Recursive Partitioning and Amalgamation Algorithms in 1052 Pa- tients. The European Lung Cancer Working Party,” Journal of Clinical Oncology, Vol. 13, No. 5, 1995, pp. 1221-1230. [21] J. S. Paddison, J. S. Temel, G. L. Fricchione and W. F. Pirl, “Using the Differential from Complete Blood Counts as a Biomarker of Fatigue in Advanced Non-Small-Cell Lung Cancer: An Exploratory Analysis,” Palliat Support Care, Vol. 7, No. 2, 2009, pp. 213-217. doi:10.1017/S1478951509000273 [22] D. Ferrigno and G. Buccheri, “Hematologic Counts and Clinical Correlates in 1201 Newly Diagnosed Lung Can- cer Patients,” Monaldi Archives for Chest Disease, Vol. 59, No. 3, 2003, pp. 193-198. [23] T. Win, L. Sharples, A. M. Groves, A. J. Ritchie, F. C. Wells and C. M. Laroche, “Predicting Survival in Poten- tially Curable Lung Cancer Patients,” Lung, Vol. 186, No. 2, 2008, pp. 97-102. doi:10.1007/s00408-007-9067-1 [24] F. Tas, A. Aydiner, E. Topuz, H. Camlica, P. Saip and Y. Eralp, “Factors Influencing the Distribution of Metastases and Survival in Extensive Disease Small Cell Lung Can- cer,” Acta Oncologica, Vol. 38, No. 8, 1999, pp. 1011- 1015. doi:10.1080/028418699432275 [25] S. L. Vanhees, R. Paridaens and J. F. Vansteenkiste, “Syndrome of Inappropriate Antidiuretic Hormone Asso- ciated with Chemotherapy-Induced Tumour Lysis in Copyright © 2012 SciRes. JCT  Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) 422 Small-Cell Lung Cancer: Case Report and Literature Re- view,” Annals of Oncology, Vol. 11, 2000, pp. 1061-1065. doi:10.1023/A:1008369932384 [26] O. Hansen, P. Sorensen and K. H. Hansen, “The Occur- rence of Hyponatremia in SCLC and the Influence on Prognosis: A Retrospective Study of 453 Patients Treated in a single Institution in a 10-Year Period,” Lung Cancer, Vol. 68, No. 1, 2010, pp. 111-114. doi:10.1016/j.lungcan.2009.05.015 [27] N. S. Rawson and J. Peto, “An Overview of Prognostic Factors in Small Cell Lung Cancer. A Report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Re- search,” British Journal of Cancer, Vol. 61, No. 4, 1990, pp. 597-604. doi:10.1038/bjc.1990.133 [28] J. Luo, Y. J. Chen and L. J. Chang, “Fasting Blood Glu- cose Level and Prognosis in Non-Small Cell Lung Cancer (NSCLC) Patients,” Lung Cancer, Vol. 76, No. 2, 2011, pp. 242-247. doi:10.1016/j.lungcan.2011.10.019 [29] M. M. Oken, R. H. Creech, D. C. Tormey, J. Horton, T. E. Davis, E. T. McFadden and P. P. Carbone, “Toxicity and Response Criteria of the Eastern Cooperative Oncology Group,” American Journal of Clinical Oncology, Vol. 5, No. 6, 1982, pp. 649-655. doi:10.1097/00000421-198212000-00014 [30] M. E. Charlson, P. Pompei, K. L. Ales and C. R. MacKenzie, “A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation,” Journal of Chronic Diseases, Vol. 40, No. 5, 1987, pp. 373-383. doi:10.1016/0021-9681(87)90171-8 [31] O. Birim, A. P. Kappetein and A. J. Bogers, “Charlson Comorbidity Index as a Predictor of Long-Term Outcome after Surgery for Nonsmall Cell Lung Cancer,” European Journal Cardio-Thoracic Surgery, Vol. 28, No. 5, 2005, pp. 759-762. doi:10.1016/j.ejcts.2005.06.046 [32] J. V. Corbett, “Laboratory Tests and Diagnostic Proce- dures with Nursing Diagnoses,” Pearson/Prentice Hall, Upper Saddle River, 2004. [33] D. R. Cox and D. Oakes, “Analysis of Survival Data,” Chapman and Hall, London, 1984. [34] S. Leij-Halfwerk, P. C. Dagnelie, J. W. van Den Berg, J. D. Wattimena, C. H. Hordijk-Luijk and J. P. Wilson, “Weight Loss and Elevated Gluconeogenesis from Alanine in Lung Cancer Patients,” American Journal of Clinical Nutrition, Vol. 71, 2000, pp. 583-589. [35] H. Wertzel, H. Siebert, W. Lange, L. Swoboda, E. Graf and J. Hasse, “Results after Surgery in Stage-I Broncho- genic Carcinoma,” Journal of Thoracic and Cardiovas- cular Surgery, Vol. 46, 1998, pp. 365-369. doi:10.1055/s-2007-1010255 [36] R. R. Jennens, G. G. Giles and R. M. Fox, “Increasing Under Representation of Elderly Patients with Advanced Colorectal or Non-Small-Cell Lung Cancer in Chemo- therapy Trials,” Internal Medicine Journal, Vol. 36, 2006, pp. 216-220. doi:10.1111/j.1445-5994.2006.01033.x [37] C. Y. Wang, Y. S. Lin, C. Tzao, H. C. Lee, M. H. Huang, W. H. Hsu