 Journal of Water Resource and Protection, 2012, 4, 638-647 http://dx.doi.org/10.4236/jwarp.2012.48074 Published Online August 2012 (http://www.SciRP.org/journal/jwarp) Influence of Temperature on Mutagenicity in Plants Exposed to Surface Disinfected Drinking Water Bianca Gustavino1, Elisabetta Ceretti2, Claudia Zani2, Ilaria Zerbini2, Marco Rizzoni1, Silvano Monarca3, Donatella Feretti2* 1Department of Biology, University of Rome “Tor Vergata”, Rome, Italy 2Department of Experimental and Applied Medicine, Hygiene Epidemiology and Public Health Institute, University of Brescia, Brescia, Italy 3Department of Surgical and Medical Specialities and Public Health, University of Perugia, Perugia, Italy Email: *feretti@med.unibs.it Received May 31, 2012; revised July 2, 2012; accepted July 14, 2012 ABSTRACT Disinfection of surface drinking water, particularly water chlorination, produces by-products with potential genotoxic and/or carcinogenic activity. A study carried out at a pilot plant for drinking water disinfection of lake water revealed mutagenic activity of three different disinfectants (sodium hypochlorite, chlorine dioxide and peracetic acid) in different seasons using in situ mutagenicity assays, both in animal (micronucleus test) and in plant organisms (anaphase chro- mosomal aberration and micronucleus tests). The effects of the disinfectants appeared to be modulated by the season of exposure. In this study, we tried to understand if (and to what extent) the temperature parameter could actually play an independent role in the registered seasonal variation of mutagenic effects, neglecting the variation of other parameters, e.g. physical conditions and chemical composition of the lake water. Therefore plants (Allium cepa for chromosomal aberration test and Vicia faba for micronucleus test) were exposed to the same disinfected lake-water samples at differ- ent temperatures (10˚C, 20˚C and 30˚C), according the ones registered during the in situ experiment. Long-term expo- sure at the temperatures of 20˚C (both Vicia faba and Allium cepa) and 30˚C (Vicia faba only) to disinfected waters in- duced clear mutagenic effects. These results show that temperature is an important variable which should be taken into account when in situ exposure of plants is planned for mutagenicity testing. Also, different plant systems clearly show specific temperature ranges suitable for their growth, thereby indicating the need for an accurate selection of the test organism for a specific experimental plan. Keywords: Clastogenicity/Aneugenicity; Al lium cepa Aberration Test; Vicia faba Micronucleus Test; Temperature Exposure; Water Disinfection 1. Introduction Drinking water disinfection may produce toxic com- pounds, particularly when water is obtained from surface sources. Such disinfection by-products (DBP) are formed when disinfectants (e.g. chlorine, ozone, chlorine dioxide, or chloramines) react with naturally occurring organic matter, anthropogenic contaminants, bromide, and iodide. It has been demonstrated that chlorination, the most widely used method of disinfecting water, leads to the formation of numerous mutagenic and/or carcinogenic DBPs [1-4]. Despite the numerous laboratory evidences on the mutagenic or carcinogenic properties for many DBPs, epidemiologic studies to date have revealed only a mod- est association between DBP exposure and cancer in humans [4,5]. Interesting findings for their significant implications for cancer prevention come from a recent case-control study carried out in hospital which showed strong associations between DBP exposure and bladder cancer among individuals carrying inherited variants in three genes (GSTT1, GSTZ1, and CYP2E1) that code for key enzymes that metabolize DBPs [6]. As far as the mutagenic and genotoxic potential of DPBs is concerned, still incomplete information for many of them is available, particularly for the emerging ones, the levels of which are increased by alternative disinfectants that are being employed (primarily ozone or chloramines) compared to chlorination. However, many emerging DBPs are more genotoxic than some of the DBPs that are submitted to regulation [4,7]. Since the growing body of evidence about the adverse effects of DPBs produced by common disinfectants, alternative disinfection practices have been implemented and in some instances results from extracted organic material in *Corresponding author. C opyright © 2012 SciRes. JWARP  B. GUSTAVINO ET AL. 639 drinking water showed to be less mutagenic than extracts from chlorinated water [4,8]. Nevertheless, the full toxicological effects of the com- plex mixtures of DBPs present in drinking water are largely unknown, except through epidemiologic studies [5], because the greatest majority of mutagenicity studies are carried out on drinking-water extracts or concentrates, largely in Salmonella [9]. In general, only a few of them involved drinking waters prepared by alternative disin- fection methods, which showed that alternative disinfect- tion methods may be considerably less mutagenic than chlorinated drinking water [10]. For this kind of studies an environmental biomonitoring approach can be adopt- ed, in which in situ exposure of bioindicator organisms, principally aquatic animals (fish, mollusks) and plants [9,11,12] is performed. Plants are unique in their ability to serve as in situ monitors