Food and Nutrition Sciences
Vol.4 No.8A(2013), Article ID:35298,9 pages DOI:10.4236/fns.2013.48A022
A Comparative Study on the Total Antioxidant and Antimicrobial Potentials of Ethanolic Extracts from Various Organ Tissues of Allium spp.
![]()
1Department of Nursing, Mackay Medicine, Nursing and Management College, Taipei, Chinese Taipei; 2School of Forestry and Resource Conservation, National Taiwan University, Taipei, Chinese Taipei; 3Sunshin Area Farmer’s Association, Yi-Lan County, Chinese Taipei; 4Department of Food Science, Yuanpei University, Hsinchu, Chinese Taipei.
Email: *hungder@mail.ypu.edu.tw
Copyright © 2013 Tsan-Chang Chang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 12th, 2013; revised April 12th, 2013; accepted April 19th, 2013
Keywords: Allium spp.; Antioxidants; Antimicrobial Activity; DPPH IC50; TEAC; Total Phenolic Content
ABSTRACT
The extracts from different tissues of Allium fistulosum L. and Allium sativum L. were investigated to evaluate their antioxidant and antimicrobial capacity. The highest yields of the Allium extracts were prepared from the extraction of 30% ethanol solution. The DPPH scavenging activity was the highest in A. fistulosum L. leaves, which IC50 is 14.61 μg·mL−1. The highest antioxidant activity using TEAC assay and total phenolic content were observed in A. sativum L. stems and A. fistulosum L. stems, where they are determined to be 15.51 mM and 191.04 mg GAE·g−1, and 14.59 mM and 182.60 mg GAE·g−1, respectively. Statistic analysis revealed that the DPPH IC50 value was significantly correlated with total phenolic contents and antioxidant activity using TEAC assay. The extracts of A. sativum L. bulbs were found to exhibit antimicrobial activity against Staphylococcus aureus with the MIC and MBC of 0.2 mg·mL−1 and 0.4 mg·mL−1, respectively. In addition, the extract of A. fistulosum L. stems was more active against Bacillus subtilis, with an MIC and MBC of 0.2 and 0.4 mg·mL−1, respectively. The inhibitory activity of various Allium extracts against the test bacteria was greater than that of 10 μg·mL−1 allicin. The results indicated that Allium spp. extracts could be used as a potential source of natural antioxidants and antimicrobial agents.
1. Introduction
The economically important Allium spp. (onion and garlic) are used worldwide as spices, vegetables, and medicinal plants. Traditionally, they also play a very essential role in the daily diet in many Asia countries, and many used to flavor foods. The green onions (Allium fistulosum L.), also referred to as the Welsh onion, and garlic (Allium sativum L.) belong to the family Alliaceae, which are very economically important vegetables in most East Asian countries [1-3]. The Allium spp. materials were acquired from Sunshin Area, Yi-Lan, Taiwan. This area, mostly run by local small-scale farmers, is famous for its top-quality, fragrance-rich green onions. Green onions and garlic are commonly consumed vegetables that possess markedly bioactive potentials, such as antioxidant capacity [4], antimicrobial activity [5,6], antiplatelet activities [7] and anticancer activity [8]. With successful demonstration of the functioning properties of the Sunshin green onions, this study would help the local farmers to market their produce with value adding strategies and benefit economically from their production.
Polyphenols and quercetin in plants have been reported to be the major compounds exhibiting antioxidant activity [4,9]. In addition, the antioxidant activity of quercetin and its functional effects have been fully demonstrated in a few previous studies [10,11]. On the other hand, Allium spp. contains other antioxidant components, such as flavonoids and volatile sulfur compounds [10,12]. Statistical analysis revealed that the total phenolic contents and the DPPH free radical-scavenging activity were positively correlated [13]. Besides, it had been showed a positive correlation between the dynamics of DPPH free radical-scavenging activity and the antioxidant capacity to scavenge the ABTS+ radicals [14]. Studies of the antioxidant activity of vegetables in South America also pointed out that the DPPH free radical-scavenging ability and total phenolic contents showed a good linear relationship and that both methods could be used to measure the antioxidant activity in vegetables [15].
Antioxidant and antimicrobial effects of Allium plants have been reported for times [1,4,5,10]; however, a study on the top-quality green onions and garlic produced in Taiwan is still scarce. In this study, different types of tissue of Taiwan Sun-Shin green onion and garlic were extracted from roots, stems and leaves using ethanol. Methods of determining the antioxidant activities of ethanol extracts from various Allium tissues include the 2,2’- dipheny-1-picrylhydrazyl (DPPH) radical-scavenging assay and trolox equivalent antioxidant capacity (TEAC) assay, and total phenolics were determined using the Folin-Ciocalteu method. In order to evaluate the potential use of different tissues of Allium spp. in Taiwan, their antioxidant capacity and antimicrobial activity were measured. The objectives of this study were to compare the antioxidant activity of the different parts of Allium spp. from Taiwan, to compare the antimicrobial activities of various Allium extracts, and to correlate the antioxidant load with the antioxidant activity of the extracts.