and H. S. Hsu, “Comparison of Charlson Co- morbidity Index And Kaplan-Feinstein Index in Patients with Stage I Lung Cancer after Surgical Resection,” European Journal Cardio-Thoracic Surgery, Vol. 32, No. 6, 2007, pp. 877-881. doi:10.1016/j.ejcts.2007.09.008 [38] S. Firat, M. Bousamra, E. Gore and R. W. Byhardt, “Co- morbidity and KPS Are Independent Prognostic Factors in Stage I Non-Small-Cell Lung Cancer,” International Journal of Radiation Oncology*Biology*Physics, Vol. 52, 2002, pp. 1047-1057. [39] D. Moro-Sibilot, A. Aubert, S. Diab, S. Lantuejoul, P. Fourneret, E. Brambilla, C. Brambilla and P. Y. Brichon, “Comorbidities and Charlson Score in Resected Stage I nonsmall Cell Lung Cancer,” European Respiratory Journal, Vol. 26, No. 3, 2005, pp. 480-486. doi:10.1183/09031936.05.00146004 [40] A. Shankar, J. J. Wang, E. Rochtchina, M. C. Yu, R. Kefford and P. Mitchell, “Association between Circulat- ing White Blood Cell Count and Cancer Mortality: A Population-Based Cohort Study,” Archives of Internal Medicine, Vol. 166, 2006, pp. 188-194. doi:10.1001/archinte.166.2.188 [41] L. M. Coussens and Z. Werb, “Inflammation and Can- cer,” Nature, Vol. 420, No. 6917, 2002, pp. 860-867. doi:10.1038/nature01322 [42] F. Balkwill, “Cancer and the Chemokine Network,” Na- ture Reviews Cancer, Vol. 4, 2004, pp. 540-550. doi:10.1038/nrc1388 [43] F. Balkwill and L. M. Coussens, “Cancer: An Inflamma- tory Link,” Nature, Vol. 431, No. 7007, 2004, pp. 405- 406. doi:10.1038/431405a [44] E. Pikarsky, R. M. Porat, I. Stein, R. Abramovitch, S. Amit, S. Kasem, E. Gutkovich-Pyest, S. Urieli-Shoval, E. Galun and Y. Ben-Neriah, “NF-kappaB Functions as a Tumour Promoter in Inflammation-Associated Cancer,” Nature, Vol. 431, No. 7007, 2004, pp. 461-466. doi:10.1038/nature02924 [45] M. D. Brundage, D. Davies and W. J. Mackillop, “Prog- nostic Factors in Non-Small Cell Lung Cancer: A Decade of Progress,” Chest, Vol. 122, No. 3, 2002, pp. 1037-1057. doi:10.1378/chest.122.3.1037 [46] S. Sugiura, Y. Ando, H. Minami, M. Ando, S. Sakai and K. Shimokata, “Prognostic Value of Pleural Effusion in Patients with Non-Small Cell Lung Cancer,” Clinical Cancer Research, Vol. 3, 1997, pp. 47-50. [47] N. Takigawa, Y. Segawa, M. Okahara, Y. Maeda, I. Ta- kata, M. Kataoka and M. Fujii, “Prognostic Factors for Patients with Advanced Non-Small Cell Lung Cancer: Univariate and Multivariate Analyses Including Recur- sive Partitioning and Amalgamation,” Lung Cancer, Vol. 15, No. 1, 1996, pp. 67-77. doi:10.1016/0169-5002(96)00571-5 [48] K. S. Albain, J. J. Crowley, M. LeBlanc and R. B. Livingston, “Survival Determinants in Extensive-Stage Non-Small-Cell Lung Cancer: The Southwest Oncology Group Experience,” Journal of Clinical Oncology, Vol. 9, No. 9, 1991, pp. 1618-1626. [49] A. Phillips, A. G. Shaper and P. H. Whincup, “Associa- tion between Serum Albumin and Mortality from Car- diovascular Disease, Cancer, and Other Causes,” Lancet, Copyright © 2012 SciRes. JCT  Clinical Biomarkers and Prognosis in Taiwanese Patients with Non-Small Cell Lung Cancer (NSCLC) Copyright © 2012 SciRes. JCT 423 Vol. 2, 1989, pp. 1434-1436. doi:10.1016/S0140-6736(89)92042-4 [50] B. R. Don and G. Kaysen, “Serum Albumin: Relationship to Inflammation and Nutrition,” Seminars in Dialysis, Vol. 17, No. 6, 2004, pp. 432-437. doi:10.1111/j.0894-0959.2004.17603.x [51] B. L. Sprague, A. Trentham-Dietz, B. E. Klein, R. Klein, K. J. Cruickshanks, K. E. Lee and J. M. Hampton, “Physical Activity, White Blood Cell Count, and Lung Cancer Risk in a Prospective Cohort Study,” Cancer Epidemiology, Biomarkers & Prevention, Vol. 17, No. 10, 2008, pp. 2714-2722. doi:10.1158/1055-9965.EPI-08-0042 [52] Y. H. Li, S. H. Shieh and C. Y. Chen, “The Influence of Health Behaviors on Survival in Lung Cancer Patients in Taiwan,” Japanese Journal of Clinical Oncology, Vol. 41, No. 3, 2011, pp. 365-372. doi:10.1093/jjco/hyq188 [53] T. A. Chiang, P. H. Chen, P. F. Wu, T. N. Wang, P. Y. Chang, A. M. Ko, M. S. Huang and Y. C. Ko, “Important Prognostic Factors for the Long-Term Survival of Lung Cancer Subjects in Taiwan,” BMC Cancer, Vol. 8, No. 324, 2008.
|