for environmental genotoxins that exist either as single or complex forms [13] and to date new plant systems are being proposed for assessing the genotoxic potential on living organisms as a sensitive indicator of water quality complex-mixtures [14-16], besides the tra- ditional plant systems routinely employed for cytogenetic damage. Plant bioassays can detect a wide range of ge- netic damage, including gene mutations and chromoso- mal aberrations; in particular, mitotic cells in meristems of plant roots are considered excellent experimental tool for assessing the cytogenetic damage induced by clasto- genic and aneugenic environmental pollutants [13,17]. Cytogenetic tests have been successfully performed in plant systems for assessing genotoxic contamination of aquatic environments—especially polluted rivers [18,19] and soil [20-23], as well as for detection of drinking- water mutagenic potential [13,15,24-31]. Among plants, Vicia faba and Allium cepa are routinely used especially because of their sensitivity to a wide variety of muta- genic compounds [32] and to extremely low doses of X-rays [33]. Other factors, such as high percentage of dividing cells in root tips, uniform size of chromosomes, easy culturing in laboratory conditions and growth under in situ exposure, are further advantages which encourage the use of these systems [34]. Vicia faba and Allium cepa are widely used for the evaluation of genotoxic potential of disinfected waste- water, surface water, and drinking water [12,16,34-40]. A previous study was carried out to evaluate the genotoxic potential induced by surface water disinfected for drinking usage in root cells of plant organisms ex- posed in situ to unconcentrated water samples, using the Allium cepa anaphase chromosomal aberration test and the Vicia faba micronucleus test [11,29]. This approach allows waters to be tested without requiring extraction processes/concentration methods of water samples, so as to expose test organisms both in the laboratory and in situ. That study was performed at a pilot drinking water treatment plant, located near Lake Trasimeno (Central Italy), where lake water was experimentally disinfected using three alternative compounds: sodium hypochlorite (NaClO), chlorine dioxide (ClO2) and peracetic acid (PAA). Results indicated that all the disinfection treat- ments, especially ClO2 and NaClO, induce a clasto- genic/aneugenic effect. All the plant tests gave overall similar results, yet a seasonal correlation was also ob- served, since a noticeable variability of the mutagenic effect was found among the different sampling months (October, February and June): while in Allium cepa raw water resulted to be weakly genotoxic in October and February, the in situ exposures carried out in October to disinfected waters exerted the strongest mutagenic ef- fects both in Vicia faba and in Allium cepa, which were induced by all the compounds employed for drinking water treatment [11]. These differences were thought to be due to either temperature or chemical composition of disinfected water, being the two factors closely correlated. To verify the first hypothesis, i.e., the influence of temperature on the expression of mutagenic damage, the same samples of raw and NaClO-, ClO2- and PAA-dis- infected water were supplied to the plants (Allium cepa for chromosomal aberration test and Vicia faba for mi- cronucleus test) at different temperatures (10˚C, 20˚C and 30˚C) in the present laboratory tests following the same protocol as that adopted for in situ exposures. 2. Materials and Methods 2.1. Lake Water Sampling and Treatment with Disinfectants Water taken from Lake Trasimeno underwent sedimenta- tion, filtration and acidification (H2SO4, pH 7.0), fol- lowed by disinfection with 3 mg/L of sodium hypochlo- rite (NaClO), chlorine dioxide (ClO2) and peracetic acid (PAA). Water samples were taken in springtime and used immediately for plant exposures. The disinfection treatments were as follows: 1) Chlorine dioxide (ClO2): produced directly in treat- ed water from an 8% NaClO2 solution and a 10% HCl solution using an automatic generator (Tecme S.r.l., Gardolo di Trento, TN, Italy); 2) Sodiumhypochlorite (NaClO), (Solvay S.p.A., Rosi- gnano, LI, Italy): supplied as a 14.5% - 15.5% solution via a membrane pump; 3) Peracetic acid (CH3COO2H), (Promox S.r.l., Leggi- uno, VA, Italy): supplied as a 15% solution via a mem- brane pump. 2.2. Water Quality Measurements Total Organic Carbon (TOC), Adsorbable Organic Halo- Copyright © 2012 SciRes. JWARP  B. GUSTAVINO ET AL. Copyright © 2012 SciRes. JWARP 640 gens (AOX), UV absorbance at 254 nm and UV absorb- ance at 254 nm after filtration on a metallic filter at 0.45 µm (DUV) were measured in raw and treated water [29,41]. All these parameters indicate the total organic content that can react with disinfectants to produce po- tentially toxic and carcinogenic compounds. 