2. Materials and Methods
2.1. Plant Materials
Plant materials including Allium fistulosum L. (the Welsh onion) and A. sativum L. (Garlic) were collected and purchased from the Farmer’s Association in the Yi-Lan Sunshin Area in Taiwan. The plant tissues, which included bulbs, roots, stems and leaves of Allium spp., were dried in an oven at 50˚C for 7 d. The lengths of the root, stem and leaf from A. fistulosum L. were 8.2 ± 4.6 cm, 16.9 ± 1.9 cm and 46.1 ± 6.3 cm, respectively, and those from A. sativum L. were 9.7 ± 4.1 cm, 21.9 ± 5.4 cm and 50.4 ± 12.2 cm, respectively. The dried plant tissues were then extracted using various concentrations of ethanol and 100˚C boiling water. Adequate amounts (10 g) of the dried tissues were extracted with 30%, 60%, 95% ethanol for 7 d at ambient temperature and boiling water for 30 min, respectively. Fractions of the extracted material were stored at 4˚C prior to analysis. The abbreviations AFR, AFS and AFL represent respectively represent the roots, stems, and leaves of A. fistulosum, and ASB, ASR, ASS, and ASL respectively represent the bulbs, roots, stems, and leaves of A. sativum.
2.2. Chemicals
2,2’-Diphenyl-1-picrylhydrazyl radical (DPPH), 2,2’- azinobis-(3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), Folin-Ciocalteu reagent quercetin and (+)-catechin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Potassium persulfate was purchased from Merck. All other solvents and reagents were purchased from Sigma Chemical Co.
2.3. Antioxidant Activity Assays
The DPPH radical-scavenging capacity of Allium spp. was determined according to the method described by Gyamfi et al. [16]. One milliliter of ethanolic extract and 5 mL of freshly prepared 0.1 mM DPPH ethanolic solution were mixed thoroughly and kept in the dark. After 30 min of incubation at room temperature, the absorbance was read against a blank at 517 nm by a Jasco V- 550 UV-visible spectrophotometer (Tokyo, Japan). The blank was prepared by replacing the extract with various concentrations of ethanol or water. The percentage of free radical scavenging activity was calculated as follows:
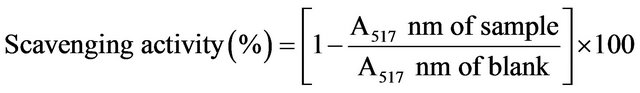
The decrease in absorbance, which was induced by the tested sample and illustrated by a change of color from deep-violet to light-yellow, was compared to that of the positive control using (+)-catechin as a standard. The IC50 value, representing the concentration of extract that required for 50% inhibition of DPPH radicals, was determined. The assay was carried out in triplicate and results were averaged.
In order to determine the free radical scavenging capacity, the TEAC assay, using the ABTS•+ radical cation, was carried out according to the method of Re et al. [17], with the following slight modification. The relative antioxidant capacity of the test tissues was measured in comparison to that of Trolox (as the control) by scavenging the 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS•+) radical. The ABTS•+ radical was generated by mixing a solution of 7 mM ABTS•+ with 2.45 mM K2S2O8. The ABTS•+ solution was diluted with water prior to use and an absorbance of 0.70 ± 0.02 at 734 nm was obtained. Upon adding 1485 μL of the diluted ABTS•+ solution to 15 μL of tested sample or Trolox, the absorbance at 734 nm was read by a Jasco V-550 UV-visible spectrophotometer after incubating the 1.5 mL mixed solution for 6 min. Decreases in absorbance were recorded and the concentrations of the standard and samples were calculated with respect to the calibration plot. The final TEAC value of the tested samples was calculated by comparing ABTS•+ decolorisation with that of Trolox. The Trolox solution was used as a reference standard and the results were expressed as mmol equivalent.
2.4. Determination of Total Phenolics Contents
The amount of total phenolics was measured by the Folin-Ciocalteu method described by Kujala et al. [18], using gallic acid as the standard. A calibration curve was obtained using concentrations of standard solutions in the range of 0.005 - 0.08 mg·mL−1. A 0.4 mL aliquot of diluted extract (all fractions were diluted with methanol to adjust the absorbance within the calibration limits), 0.4 mL of 1 M Folin-Ciocalteu reagent, and 0.8 mL of Na2CO3 (20%, w/v) were mixed. After 8 min of incubation, the mixture was centrifuged at 15,000 × g for 10 min. Then the absorbance of the supernatant was measured at 730 nm against a blank (using distilled water) with a Jasco V-550 UV-visible spectrophotometer. The concentration of phenolics thus obtained was multiplied by the dilution factor, and the results were expressed as the equivalent of milligrams of gallic acid per gram of extract (mg GAE·g−1).
