American Journal of Plant Sciences
Vol.07 No.03(2016), Article ID:65098,8 pages
10.4236/ajps.2016.73057
Enzymatic Characterisation of Lignin Peroxidase from Luffa aegyptiaca Fruit Juice
Nivedita Rai, Meera Yadav*, Hardeo Singh Yadav
Department of Chemistry, NERIST, Itanagar, India

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 11 February 2016; accepted 25 March 2016; published 29 March 2016
ABSTRACT
Luffa aegyptiaca fruit has been assayed for the presence of lignin peroxidase activity using veratryl alcohol as the substrate. The fruit juice contained activity of 3.14 U/ml which was much higher than 0.075 U/ml reported in the culture filtrate of Phanarochaete chrysosporium ATCC-24725. The Km value of the lignin peroxidase using veratryl alcohol as the variable substrate in 50mM phosphate buffer pH 2.5 at 25˚C was found to be 50 μM respectively. The pH and temperature optima of the lignin peroxidase were 2.4 and 22˚C, respectively. The present article reports viable method to explore rich sources of lignin peroxidase from plants which can be used as a mediator in oxidative organic transformations within green chemistry domain ensuring ecofriendly synthesis of bioorganic molecules of pharmaceutical value.
Keywords:
Lignin Peroxidase, Metalloenzyme, Heme, Biomass, Bioconversion, Luffa aegyptiaca

1. Introduction
Rising energy consumption, depletion of fossil fuels and increased environmental concern have shifted the focus of energy generation towards biofuel use. The development of alternative energy technology is important because of the rising prices of crude oil and environmental issues such as global warming and air pollution. Bioconversion of biomass has significant advantages over other alternative energy strategies because biomass is the most abundant and also the most renewable biomaterial on our planet. They are renewable alternative to depleting oil reserves which are basis of our present chemical industries. Lignocellulose biomass has a great potential as feed stock for production of value added products such as low price chemicals, eg xylose, glucose, furfural, fuels, biofibres, ruminant feed, biopulp or even for enzyme production [1] . However, these carbohydrates are generally infiltrated by lignin. Lignin is a complex, large molecular structure containing cross-linked polymer of phenolic monomers. It is a natural polymer consisting of arylpropyl unit linked primarily with the β-O-4 bond but also with various other C-C and C-O linkages as shown in Figure 1.
Lignocellulose is resistant to microbial attack and chemical conversion to feed stock for chemical industries requires dratic reaction conditions which are expensive in energy and pollute the environment. Breakdown of the lignin barrier will alter lignocelluloses structures and make the carbohydrates accessible for more efficient bioconversion. White rot fungi produce ligninolytic enzymes [3] which efficiently delignify lignin and make it biotransformable by microbes and enzymes [4] . Moreover, ligninolytic enzymes have potential applications in a large number of fields, including the chemical, fuel, food, agricultural, paper, textile, cosmetic industries [5] . The ligninolytic system of white rot fungi [6] is also involved in the degradation of various xenobiotic compounds and dyes. Their capacities to remove xenobiotic substances and produce polymeric products make them a useful tool for bioremediation purposes [7] . Keeping these points in view we have initiated research work on lignin peroxidase enzyme from Luffa aegyptiaca fruit juice which has not been reported so far.
Lignin peroxidases (LiP, EC 1.11.1.7], which in presence of H2O2, degrades lignin and lignin model compounds [8] . The sequence of catalysis is given below:
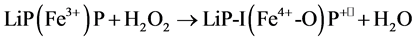 (1)
(1)
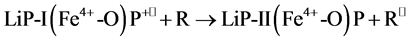 (2)
(2)
 (3)
(3)
Figure 1. Structure of lignin [2] .