2.3. Allium cepa Test After root germination under controlled laboratory con- ditions (20˚C in mineral water) young bulbs of Allium cepa of equal size (2 - 2.5 cm in diameter) were exposed to disinfected and raw water for 24 and 72 hours at 10˚C, 20˚C and 30˚C. At the end of exposure roots were cut and fixed in 1:3 acetic acid-ethanol solution and stored in 70% ethanol. Clastogenic effects (chromatin bridges, fragments) and spindle disturbance (vagrants, c-mitosis, multipolar anaphases) were studied in root cells after Feulgen staining [42,43]. The mitotic index (MI) was also evaluated as a measure of cell division rate; MI val- ues lower than 10/1000 was not considered as they are indicative of toxicity. At least 800 anaphase cells per experimental point (40 for each root, 20 slides) for ana- phase aberrations and 5000 cells per experimental point (1000 for each root, 5 slides) for the mitotic index were analyzed. Root length was used as an index of toxicity, and modifications in root form (formation of tumours, hook roots, twisted roots) and root consistency were ob- served [44]. Statistical data analysis was performed by means of the 2 test. Raw and treated waters were com- pared to a negative control (mineral water stored in glass bottles). Maleic hydrazide (10 mg/L) was used as a posi- tive control. 2.4. Vicia faba Micronucleus Test The micronucleus test was performed in secondary root tips of Vicia faba [32] which have been exposed to raw and disinfected lake waters for 6 and 72 hours at 10˚C, 20˚C and 30˚C. After the initial 6-hour exposure to the disinfected waters, part of the seedlings were transferred in Hoagland’s solution for the next 66 hours (recovery time) and maintained at the corresponding temperatures, according to the experimental protocol (10˚C, 20˚C and 30˚C). Hoagland’s solution was also used for roots of the negative control group. After exposure, roots were fixed in 1:3 acetic acid/ethanol mixture and Feulgen stained. Root tips were cut and squashed onto microscope slides. Micronucleus frequency was studied in the proliferating tissue of each root, analysing 5 × 103 cells/root tip, 10 tips/experimental point of secondary roots (5 × 104 pro- liferating cells). This analysis was carried out after checking the mitotic index, which allowed the study of cell populations with comparable proliferation activity (data not shown). Statistical analysis of data was carried out through the Mann-Whitney test; pair-wise compare- sons of micronucleus frequencies were carried out be- tween root cells exposed to the differently-disinfected waters and either raw waters or Hoagland’s solution. In addition, a comparison was made between data from 6- hour and 72-hour exposure to the same samples of water. Regression analyses were also performed between tem- peratures and micronucleus frequencies for each treat- ment and time of exposure. Maleic hydrazide (10 mg/L) was used as a positive control. 3. Results 3.1. Water Quality Measurements The results of the physical and chemical analyses are set out in Table 1. A high concentration of TOC was ob- served in raw lake water (7.8 mg/L), similar to that for water disinfected with NaClO and ClO2. The light in- crease registered in PAA treated water may be a cones- quence of the carbon content of the compound itself. Organic carbon in water is composed of organic com- pounds in various oxidation states and TOC is the direct expression of total organic content. For disinfected wa- ters, organic compounds may react with disinfectants to produce potentially toxic and carcinogenic compounds. UV-absorbing organic constituents in a sample absorb UV light in proportion to their concentration. Samples are filtered to control variations in UV absorption caused by particles. UV254 absorbance is 0.074 abs/cm and 0.069 abs/cm in raw water and filtered raw water (DUV), re- spectively. The absorbance value of ClO2-disinfected water was similar to that of raw water. NaClO- and Table 1. Physical and chemical analyses of disinfected and raw lake water. Water samples Parameters Raw water ClO2-treated NaClO-treated PAA-treated UV254 nm (abs/cm) 0.074 0.072 0.083 0.090 DUV254 nm (abs/cm) 0.069 0.048 0.052 0.056 TOC (mg/L) 7.8 7.6 7.6 9.2 AOX (g/L) nd 22 166 16 nd = not detected. 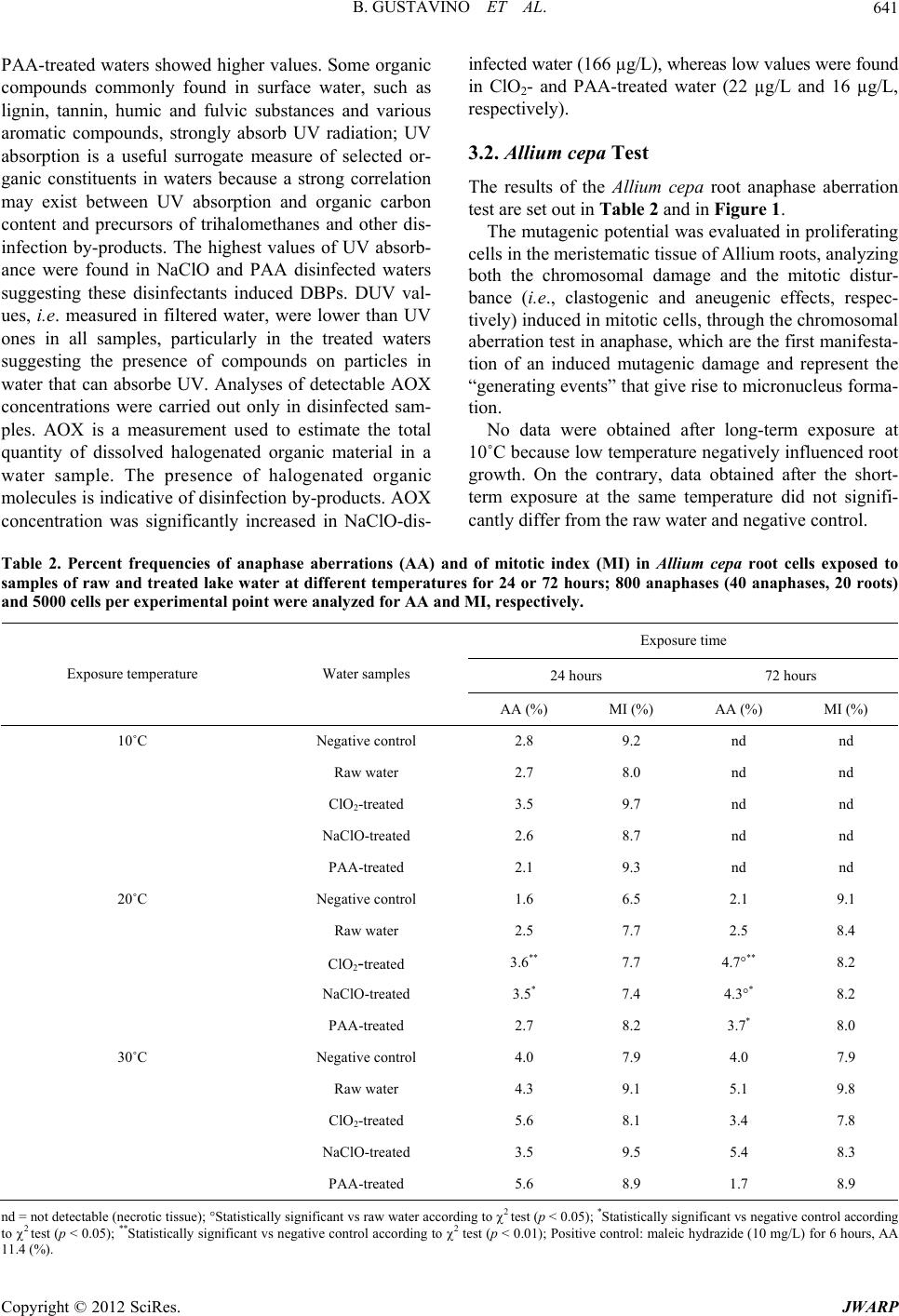 B. GUSTAVINO ET AL. 641 PAA-treated waters showed higher values. Some organic compounds commonly found in surface water, such as lignin, tannin, humic and fulvic substances and various aromatic compounds, strongly absorb UV radiation; UV absorption is a useful surrogate measure of selected or- ganic constituents in waters because a strong correlation may exist between UV absorption and organic carbon content and precursors of trihalomethanes and other dis- infection by-products. The highest values of UV absorb- ance were found in NaClO and PAA disinfected waters suggesting these disinfectants induced DBPs. DUV val- ues, i.e. measured in filtered water, were lower than UV ones in all samples, particularly in the treated waters suggesting the presence of compounds on particles in water that can absorbe UV. Analyses of detectable AOX concentrations were carried out only in disinfected sam- ples. AOX is a measurement used to estimate the total quantity of dissolved halogenated organic material in a water sample. The presence of halogenated organic molecules is indicative of disinfection by-products. AOX concentration was significantly increased in NaClO-dis- infected water (166 µg/L), whereas low values were found in ClO2- and PAA-treated water (22 µg/L and 16 µg/L, respectively). 