2.5. Assay of Antibacterial Activity
The tested bacteria were Gram-negative Escherichia coli ATCC 10536, Pseudomonas aeruginosa ATCC 9027, and Gram-positive Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6051. The strains were purchased from the Bioresource Collection and Research Center (BCRC) of the Food Industry Research Institute in Hsinchu, Taiwan. These bacteria were cultured on a nutrient agar plate for 24 h at 37˚C, and the colonies that formed were picked up twice with a platinum loop and inoculated with a sterilized cotton stick onto the surface of a new nutrient agar plate. Then 50 μL of 1 mg·mL−1 Allium extract were applied to an ethanol-sterilized paper disc (8 mm in diameter), and were placed on the plate. After incubation at 37˚C for 24 h, the inhibition zone around the disc was measured [6]. Penicillin and tetracycline were used as reference controls in the antimicrobial assay. The experiments were run in triplicate, and the developing inhibition zones were compared with those of the reference discs. In addition, the minimal inhibitory concentration (MIC) of the samples was determined by the broth dilution method using the serially diluted Allium extracts as described [6,9]. Then, bacterial cultures were prepared in a nutrient broth and incubated at 37˚C for 24 h. The bacterial cultures were adjusted with sterilized saline to bring the concentration to 107 mL−1. The media containing various Allium extracts were diluted with distilled water to give concentrations ranged from 2 to 0.05 mg·mL−1. One hundred microlitres of the diluted bacterial culture, 0.6 mL of nutrient broth, and 0.6 mL of the Allium extract were mixed well in a test tube. The mixture was then incubated at 37˚C for 24 h to determine the minimal concentration at which growth of bacterial cells was fully inhibited. The MIC and MBC, the lowest concentration of Allium extracts that inhibits the growth of the test microorganism, was determined.
2.6. Statistical Analysis
The triplicate data were subjected to an analysis of variance for a completely random design, using SAS statistics software. The data were presented as mean ± standard deviation of three determinations. Comparison of means was analyzed by Scheffe’s test of the SAS system, and differences were considered significant when p < 0.05.
3. Results and Discussion
3.1. The Characteristics of Plant Material
Both the water contents and extraction yields with ethanol of different parts of plant tissues were determined to comprehend the general characteristics of the A. fistulosum L. and A. sativum L. The water contents of different plant tissues, including root, stem, and leave, on a drying basis, obtained from A. fistulosum and A. sativum were analyzed and found to be in the range of 83.47% to 94.76%, whereas the bulb of Allium sativum L. had the lowest water content of 69.80% (data not shown).
Next, the extraction yields from whole Allium spp. plants prepared with various concentrations of ethanol (30%, 60%, and 95%) were examined. These extraction yields were then compared with those of the same Allium spp. extracted with 100˚C hot water. The results show that the highest extraction yields, which were 42.87% ± 8.74% and 42.43% ± 4.63% for A. fistulosum and A. sativum, respectively, were prepared from 100˚C hot water. The second highest extraction yields, which were 39.06% ± 6.89% and 41.39% ± 3.63% for A. fistulosum and A. sativum, were extracted from 30% ethanol solution. Nevertheless, these extraction yields of the tissues extracted from hot water and 30% ethanol solution were not significantly different at 95% confidence interval. However, it was found in our experiments that the hot water extracts contain many visible remnants of plants, and that their antioxidant and antimicrobial activities were less than those of the ethanolic extracts (results not shown). Ethanol has been proved to be a better solvent than water for the extraction of allicin in plant tissues [6]. The subsequent extraction of Allium spp. in the study was prepared with a solution that contained 30% of ethanol to generate the high extraction yields. The extraction yields of various parts of the plant tissues extracted from A. fistulosum and A. sativum with 30% (w/w) ethanol, on a drying weight basis, were also investigated. The extraction yields of ethanol ranged from 15.47% to 43.56% for A. fistulosum, while it ranged from 25.31% to 39.05% for A. sativum (data not shown). The results also show that the extract of stem had the highest yields, followed by that of the leaf and root.
3.2. The DPPH Free Radical-Scavenging Activity of Different Allium Extracts
The DPPH free radical-scavenging activities of different Allium extracts are presented in Table 1. The DPPH free radical-scavenging activity of Allium spp. ranged from 67.34% to 90.03% for A. fistulosum, while it ranged from 63.63% to 88.33% for A. sativum. AFL extracts (90.0%) and ASL (88.3%) had the highest DPPH free radicalscavenging activity among the extracts of various parts of the Allium spp. Furthermore, the leaf extracts had higher DPPH free radical-scavenging activity than that of the stem, bulb and root. The corresponding IC50 values of the various Allium spp. required to scavenge 50% of free radicals are also shown in Table 1. Compared to the IC50 values of various Allium spp., the free radical-scavenging potency of the AFL and ASL was 14.61 and 14.89 μg·mL−1, respectively, which were higher than that of (+)-Catechin (2.19 μg·mL−1). In addition, the extracts of leaf had higher DPPH free radical-scavenging activity than that of the other parts of Allium spp.