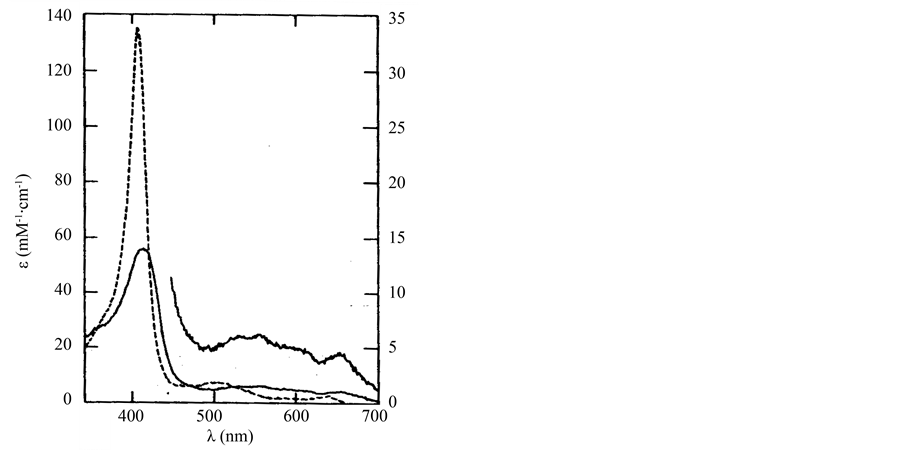
where R is the organic substrate and P is porphyrin. LiP compound I (LiP-I) carries both oxidizing equivalents of H2O2, one as an oxyferryl (Fe4+-O) center and one as a porphyrin π cation radical (P+•), whereas LiP compound II (LiP-II) carries only one oxidizing equivalent. The substrate R is oxidized by compound I to an aryl cation radical which with subsequent nonenzymatic reactions yields the final products. Renganathan and Gold [8] characterized the H2O2 oxidized forms of lignin peroxidase compounds I, II and III using electronic absorption spectroscopy. The native enzyme contains high-spin ferric iron and has its Soret maximum at 407.6 nm and α and β bands at 496 and 630 nm (Figure 2)
It also has indicated that the dehydrogenation of veratryl alcohol by lignin peroxidase proceeds via two single-electron oxidation steps rather than through a single two electron oxidation mechanism. The different enzyme intermediates could be depicted by Figure 3.
Figure 2. Electronic absorption spectrum of the native enzyme (---) and of the compound I ( __ ) [6] .
Figure 3. Interrelationship between the five redox states of lignin peroxidase [5] . Reaction paths 3→5→4 indicate one catalytic cycle of the enzyme. AH2 = substrate.
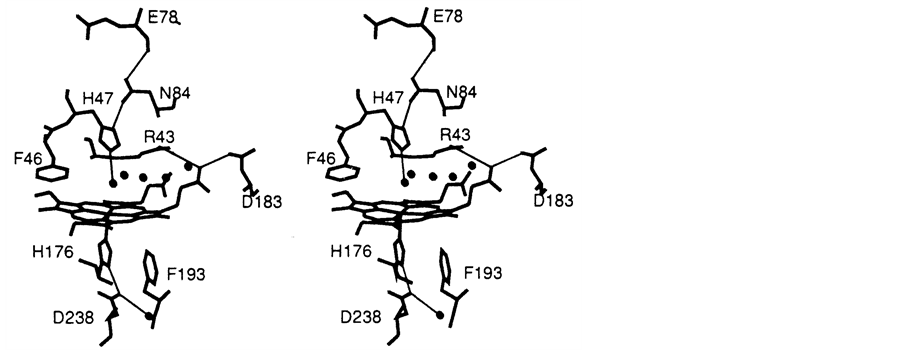
The active sites as shown in Figure 4 are similar because both contain a proximal histidine haem ligand H176 hydrogen bonded to a buried aspartate residue D238 and both contain histidine H47 and arginine R43 residues in the distal peroxide binding pocket. The most obvious difference in the active site is that whereas cytochrome c peroxidase has tryptophan residues located in the proximal and distal haem pockets, lignin peroxidase has phenylalanines. There are four other differences in the two structures. The haem pocket in cytochrome c peroxidase is open to solvent whereas in lignin peroxidase this opening is smaller. Lignin peroxidase has a carboxylate- carboxylate hydrogen bond important for haem binding that is not present in cytochrome c peroxidase. Lignin peroxidase contains 2 structural calcium ions while cytochrome c peroxidase does not contain any calcium ion. The extra 49 residues in lignin peroxidase which is not present in cytochrome c peroxidase constitute the C terminal end of the molecule situated between the two haem propionates.