3.2. Allium cepa Test The results of the Allium cepa root anaphase aberration test are set out in Table 2 and in Figure 1. The mutagenic potential was evaluated in proliferating cells in the meristematic tissue of Allium roots, analyzing both the chromosomal damage and the mitotic distur- bance (i.e., clastogenic and aneugenic effects, respec- tively) induced in mitotic cells, through the chromosomal aberration test in anaphase, which are the first manifesta- tion of an induced mutagenic damage and represent the “generating events” that give rise to micronucleus forma- tion. No data were obtained after long-term exposure at 10˚C because low temperature negatively influenced root growth. On the contrary, data obtained after the short- term exposure at the same temperature did not signifi- cantly differ from the raw water and negative control. Table 2. Percent frequencies of anaphase aberrations (AA) and of mitotic index (MI) in Allium cepa root cells exposed to samples of raw and treated lake water at different temperatures for 24 or 72 hours; 800 anaphases (40 anaphases, 20 roots) and 5000 cells per experimental point were analyzed for AA and MI, respectively. Exposure time 24 hours 72 hours Exposure temperature Water samples AA (%) MI (%) AA (%) MI (%) 10˚C Negative control 2.8 9.2 nd nd Raw water 2.7 8.0 nd nd ClO2-treated 3.5 9.7 nd nd NaClO-treated 2.6 8.7 nd nd PAA-treated 2.1 9.3 nd nd 20˚C Negative control 1.6 6.5 2.1 9.1 Raw water 2.5 7.7 2.5 8.4 ClO2-treated 3.6** 7.7 4.7°** 8.2 NaClO-treated 3.5* 7.4 4.3°* 8.2 PAA-treated 2.7 8.2 3.7* 8.0 30˚C Negative control 4.0 7.9 4.0 7.9 Raw water 4.3 9.1 5.1 9.8 ClO2-treated 5.6 8.1 3.4 7.8 NaClO-treated 3.5 9.5 5.4 8.3 PAA-treated 5.6 8.9 1.7 8.9 nd = not detectable (necrotic tissue); °Statistically significant vs raw water according to 2 test (p < 0.05); *Statistically significant vs negative control according to 2 test (p < 0.05); **Statistically significant vs negative control according to 2 test (p < 0.01); Positive control: maleic hydrazide (10 mg/L) for 6 hours, AA 11.4 (%). Copyright © 2012 SciRes. JWARP 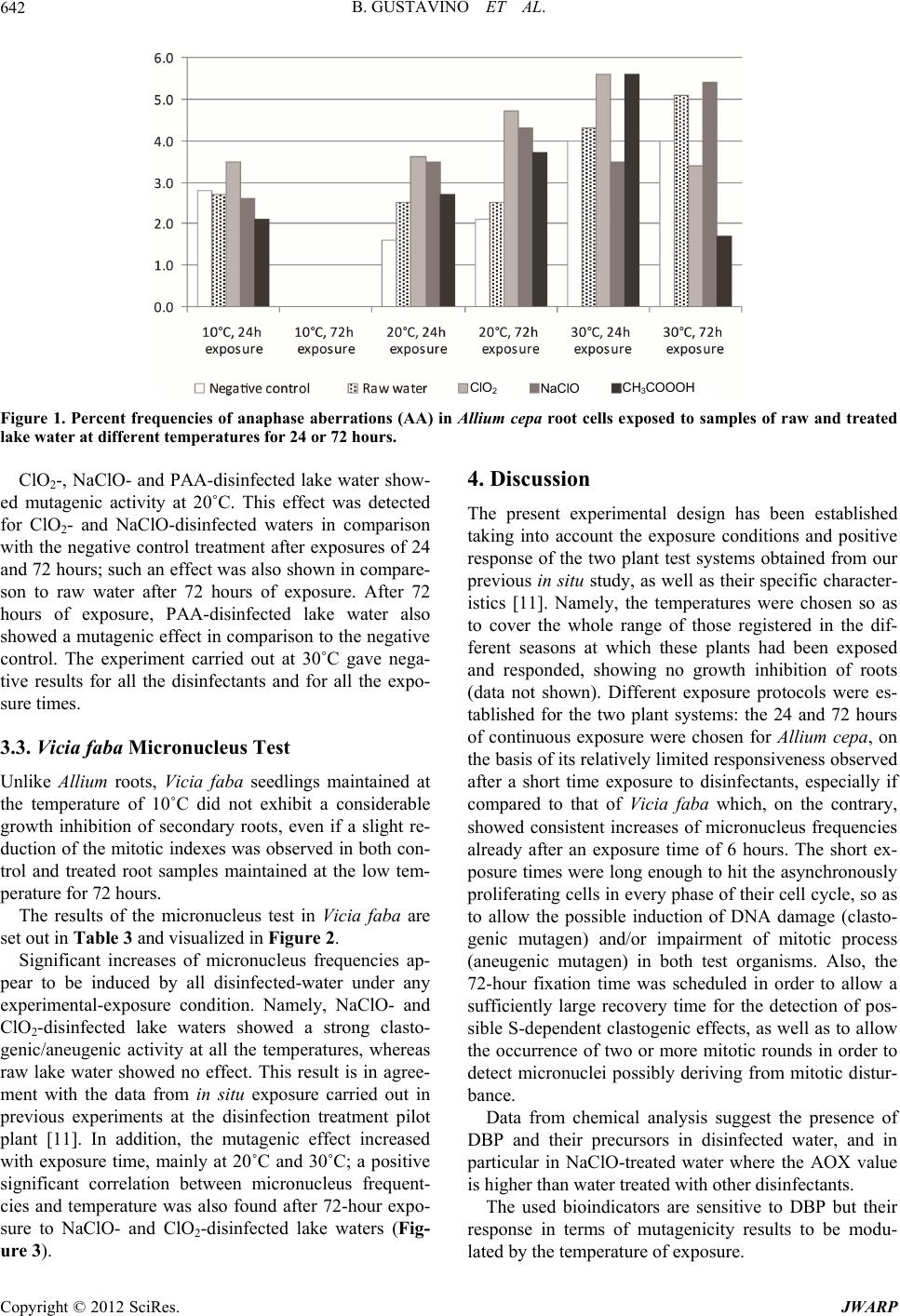 B. GUSTAVINO ET AL. 642 ClO 2 NaClO CH 3 COOOH Figure 1. Percent frequencies of anaphase aberrations (AA) in Allium cepa root cells exposed to samples of raw and treated lake water at different temperatures for 24 or 72 hours. ClO2-, NaClO- and PAA-disinfected lake water show- ed mutagenic activity at 20˚C. This effect was detected for ClO2- and NaClO-disinfected waters in comparison with the negative control treatment after exposures of 24 and 72 hours; such an effect was also shown in compare- son to raw water after 72 hours of exposure. After 72 hours of exposure, PAA-disinfected lake water also showed a mutagenic effect in comparison to the negative control. The experiment carried out at 30˚C gave nega- tive results for all the disinfectants and for all the expo- sure times. 3.3. Vicia faba Micronucleus Test Unlike Allium roots, Vicia faba seedlings maintained at the temperature of 10˚C did not exhibit a considerable growth inhibition of secondary roots, even if a slight re- duction of the mitotic indexes was observed in both con- trol and treated root samples maintained at the low tem- perature for 72 hours. The results of the micronucleus test in Vicia faba are set out in Table 3 and visualized in Figure 2. Significant increases of micronucleus frequencies ap- pear to be induced by all disinfected-water under any experimental-exposure condition. Namely, NaClO- and ClO2-disinfected lake waters showed a strong clasto- genic/aneugenic activity at all the temperatures, whereas raw lake water showed no effect. This result is in agree- ment with the data from in situ exposure carried out in previous experiments at the disinfection treatment pilot plant [11]. In addition, the mutagenic effect increased with exposure time, mainly at 20˚C and 30˚C; a positive significant correlation between micronucleus frequent- cies and temperature was also found after 72-hour expo- sure to NaClO- and ClO2-disinfected lake waters (Fig- ure 3). 4. Discussion The present experimental design has been established taking into account the exposure conditions and positive response of the two plant test systems obtained from our previous in situ study, as well as their specific character- istics [11]. Namely, the temperatures were chosen so as to cover the whole range of those registered in the dif- ferent seasons at which these plants had been exposed and responded, showing no growth inhibition of roots (data not shown). Different exposure protocols were es- tablished for the two plant systems: the 24 and 72 hours of continuous exposure were chosen for Allium cepa, on the basis of its relatively limited responsiveness observed after a short time exposure to disinfectants, especially if compared to that of Vicia faba which, on the contrary, showed consistent increases of micronucleus frequencies already after an exposure time of 6 hours. The short ex- posure times were long enough to hit the asynchronously proliferating cells in every phase of their cell cycle, so as to allow the possible induction of DNA damage (clasto- genic mutagen) and/or impairment of mitotic process (aneugenic mutagen) in both test organisms. Also, the 72-hour fixation time was scheduled in order to allow a sufficiently large recovery time for the detection of pos- sible S-dependent clastogenic effects, as well as to allow the occurrence of two or more mitotic rounds in order to detect micronuclei possibly deriving from mitotic distur- bance. Data from chemical analysis suggest the presence of DBP and their