The DPPH free radical-scavenging activity of A. fistulosum extracts in this study was higher than the results described by Huang et al. [15]. In their study, the green onion extract showed a lower antioxidant capacity, at a
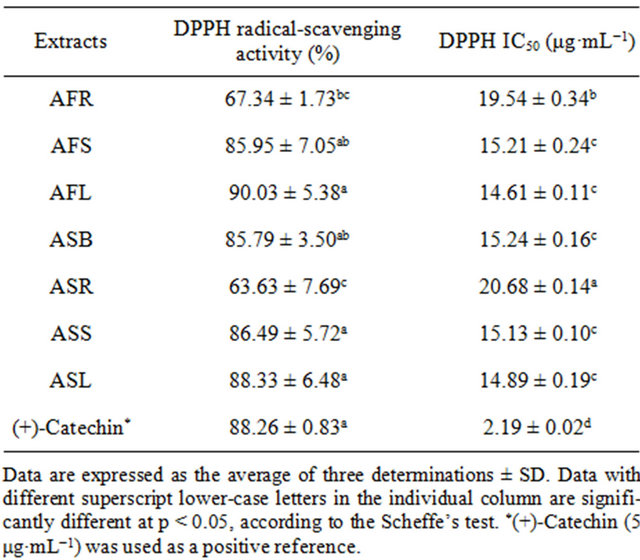
Table 1. Effects of ethanolic extracts on the in vitro DPPH radical-scavenging activity and DPPH free radical IC50 value of various Allium spp.
concentration of 10.0 mg dried vegetable equivalent mL−1, about only 60% of the DPPH free radical-scavenging activity obtained from our study. The discrepancy among the DPPH radical-scavenging activities in different studies may be partially due to different antioxidant potentials of different compounds, the antioxidant activeity of the plant extracts strongly depends on the extraction solvent. For example, the DPPH radical-scavenging activity of Cynara cardunculus EtOAc extracts showed the strongest activity (IC50 = 21.50 μg·mL−1), when compared to those of other extraction solvents [19].
3.3. TEAC Assay of Different Allium Extracts
The antioxidant activity obtained from the TEAC assay is presented in Figure 1. The TEAC values of the various Allium extracts ranged from 6.74 to 14.59 mM for A. fistulosum, while it ranged from 6.79 to 15.51 mM for A. sativum. ASS had the highest antioxidant activity, followed by AFS, ASL, AFL, ASB, while ASR and AFR appeared to have the lowest ability to scavenge the ABTS+. The most interesting result is that the TEAC values of the leaf and stem were significantly higher than those of the root (Figure 1). Another significant finding is that the extracts from the stem had higher antioxidant activities than those taken from other tissues of Allium spp. This finding adheres to the finding of Aoyama and Yamamoto [10], showing that the flavonoid-rich Allium vegetables demonstrated a high antioxidant activity using
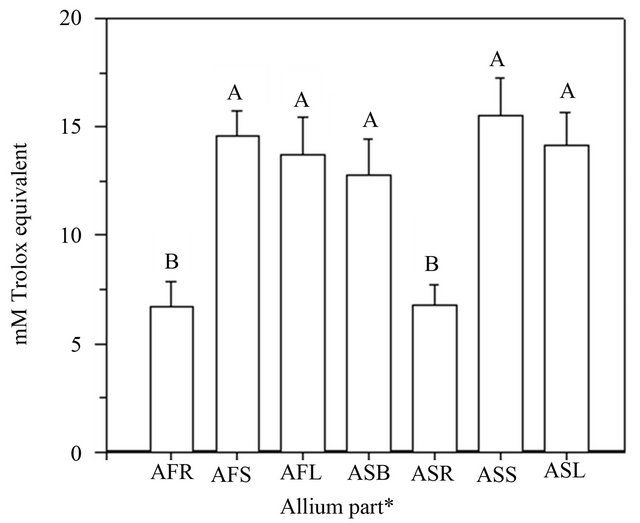
Figure 1. Antioxidant activity of various Allium extracts using a TEAC assay. Data are expressed as the average of three determinations ± SD. Data that do not share the same capital case letter on the top of vertical bar are significantly different at p < 0.05, according to the Scheffe’s test. The symbols AFR, AFS, AFL represent the roots, stems and leaves of Allium fistulosum, respectively; ASB, ASR, ASS, ASL represent the bulbs, roots, stems and leaves of Allium sativum, respectively.
the TEAC assay. The finding of the current study is also consistent with that of Jang et al. [4], who found that the antioxidant activity of the Welsh onion extract, with a concentration of 50 μg·mL−1, was higher than that of other Allium spp. Moreover, Vågen & Slimestad [20] analyzed the antioxidant capacities of Allium spp. using a TEAC assay and also found that TEAC values of the methanol extracts from 15 different onion cultivars were in the range of 0.7 to 5.1 μmol·g−1 fresh sample weight. The discussion above shows that the antioxidant capacity of green onion can differ from cultivar to cultivar. Further, the antioxidant capacity also varies depending on the location of the onion. In general, the onion extracts were proven to act as excellent antioxidants, especially the stem part of the onion.