precursors in disinfected water, and in particular in NaClO-treated water where the AOX value is higher than water treated with other disinfectants. The used bioindicators are sensitive to DBP but their response in terms of mutagenicity results to be modu- ated by the temperature of exposure. l Copyright © 2012 SciRes. JWARP 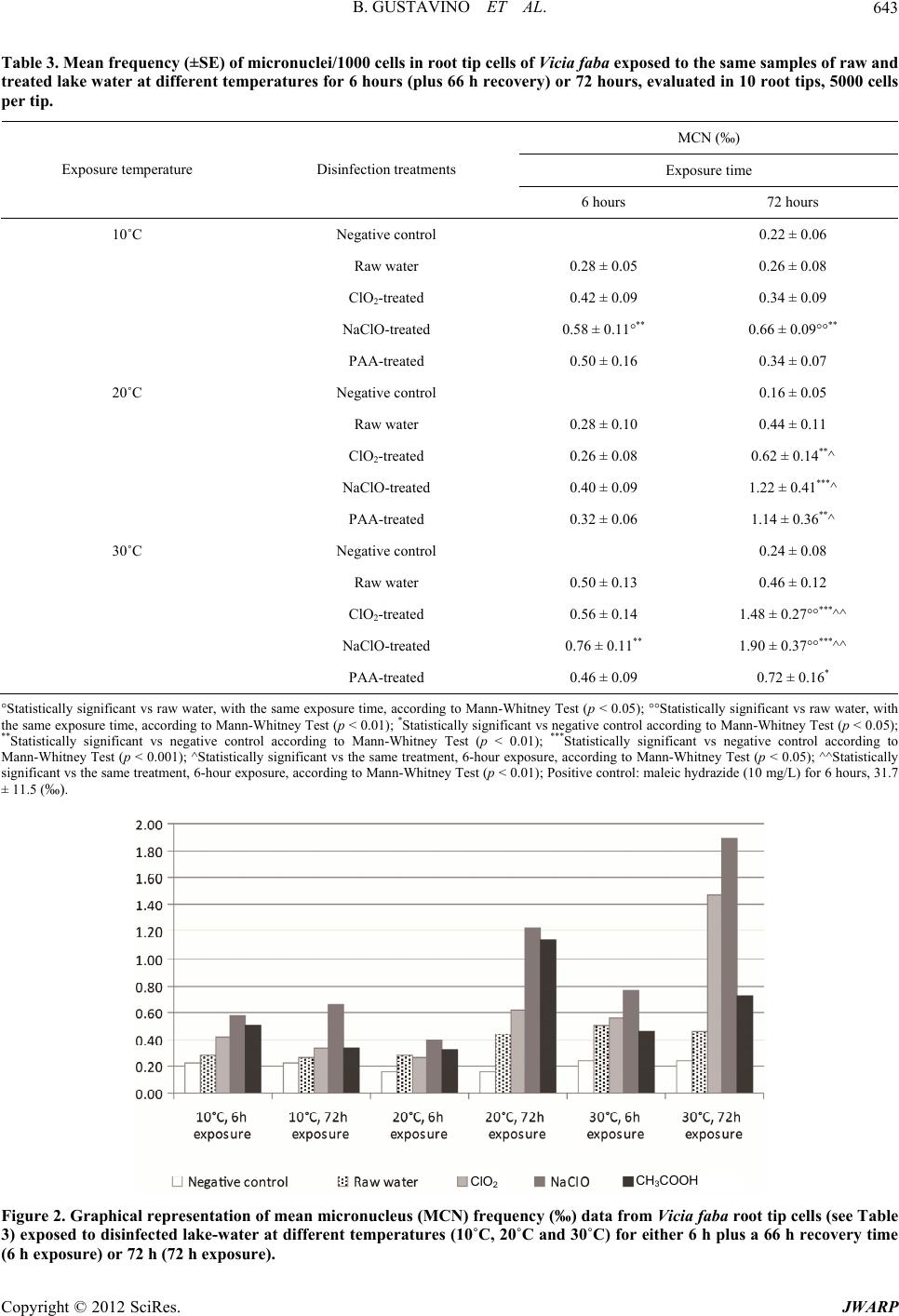 B. GUSTAVINO ET AL. 643 Table 3. Mean frequency (±SE) of micronuclei/1000 cells in root tip cells of Vicia faba exposed to the same samples of raw and treated lake water at different temperatures for 6 hours (plus 66 h recovery) or 72 hours, evaluated in 10 root tips, 5000 cells per tip. MCN (‰) Exposure time Exposure temperature Disinfection treatments 6 hours 72 hours 10˚C Negative control 0.22 ± 0.06 Raw water 0.28 ± 0.05 0.26 ± 0.08 ClO2-treated 0.42 ± 0.09 0.34 ± 0.09 NaClO-treated 0.58 ± 0.11°** 0.66 ± 0.09°°** PAA-treated 0.50 ± 0.16 0.34 ± 0.07 20˚C Negative control 0.16 ± 0.05 Raw water 0.28 ± 0.10 0.44 ± 0.11 ClO2-treated 0.26 ± 0.08 0.62 ± 0.14**^ NaClO-treated 0.40 ± 0.09 1.22 ± 0.41***^ PAA-treated 0.32 ± 0.06 1.14 ± 0.36**^ 30˚C Negative control 0.24 ± 0.08 Raw water 0.50 ± 0.13 0.46 ± 0.12 ClO2-treated 0.56 ± 0.14 1.48 ± 0.27°°***^^ NaClO-treated 0.76 ± 0.11** 1.90 ± 0.37°°***^^ PAA-treated 0.46 ± 0.09 0.72 ± 0.16* °Statistically significant vs raw water, with the same exposure time, according to Mann-Whitney Test (p < 0.05); °°Statistically significant vs raw water, with the same exposure time, according to Mann-Whitney Test (p < 0.01); *Statistically significant vs negative control according to Mann-Whitney Test (p < 0.05); **Statistically significant vs negative control according to Mann-Whitney Test (p < 0.01); ***Statistically significant vs negative control according to Mann-Whitney Test (p < 0.001); ^Statistically significant vs the same treatment, 6-hour exposure, according to Mann-Whitney Test (p < 0.05); ^^Statistically significant vs the same treatment, 6-hour exposure, according to Mann-Whitney Test (p < 0.01); Positive control: maleic hydrazide (10 mg/L) for 6 hours, 31.7 ± 11.5 (‰). ClO 2 CH 3 COOH Figure 2. Graphical representation of mean micronucleus (MCN) fre quency (‰) data fr om Vicia faba root tip cells (see Table 3) exposed to disinfected lake-w ater at different temperatures (10˚C, 20˚C and 30˚C) for either 6 h plus a 66 h recovery time (6 h exposure) or 72 h (72 h exposure). Copyright © 2012 SciRes. JWARP  B. GUSTAVINO ET AL. 644 3028262422201816141210 14 12 10 8 6 4 2 0 3028262422201816141210 20 15 10 5 0 (a) (b) 3028262422201816141210 18 16 14 12 10 8 6 4 2 0 3028262422201816141210 6 5 4 3 2 1 0 (c) (d) 26 28 302422201816141210 3 2 1 0 (e) Figure 3. Regression analysis curves between temperature values (˚C, abscissa) and micronucleus frequencies (ordinate) ob- tained from 72 h exposure of secondary root tips of Vicia faba to samples of disinfected lake water (a)-(c), raw water (d) and negative con trol water (e). (a) ClO2; (b) NaClO; (c) PAA; (d) Lake raw water; (e) Hoagland’s solution. The results of this study indicate that temperature may play an important role in mitotic cell cycle progression, at least in Allium cepa and Vicia faba root cells, and in modulating the expression of clastogenic/aneugenic da- mage. Despite the positive results obtained in Allium cepa from the field study carried out at the Trasimeno wa- ter-treatment plant, where roots not only survived the 72-hour exposure at temperature as low as 10˚C, but also gave the most powerful mutagenicity data, in the present experiment the same temperature showed to be not per- missive for root growth. Indeed, mutagenic effects were only observed at 20˚C, which shows to be the optimal temperature for cell pro- liferation of this plant roots. In this organism, mitotic cycle duration has been determined by several authors, who showed that the optimal temperature for root growth is 20˚C ± 2˚C; under such conditions mitotic cycle dura- tion is about 24 hours [42]. The observed variations re- ported in the present study could largely be explained by the different exposure temperatures, which appear to influence root growth [45]. Treatments of Allium cepa with NaClO- and ClO2- disinfected lake waters performed at 20˚C induced a clear aneugenic/clastogenic effect, detectable at both exposure times. This effect seemed to be more pro- nounced at the long-term exposure (72 hours), at which PAA-disinfected lake water also appears to exert a mutagenic effect. Short-term exposure in the Allium cepa test gave generally negative results for all disinfectants, although NaClO- and ClO2-disinfected lake water pro- duced mutagenic effects compared to the negative con- trol. On the other hand, when the exposure time was in- creased (72 hours, about two mitotic cycles), mutagenic effects were detected for the NaClO- and ClO2-disin- fected lake water compared to raw water. Low temperature (10˚C) is likely to cause a delay in cell proliferation in Allium cepa roots, which may ex- Copyright © 2012 SciRes. JWARP  B. GUSTAVINO ET AL. 645 plain the long-term exposure inhibition of root germina- tion and growth. At the same temperature, short-term ex- posure produced no genotoxic effects since mitosis rate was reduced, and possibly aberration frequency as well. The highest temperature (30˚C) did not cause any re- markable mutagenic effects, despite the increase in