3.4. Total Phenolic Contents of Different Allium Extracts
The total phenolic content of various Allium extracts is presented in Figure 2. Total phenolic contents from different Allium extracts ranged from 75.27 to 191.04 mg·GAE·g−1 for A. fistulosum, while they ranged from 82.86 to 182.60 mg·GAE·g−1 for A. sativum. Our results showed the total phenolic contents in the various tissue extracts of Allium spp. as: AFS (191.04 ± 8.02 mg·GAE·g−1) > ASS (182.60 ± 1.73 mg·GAE·g−1) > ASB (179.68 ± 8.48 mg·GAE·g−1) > ASL (176.94 ± 7.67 mg·GAE·g−1)
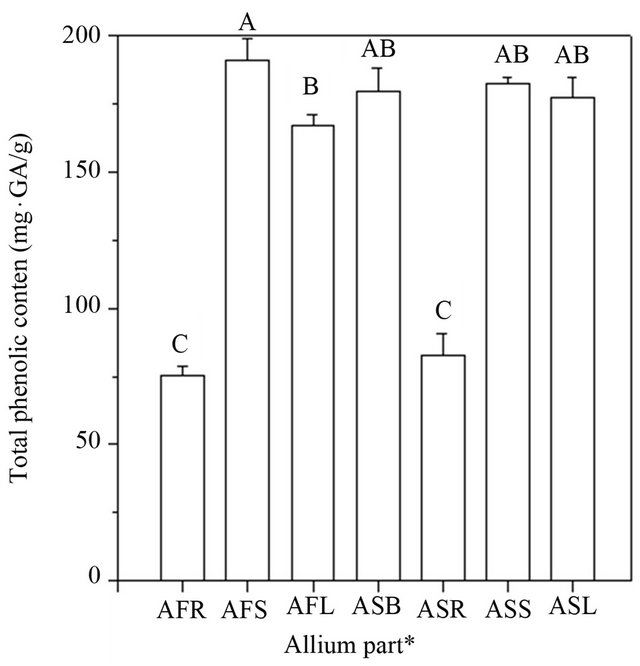
Figure 2. Total phenolic contents of various Allium extracts. Data are expressed as the average of three determinations ± SD. Data that do not share the same capital case letter on the top of vertical bar are significantly different at p < 0.05, according to the Scheffe’s test. The symbols AFR, AFS, AFL represent the roots, stems and leaves of Allium fistulosum, respectively; ASB, ASR, ASS, ASL represent the bulbs, roots, stems and leaves of Allium sativum, respectively.
> AFL (167.16 ± 3.78 mg·GAE·g−1) > ASR (82.86 ± 7.82 mg·GAE·g−1) > AFR (75.27 ± 3.56 mg·GAE·g−1). The highest total phenolic content was observed in the stem extracts and the lowest in the root extracts of Allium spp. with a 30% ethanol extract solution. The results also showed that various tissue extracts of Allium spp. contain significantly high concentrations of phenolic compounds, which could act as the potential antioxidants. In recent studies, it was proven that free radical-scavenging activity and antioxidant activity are greatly influenced by the phenolic composition of the tested sample [13,21]. However, phenolic compounds can act as antioxidants and can also easily be oxidized. In addition, according to the study of Aoyama & Yamamoto [10], which was about the determination of the phenolic compound contents, suggested that the antioxidant activity of the green Welsh onion was higher than that of the yellow onion. These results are in agreement with our observations, which showed that the antioxidant activity of A. fistulosum stem extracts was higher than that of A. sativum extracts.
3.5. Correlation Analysis of the DPPH IC50 Value, Total Phenolic Contents or TEAC with Antioxidant Properties
Correlation analysis was used to determine any possible relation between the DPPH IC50 value, the total phenolic contents, or the TEAC assay of the antioxidant activity of different extracts from various parts of Allium spp. Statistical analysis revealed that the DPPH IC50 value, the total phenolic contents, as well as the TEAC assay significantly correlated in the Allium extracts (Figure 3). As the results show in Figure 3(a), statistical analysis revealed that the total phenolic contents in the Allium extracts and the DPPH IC50 value were inversely correlated; indicating that higher phenolic content leads to stronger antioxidant capacity in the Allium extracts. Correlation between the DPPH IC50 of the extracts obtained from various tissues of Allium spp. and the total phenolic content was significant (r2 = 0.92). The antioxidant activities measured in the TEAC assay also significantly correlate with the DPPH IC50 value (r2 = 0.92), as shown in Figure 3(b). This finding, which indicated that both the DPPH IC50 value and the TEAC assay could be used simultaneously to determine the antioxidant activities of Allium extracts, is supported by the findings of many other similar experiments [9,14,22,23].
In addition, the correlation between the total phenolic content and the Trolox equivalent antioxidant capacity of the extracts from various parts of Allium spp. was showed in Figure 3(c). Correlation between the total phenolic content and the Trolox equivalent antioxidant capacity of Allium spp. extracts was significant (r2 = 0.95). The anti-
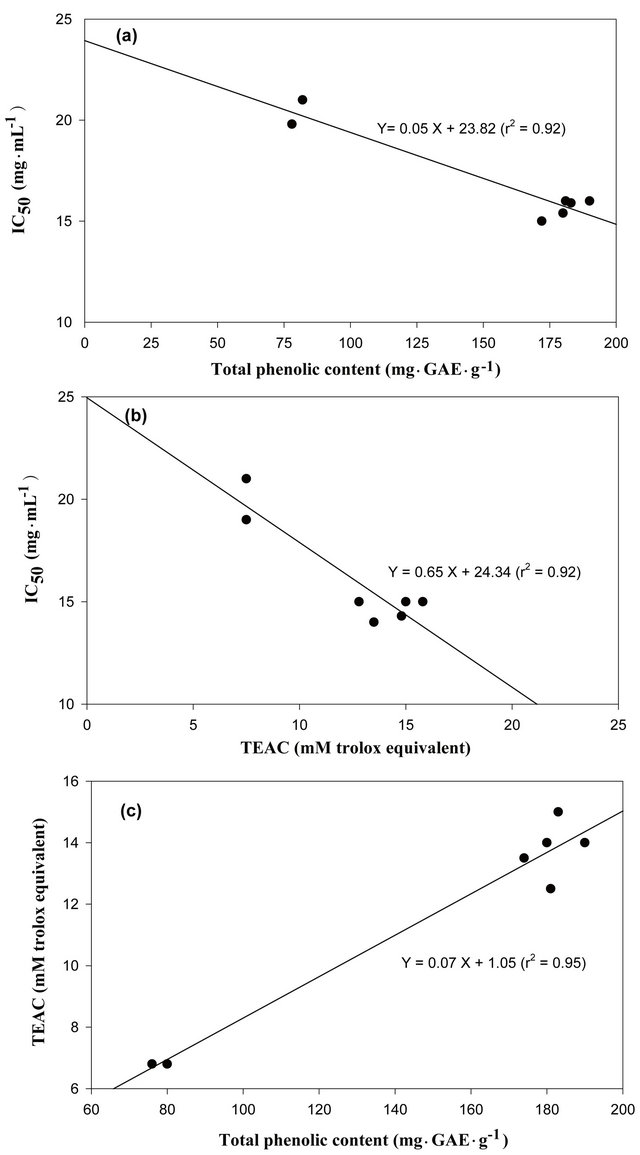
Figure 3. Correlation analysis of the DPPH IC50 value, total phenolic contents or TEAC with antioxidant properties. (a) Linear correlation of total phenolic contents with respect to the DPPH IC50, (b) Linear correlation of the TEAC with respect to the DPPH IC50 and (c) Linear correlation of total phenolic contents with respect to the TEAC, of the tissues from various parts of the Allium spp. Data are expressed as the average of three determinations.
oxidant activity exhibited by the Allium extracts may be due to high levels of phenolic compounds. Therefore, it might be concluded that the total phenolic content could be used as an index for free radical-scavenging and antioxidant capacity in the Allium extracts. These results, which proved that higher phenolic content could elucidate stronger antioxidant capacity, were consistent with those of other studies [13,15,20,23].
3.6. Antimicrobial Activity of Allium Extracts
The antimicrobial activity of Allium extracts was tested in vitro by using the disc diffusion and broth dilution method. According to the results given in Table 2, most Allium extracts exhibited antimicrobial activity against all tested microorganisms. While the results indicated that AFR and ASR extracts displayed no inhibition activity against all tested microorganisms, AFS, AFL, ASB, ASS and ASL obviously inhibited growth of the tested microorganisms. The current study revealed that the extract of ASB was more active against S. aureus, with an MIC and minimal bactericidal concentration (MBC) of 0.2 mg·mL−1 and 0.4 mg·mL−1, respectively (Table 3). The extract of AFS was more active against B. cereus, with an MIC and MBC of 0.2 and 0.4 mg·mL−1, respectively. Among these various extracts, only ASB was more effective in inhibiting the growth of E. coli, with an MIC of 0.4 mg·mL−1 and an MBC of 0.8 mg·mL−1. According to the results of this study, the extracts from various parts of Allium spp. also exhibited strong inhibitory activity against the tested bacteria. However, the Allium extracts showed higher inhibitory responses towards B. cereus, S. aureus and P. aeruginosa, but they were less effective in inhibiting E. coli. The mechanisms as how natural compounds in green onion and garlic exert their function have been previously discussed and the antimicrobial activity of the allicin contained in the garlic and the onion has been proposed in many studies [6, 24-26]. The sulfur-containing compounds, such as allicin contained in green onion and garlic, could be responsible for the antimicrobial potential. The allicin exhibited
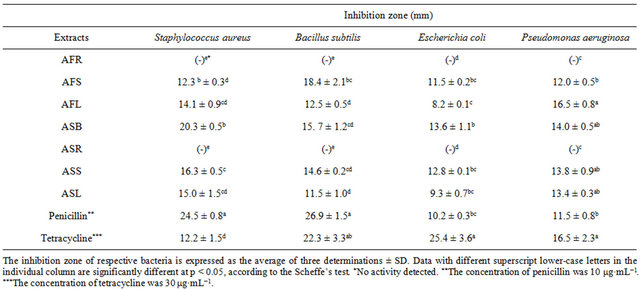
Table 2. Antimicrobial activities of various Allium extracts, penicillin and tetracycline.