fre- quency in some samples. The negative control also gave a high frequency of aberrations at the highest tempera- ture. High temperature is likely to impair root proliferate- ing cells, inducing a sort of stress that may be the cause of most mutagenic damage. In this study the Allium cepa test was less sensitive to mutagenic effects at high tem- perature conditions, which is in agreement with the data reported in a previous study on in situ exposure in June, which corresponded to a very warm period [11]. In Al- lium cepa the highest temperature did not seem to influ- ence the mitotic index, and hence the cell cycle was not influenced. This means that cell division rate was not changed, but root length was on average shorter than the negative control. Moreover, other signs of toxicity were observed in Allium roots (form and consistency of roots) and the toxicity may have concealed the genotoxic ef- fects. In Vicia faba temperature also plays an important role in modulating the expression of clastogenic/aneugenic damage. Low temperature may slow down the progress- sive reduction in micronucleus frequency after short-term treatments, which is expected from their dilution/de- struction [32,46], as a consequence of the lengthening of the cell cycle. This could explain why 72-hour exposure produced higher clastogenic/aneugenic effects than 6- hour exposure at a higher temperature (Figure 2). In- creasing clastogenic/aneugenic activity of NaClO- and ClO2-disinfected lake waters at increasing temperatures is suggested by the increase in micronucleus frequencies after 72 hours of exposure. Equilibrium value of micro- nucleus frequency is reached after long-term treatments (72 hours) for the rise of new micronuclei and the dilu- tion/destruction of the old ones [32,46]. An increase in the equilibrium frequency of micronuclei therefore sug- gests a higher rate of new micronucleus formation. A comparison of these findings with those from in situ ex- posure [11] confirmed that NaClO- and ClO2-disinfected lake waters have stronger clastogenic/aneugenic effects than PAA-disinfected waters; the lack of a difference in micronucleus frequency after 6-hour and 72-hour expo- sure in the cold season is also confirmed. The hypothesis that low temperatures slow down micronucleus dilu- tion/destruction with time relenting cell cycle progress- sion is therefore supported. The importance of cell cycle duration in evaluating genotoxic damage has been seen for other genotoxicity plant tests as well. In the Tradescantia/micronuclei test, exposure to some toxic compounds or to overdoses causes a delay in the meiotic cycle, and the induced damage may be not recognized using the standard time of test protocol [47]. The temperature affecting cell cycle duration may in- fluence sensitivity and the possibility of revealing low levels of environmental genotoxins. The results of this study show that temperature plays an important role in mitotic cell cycle progression in Allium cepa and Vicia faba root tips. Vicia faba micronucleus test proved to be more sensitive than Allium cepa chromosomal aberra- tion test with regard to temperature influence on geno- toxic damage expression. The different temperatures at which the plant tests were carried out could have im- paired the plausibility of the experimental data obtained. In conclusion, temperature is an important variable to be taken into account when planning in situ exposure of plants for mutagenicity tests. This poses the question of selecting appropriate test organisms, taking into account their tolerance and viability under relatively wide thermal ranges among environmental variables. REFERENCES [1] IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans, “Chlorinated Drinking-Water, Chlori- nation By-Products, Some Other Halogenated Compounds, Cobalt and Cobalt Compounds,” International Agency for Research on Cancer, Lyon, Vol. 52, 1991. [2] WHO, “Revision of the WHO Guidelines for Drinking Water Quality,” World Health Organization, Geneva, 1996. [3] WHO, “Guidelines for Drinking-Water Quality. Volume 1: Recommendations,” 3rd Edition, World Health Or- ganization, Geneva, 2004. [4] S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. Demarini, “Occurrence, Genotoxicity, and Car- cinogenicity of Regulated and Emerging Disinfection By-Products in Drinking Water: A Review and Roadmap for Research,” Mutation Research, Vol. 636, No. 1-3, 2007, pp. 178-242. doi:10.1016/j.mrrev.2007.09.001 [5] IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, “Some Drinking-Water Disinfectants and Contaminants, Including Arsenic,” International Agency for Research on Cancer, Lyon, Vol. 84, 2004. [6] K. P. Cantor, C. M. Villanueva, D. T. Silverman, J. D. Figueroa, F. X. Real, M. Garcia-Closas, N. Malats, S. Chanock, M. Yeager, A. Tardon, R. Garcia-Closas, C. Serra, A. Carrato, G. Castaño-Vinyals, C. Samanic, N. Rothman and M. Kogevinas, “Polymorphisms in GSTT1, GSTZ1, and CYP2E1, Disinfection By-Products, and Risk of Bladder Cancer in Spain,” Environmental Health Perspectives, Vol. 118, No. 11, 2010, pp. 1545-1550. doi:10.1289/ehp.1002206 [7] R. J. Bull, D. A. Reckhowb, X. Li, A. R. Humpage, C. Joll and S. E. Hrudey, “Potential Carcinogenic Hazards of Non-Regulated Disinfection By-Products: Haloquinones, Halo-Cyclopentene and Cyclohexene Derivatives, N-Hala- Copyright © 2012 SciRes. JWARP  B. GUSTAVINO