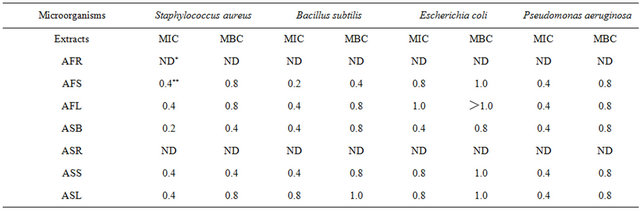
Table 3. The minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of various Allium extracts.
dose-dependent antimicrobial activity against both S. aureus and E. coli, where the concentration of the allicin was from 5 to 20 mM; yet, E. coli was less sensitive to allicin than S. aureus was [6]. However, the antimicrobial mechanism of the Allium extracts has not been clearly elucidated and analyses of the active compounds which inhibit the growth of tested bacteria were under the way in our laboratory.
4. Conclusion
This paper presented the potential use of the antioxidant and antimicrobial properties of the ethanolic extracts of Allium spp. In our studies, it has also been noted that the Allium extracts show high radical-scavenging activity (63% - 90%) and can be considered a good source of natural antioxidants and antimicrobials. It was also found that the DPPH IC50 value, the total phenolic contents, and the TEAC assay were strongly correlated. Evaluation of these three variables and their correlationships could be used as an index for measuring the net effective antioxidant activity in Allium extracts. Furthermore, the results from the Allium extracts could be used in the development of food preservatives and antioxidants obtained from natural sources that are effective against foodborne pathogens.
REFERENCES
- M. L. M. C. Dissanayake, R. Kashima, S. Tanaka and S. Ito, “Pathogenic Variation and Molecular Characterization of Fusarium Species Isolated from Wilted Welsh Onion in Japan,” Journal of General Plant Pathology, Vol. 75, No. 1, 2009, pp. 37-45.
- H. Indon and T. Asahira, “Japanese Bunching Onion (Allium fistulosum L.),” In: H. D. Rabinowitch and J. L. Brewster, Eds., Onions and Allied Crops, Vol. 3, CRC Press, Boca Raton, 1990, pp. 159-178.
- T. Yakuwa, “Welsh Onion or Japanese Bunching Onion,” In: The Japanese Society for Horticultural Science Horticulture in Japan, Shokabo Publications, Tokyo, 2006, pp. 165-166.
- H. W. Jang, M. H. Ka and K. G. Lee, “Antioxidant Activity and Characterization of Volatile Extracts of Capsicum annuum L. and Allium spp.,” Flavour and Fragrance Journal, Vol. 23, No. 3, 2008, pp. 178-184. doi:10.1002/ffj.1872
- R. S. Feldberg, S. C. Chang, A. N. Kotik, M. Nadler, Z. I. Neuwirth, D. C. Sundstrom and N. H. Thompson, “In Vitro Mechanism of Inhibition of Bacterial Cell Growth by Allicin,” Antimicrobial Agents and Chemotherapy, Vol. 32, No. 12, 1988, pp. 1763-1768. doi:10.1128/AAC.32.12.1763
- H. Fujisawa, K. Suma, K. Origuchi, H. Kumagai, T. Seki and T. Ariga, “Biological and Chemical Stability of Garlic-derived Allicin,” Journal of Agricultural and Food Chemistry, Vol. 56, No. 11, 2008, pp. 4229-4235. doi:10.1021/jf8000907
- A. N. Makheja, J. Y. Vanderhoek and J. M. Bailey, “Inhibition of Platelet Aggregation and Thromboxane Synthesis by Onion and Garlic,” Lancet, Vol. 1, No. 8119, 1979, p. 781.
- J. A. Milner, “Garlic: Its Anticarcinogenic and Antitumorigenic Properties,” Nutrition Reviews, Vol. 54, No. 11, 1996, pp. S82-S86. doi:10.1111/j.1753-4887.1996.tb03823.x
- O. Boussaada, J. Chriaa, R. Nabli, S. Ammar, D. Saidana, M. A. Mahjoub, I. Chraeif, A. N. Helal and Z. Mighri, “Antimicrobial and Antioxidant Activities of Methanol Extracts of Evax pygmaea (Asteraceae) Growing Wild in Tunisia,” World Journal of Microbiology and Biotechnolology, Vol. 24, No. 8, 2008, pp. 1289-1296.
- S. Aoyama and Y. Yamamoto, “Antioxidant Activity and Flavonoid Content of Onion (Allium fistulosum) and the Effect of Thermal Treatment,” Food Science and Technology Research, Vol. 13, No. 1, 2007, pp. 67-72. doi:10.3136/fstr.13.67
- J. Duate, M. Galisteo, M. A. Ocete, F. Pérez-Vizcaino, F. Zarzuelo and J. Tamargo, “Effects of Chronic Quercetin Treatment on Hepatic Oxidative Status of Spontaneously Hypertensive Rats,” Molecular and Cellular Biochemistry, Vol. 221, No. 1-2, 2001, pp. 155-160.