ET AL. 646 mines, Halonitriles, and Heterocyclic Amines,” Toxicol- ogy, Vol. 286, No. 1-3, 2011, pp. 1-19. doi:10.1016/j.tox.2011.05.004 [8] L. Guzzella, S. Monarca, C. Zani, D. Feretti, I. Zerbini, A. Buschini, P. Poli, C. Rossi and S. D. Richardson, “In Vi- tro Potential Genotoxic Effects of Surface Drinking Wa- ter Treated with Chlorine and Alternative Disinfectants,” Mutation Research, Vol. 564, No. 2, 2004, pp. 179-193. doi:10.1016/j.mrgentox.2004.08.006 [9] T. Ohe, T. Watanabe and K. Wakabayashi, “Mutagens in Surface Waters: A Review,” Mutation Research, Vol. 567, No. 2-3, 2004, pp. 109-149. doi:10.1016/j.mrrev.2004.08.003 [10] A. Buschini, A. Martino, B. Gustavino, M. Monfrinotti, P. Poli, C. Rossi, M. Santoro, A. J. Dörr and M. Rizzoni, “Comet Assay and Micronucleus Test in Circulating Erythrocytes of Cyprinus carpio Specimens Exposed in Situ to Lake Waters Treated with Disinfectants for Pota- bilization,” Mutation Research, Vol. 557, No. 2, 2004, pp. 119-129. doi:10.1016/j.mrgentox.2003.10.008 [11] S. Monarca, M. Rizzoni, B. Gustavino, C. Zani, A. Al- berti, D. Feretti and I. Zerbini, “Genotoxicity of Surface Water Treated with Different Disinfectants Using, Plant Tests,” Environmental and Molecular Mutagenesis, Vol. 41, No. 5, 2003, pp. 353-359. doi:10.1002/em.10161 [12] S. Monarca, D. Feretti, C. Zani, M. Rizzoni, S. Casarella and B. Gustavino, “Genotoxicity of Drinking Water Dis- infectants in Plant Bioassays,” Environmental and Mo- lecular Mutagenesis, Vol. 46, No. 2, 2005, pp. 96-103. doi:10.1002/em.20137 [13] T. H. Ma, “The International Program on Plant Bioassays and the Report of the Follow-Up Study after the Hands- On Workshop in China,” Mutation Research, Vol. 426, No. 2, 1999, pp. 103-106. doi:10.1016/S0027-5107(99)00049-4 [14] W. Liu, L. S. Zhu, J. Wang, J. H. Wang, H. Xie and Y. Song, “Assessment of the Genotoxicity of Endosulfan in Earthworm and White Clover Plants Using the Comet Assay,” Archives of Environmental Contamination and Toxicology, Vol. 56, No. 4, 2009, pp. 742-746. doi:10.1007/s00244-009-9309-8 [15] M. Misík, T. H. Ma, A. Nersesyan, S. Monarca, J. K. Kim and S. Knasmueller, “Micronucleus Assays with Trades- cantia Pollen Tetrads: An Update,” Mutagenesis, Vol. 26, No. 1, 2011, pp. 215-221. doi:10.1093/mutage/geq080 [16] S. Radić, D. Stipaničev, P. Cvjetko, M. M. Rajčić, S. Sirac, B. Pevalek-Kozlina and M. Pavlica, “Duckweed Lemna Minor as a Tool for Testing Toxicity and Geno- toxicity of Surface Waters,” Ecotoxicology and Environ- mental Safety, Vol. 74, No. 2, 2011, pp. 182-187. doi:10.1016/j.ecoenv.2010.06.011 [17] W. F. Grant, “Higher Plant Assays for the Detection of Chromosomal Aberrations and Gene Mutations—A Brief Historical Background on Their Use for Screening and Monitoring Environmental Chemicals,” Mutation Resear- ch, Vol. 426, No. 2, 1999, pp. 107-112. doi:10.1016/S0027-5107(99)00050-0 [18] H. El Hajjouji, E. Pinelli, M. Guiresse, G. Merlina, J. C. Revel and M. Hafidi, “Assessment of the Genotoxicity of Olive Mill Waste Water (OMWW) with the Vicia faba Micronucleus Test,” Mutation Research, Vol. 634, No. 1- 2, 2007, pp. 25-31. doi:10.1016/j.mrgentox.2007.05.015 [19] D. M. Leme and M. A. Marin-Morales, “Chromosome Aberration and Micronucleus Frequencies in Allium cepa Cells Exposed to Petroleum Polluted Water—A Case Study,” Mutation Research, Vol. 650, No. 1, 2008, pp. 80-86. doi:10.1016/j.mrgentox.2007.10.006 [20] E. Béraud, S. Cotelle, P. Leroy and J. F. Férard, “Geno- toxic Effects and Induction of Phytochelatins in the Pres- ence of Cadmium in Vicia faba Roots,” Mutation Re- search, Vol. 633, No. 2, 2007, pp. 112-116. doi:10.1016/j.mrgentox.2006.05.013 [21] Y. F. Song, P. Gong, B. M. Wilke, W. Zhang, X. Y. Song, T. H. Sun and M. L. Ackland, “Genotoxicity Assessment of Soils from Wastewater Irrigation Areas and Bioreme- diation Sites Using the Vicia faba Root Tip Micronucleus Assay,” Journal of Environmental Monitoring, Vol. 9, No. 2, 2007, pp. 182-186. doi:10.1039/b614246j [22] A. S. Foltête, A. Dhyevre, J. F. Ferard and S. Cotelle, “Improvement of Vicia-Micronucleus Test for Assess- ment of Soil Quality: A Proposal for International Stan- dardization,” Chemosphere, Vol. 85, No. 10, 2011, pp. 1624-1629. doi:10.1016/j.chemosphere.2011.08.026 [23] A. S. Foltête, J. F. Masfaraud, J. F. Férard and S. Cotelle, “Is There a Relationship between Early Genotoxicity and Life-History Traits in Vicia faba Exposed to Cadmium- Spiked Soils?” Mutation Research, Vol. 747, No. 2, 2012, pp. 159-163. [24] K. Al-Sabti and B. Kurelec, “Chromosomal Aberrations in Onion (Allium cepa) Induced by Water Chlorination By-Products,” Bulletin of Environmental Contamination and Toxicology, Vol. 34, No. 1, 1985, pp. 80-88. doi:10.1007/BF01609706 [25] W. F. Grant, H. G. Lee, D. M. Logan and M. F. Salamone, “The Use of Tradescantia and Vicia faba Bioassays for the in Situ Detection of Mutagens in an Aquatic Environ- ment,” Mutation Research, Vol. 270, No. 1, 1992, pp. 53- 64. doi:10.1016/0027-5107(92)90101-7 [26] W. F. Grant, “The Present Status of Higher Plant Bioas- says for the Detection of Environmental Mutagens,” Mu- tation Research, Vol. 310, No. 2, 1994, pp. 175-185. doi:10.1016/0027-5107(94)90112-0 [27] S. Monarca, A. Zanardini, D. Feretti, A. Dalmiglio, E. Falistocco, P. Manica and G. Nardi, “Mutagenicity of Ex- tracts of Lake Drinking Water Treated with Different Disinfectants