- C. C. Wu, L. Y. Sheen, W. H. Chen, S. J. Tsai and C. K. Lii, “Effect of Organosulfur Compounds from Garlic Oil on the Antioxidation System in Rat Liver and Red Blood Cells,” Food and Chemical Toxicology, Vol. 39, No. 6, 2001, pp. 563-569. doi:10.1016/S0278-6915(00)00171-X
- H. D. Jang, K. S. Chang, T. C. Chang and C. L. Hsu, “Antioxidant Potentials of Buntan Pumelo (Citrus grandis Osbeck) and Its Ethanolic and Acetified Fermentation Products,” Food Chemistry, Vol. 118, No. 3, 2010, pp. 554-558. doi:10.1016/j.foodchem.2009.05.020
- P. J. Jara and S. C. Fulgencio, “Antioxidant Capacity of Dietary Polyphenols Determined by ABTS Assay: A Kinetic Expression of the Results,” International Journal of Food Sciences and Technology, Vol. 43, No. 1, 2008, pp. 185-191. doi:10.1111/j.1365-2621.2006.01425.x
- Z. Huang, B. Wang, D. H. Eaves, J. M. Shikany and R. D. Pace, “Total Phenolics and Antioxidant Capacity of Indigenous Vegetables in the Southeast United States: Alabama Collaboration for Cardiovascular Equality Project,” International Journal of Food Sciences and Nutrition, Vol. 60, No. 2, 2009, pp. 100-108. doi:10.1080/09637480701605715
- M. A. Gyamfi, M. Yonamine and Y. Aniya, “Free-Radical Scavenging Action of Medicinal Herbs from Ghana Thonningia sanguinea on Experimentally-Induced Liver Injuries,” General Pharmacology, Vol. 32, No. 6, 1999, pp. 661-667. doi:10.1016/S0306-3623(98)00238-9
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. RiceEvans, “Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay,” Free Radical Biology and Medicine, Vol. 26, No. 9-10, 1999, pp. 1231-1237. doi:10.1016/S0891-5849(98)00315-3
- T. S. Kujala, J. M. Loponen, K. D. Klika and K. Pihlaja, “Phenolics and Betacyanins in Red Beetroot (Beta vulgaris) Root: Distribution and Effect of Cold Storage on the Content of Total Phenolics and Three Individual Compounds,” Journal of Agricultural and Food Chemistry, Vol. 48, No. 11, 2000, pp. 5338-5342. doi:10.1021/jf000523q
- J. Kukić, V. Popović, S. Petrović, P. Mucaji, A. Ćirić, D. Stojkovic and M. Soković, “Antioxidant and Antimicrobial Activity of Cynara cardunculus Extracts,” Food Chemistry, Vol. 107, No. 2, 2008, pp. 861-868. doi:10.1016/j.foodchem.2007.09.005
- I. M. Vågen and R. Slimestad, “Amount of Characteristic Compounds in 15 Cultivars of Onion (Allium cepa L.) in Controlled Field Trials,” Journal of the Science of Food and Agriculture, Vol. 88, No. 3, 2008, pp. 404-411. doi:10.1002/jsfa.3100
- Y. T. Tung, J. H. Wu, Y. H. Kuo and S. T. Chang, “Antioxidant Activities of Natural Phenolic Compounds from Acacia confuse Bark,” Bioresource Technology, Vol. 98, No. 5, 2007, pp. 1120-1123. doi:10.1016/j.biortech.2006.04.017
- P. Marimuthu, C. L. Wu, H. T. Chang and S. T. Chang, “Antioxidant Activity of the Ethanolic Extract from the Bark of Chamaecyparis obtusa var. formosana,” Journal of Agricultural and Food Chemistry, Vol. 88, No. 8, 2008, pp. 1400-1405. doi:10.1002/jsfa.3231
- T. H. Tsai, T. H. Tsai, Y. C. Chien, C. W. Lee and P. J. Tsai, “In Vitro Antimicrobial Activities Against Carcinogenic Streptococci and Their Antioxidant Capacities: A Comparative Study of Green Tea Versus Different Herbs,” Food Chemistry, Vol. 110, No. 4, 2008, pp. 859-864. doi:10.1016/j.foodchem.2008.02.085
- E. C. Delaha and V. F. Garagusi, “Inhibition of Mycobacterial by Garlic Extract (Allium sativum),” Antimicrobial Agents and Chemotherapy, Vol. 27, No. 4, 1985, pp. 485- 486. doi:10.1128/AAC.27.4.485
- C. M. Marta, C. Nieves and V. Mar, “Biological Properties of Onions and Garlic,” Trends in Food Science and Technology, Vol. 18, No. 12, 2007, pp. 609-625. doi:10.1016/j.tifs.2007.07.011
- P. Wilson and R. R. Cutler, “Antibacterial Activity of a New, Stable, Aqueous Extract of Allicin against Methicillin-Resistant Staphylococcus aureus,” British Journal of Biomedical Science, Vol. 61, No. 2, 2004, pp. 71-74.
NOTES
*Corresponding author.