in Bacterial and Plant Tests,” Water Resear- ch, Vol. 32, No. 9, 1998, pp. 2689-2695. doi:10.1016/S0043-1354(98)00031-1 [28] H. Steinkellner, F. Kassie and S. Knasmuller, “Trades- cantia-Micronucleus Assay for the Assessment of the Clas- togenicity of Austrian Water,” Mutation Research, Vol. 426, No. 2, 1999, pp. 113-116. doi:10.1016/S0027-5107(99)00051-2 [29] S. Monarca, C. Zani, S. D. Richardson, A. D. Thruston Jr., M. Moretti, D. Feretti and M. Villarini, “A New Ap- proach to Evaluating the Toxicity and Genotoxicity of Disinfected Drinking Water,” Water Research, Vol. 38, No. 17, 2004, pp. 3809-3819. Copyright © 2012 SciRes. JWARP  B. GUSTAVINO ET AL. Copyright © 2012 SciRes. JWARP 647 doi:10.1016/j.watres.2004.07.003 [30] D. Feretti, I. Zerbini, E. Ceretti, M. Villarini, C. Zani, M. Moretti, C. Fatigoni, G. Orizio, F. Donato and S. Mon- arca, “Evaluation of Chlorite and Chlorate Genotoxicity Using Plant Bioassay and in Vitro DNA Damage Tests,” Water Research, Vol. 42, No. 15, 2008, pp. 4075-4082. doi:10.1016/j.watres.2008.06.018 [31] D. Feretti, E. Ceretti, B. Gustavino, I. Zerbini, C. Zani, S. Monarca and M. Rizzoni, “Ground and Surface Water for Drinking: A Laboratory Study on Genotoxicity Using Plant Tests,” Journal of Public Health Research, Vol. 1, No. 1, 2012, pp. 31-37. doi: 14.4081/jphr.2012.e7 [32] F. Degrassi and M. Rizzoni, “Micronucleus Test in Vicia faba Root Tips to Detect Mutagen Damage in Fresh-Wa- ter Pollution,” Mutation Research, Vol. 97, Vol. 1, 1982, pp. 19-33. [33] M. Rizzoni, E. Vitagliano, M. C. Marconi, A. Sottili and B. Gustavino, “Micronucleus Induction by Low Doses of X-Rays in Vicia faba Root Tips,” Mutation Research, Vol. 176, No. 2, 1987, pp. 205-209. doi:10.1016/0027-5107(87)90051-0 [34] D. M. Leme and M. A. Marin-Morales, “Allium cepa Test in Environmental Monitoring: A Review on Its Applica- tion,” Mutation Research, Vol. 682, No. 1, 2009, pp. 71- 81. doi:10.1016/j.mrrev.2009.06.002 [35] M. Rizzoni, B. Gustavino, C. Ferrari, L. G. Gatti and E. A. Fano, “An Integrated Approach to the Assessment of the Environmental Quality of the Tiber River in the Urban Area of Rome: A Mutagenesis Assay (Micronucleus Test) and Analysis of Macrobenthic Community Structure,” Science of the Total Environment, Vol. 162, No. 2-3, 1995, pp. 127-137. doi:10.1016/0048-9697(95)04444-6 [36] V. Smaka-Kincl, P. Stegnar, M. Lovka and M. J. Toman, “The Evaluation of Waste, Surface and Ground Water Quality Using the Allium Test Procedure,” Mutation Re- search, Vol. 368, No. 3-4, 1996, pp. 171-179. doi:10.1016/S0165-1218(96)90059-2 [37] S. Minissi and E. Lombi, “Heavy Metal Content and Mutagenic Activity, Evaluated by Vicia faba Micronu- cleus Test, of Tiber River Sediments,” Mutation Research, Vol. 393, No. 1-2, 1997, pp. 17-21. doi:10.1016/S1383-5718(97)00093-4 [38] S. Minissi, D. Caccese, F. Passafiume, A. Grella, C. Eleonora and M. Rizzoni, “Mutagenicity (Micronucleus Test in Vicia faba Root Tips), Polycyclic Aromatic Hy- drocarbons and Heavy Metal Content of Sediments Col- lected in Tiber River and Its Tributaries within the Urban Area of Rome,” Mutation Research, Vol. 420, No. 1-3, 1998, pp. 77-84. doi:10.1016/S1383-5718(98)00142-9 [39] C.-Q. Duan, B. Hu, X.-H. Jiang, C.-H. Wen, Z. H. Wang and Y.-X. Wang, “Genotoxicity of Water Samples from Dianchi Lake Detected by the Vicia faba Micronucleus Test,” Mutation Research, Vol. 426, No. 2, 1999, pp. 121- 125. doi:10.1016/S0027-5107(99)00053-6 [40] R. Crebelli, L. Conti, S. Monarca, D. Feretti, I. Zerbini, C. Zani, E. Veschetti, D. Cutilli and M. Ottaviani, “Geno- toxicity of the Disinfection By-Products Resulting from Peracetic Acid- or Hypochlorite-Disinfected Sewage Waste- water,” Water Research, Vol. 39, No. 6, 2005, pp. 1105- 1113. doi:10.1016/j.watres.2004.12.029 [41] APHA (American Public Health Association), “Standard Methods for the Examination of Water and Wastewater,” 17th Edition, American Public Health Association Inc., Washington DC, 1998. [42] G. Fiskesjo, “The Allium Test as a Standard in Environ- mental Monitoring,” Hereditas, Vol. 102, No. 1, 1985, pp. 99-112. doi:10.1111/j.1601-5223.1985.tb00471.x [43] J. Rank and M. H. Nielsen, “Allium cepa Anaphase-Te- lophase Root Tip Chromosome Aberration Assay on N-Methyl-N-Nitrosourea, Maleic Hydrazide, Sodium Azide, and Ethyl Methanesulfonate,” Mutation Research, Vol. 390, No. 1-2, 1997, pp. 121-127. doi:10.1016/S0165-1218(97)00008-6 [44] J. Rank and M. H. Nielsen, “Evaluation of the Allium Anaphase-Telophase Test in Relation to Genotoxicity Screening of Industrial Wastewater,” Mutation Research, Vol. 312, No. 1, 1994, pp. 17-24. [45] W. F. Grant, “Chromosome Aberration Assays in Allium. A Report of the US Environmental Protection Agency Gene-Tox Program,” Mutation Research, Vol. 99, No. 3, 1982, pp. 273-291. doi:10.1016/0165-1110(82)90046-X [46] B. Gustavino, E. Vitagliano, A. Sottili and M. Rizzoni, “A Comparison between Short-Term Evolution of Mi- cronuclei Induced by X-Rays and Colchicine in Root Tips of Vicia faba,” Mutation Research, Vol. 192, No. 2, 1987, pp. 109-119. doi:10.1016/0165-7992(87)90106-0 [47] E. Falistocco, R. Torricelli, D. Feretti, I. Zerbini, C. Zani and S. Monarca, “Enhancement of Micronuclei Frequency in the Tradescantia/Micronuclei Test Using a Long Re- covery Time,” Hereditas, Vol. 133, No. 2, 2000, pp. 171- 174. doi:10.1111/j.1601-5223.2000.t01-1-00171.x
|