Structural and functional aspects of lignin peroxidase have been studied [10] . LiP is a biotechnologicaly important enzyme having wide potential applications (1) in delignification of lignocellulosic materials [11] which are seen as an alternative to the depleting oil reserves, (2) in the conversion of coal to low molecular mass fractions [12] which could be used as a feed stock for the production of commodity chemicals, (3) in biopulping and biobleaching [13] in paper industries, (4) in removal of recalcitrant organic pollutants [14] - [18] and (5) in the enzymatic polymerization [19] [20] in polymer industries. LiP of Phanerocheate chyrosoporium has been extensively studied [21] - [23] .
The kinetics of lignin peroxidase catalysis has been studied by both steady state and transient state techniques [24] . The steady state studies of veratryl alcohol oxidation indicate that the mechanism of catalysis is Ping-Pong [24] . Veratryl alcohol in the presence of lignin peroxidase and H2O2 is oxidized to veratrylaldehyde as shown in Scheme 1.
2. Materials and Methods
2.1. Chemicals
Veratryl alcohol, which is 3,4-dimethoxy benzyl alcohol was from Aldrich (Wisconsin, USA). All other chemicals were from s.d. fine chem limited (Mumbai) and were used without further purification.
Figure 4. Stereo view of the active site environment of LiP [9] .
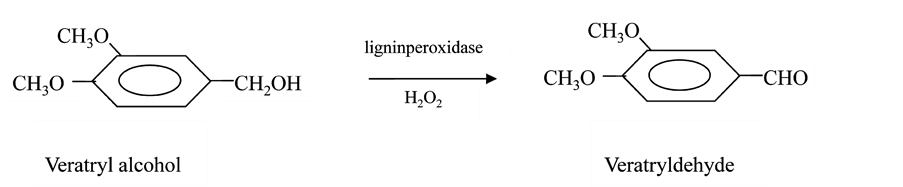
Scheme 1. Oxidation of veratryl alcohol to veratryldehyde by lignin peroxidase in presence of H2O2.
2.2. Enzyme Assay
The lignin peroxidase activity [21] has been assayed by monitoring the formation of veratraldehyde spectrophotometrically at lmax = 310 nm using veratryl alcohol as a substrate with UV/VIS spectrophotometer U-2600 SHIMADZU. Molar extinction coefficient value 9300 M−1∙cm−1 for veratraldehyde was used to calculate the enzyme unit. One unit of lignin peroxidase was defined as the amount of enzyme, which converts one m mole of veratryl alcohol to veratraldehyde under the standard assay condition. The least count of absorbance measurement was 0.001 absorbance unit.
2.3. Enzymatic Characteristics
The enzymatic characteristics of lignin peroxidases like Km, pH and temperature optima were determined using veratryl alcohol and H2O2 as the substrates. The Km values were determined by measuring steady state velocities of lignin peroxidases catalysed reactions at different substrate concentrations and drawing double reciprocal plots [25] , The reaction solution 1mL consisted of 250 μM H2O2 in 100mM phosphate buffer pH 2.5 at 25˚C, veratryl alcohol was varied in the range 0.01 mM to 0.3 mM and 5 μL of the crude enzyme was added. The Km value was calculated by the linear regression analysis of the data points of double reciprocal plots. The pH optimum was determined by measuring the steady state velocities of lignin peroxidase catalysed reaction in the reaction solutions having different pH values and plotting steady state velocity against the pH values. Similarly, the temperature optimum were determined by measuring the steady state velocities in reaction solutions at different temperatures and plotting the steady state velocities against temperatures of the reaction solutions.
3. Results and Discussion
The results of the steady state kinetic studies of the enzyme using veratryl alcohol as the variable substrate are shown in Figure 5. The calculated Km value is 50 μM respectively for veratryl alcohol. The enzymatic characteristics of lignin peroxidase of Phanarochaete chrysosporium ATCC-24725 have widely been studied [21] . The
Figure 5. Michelis-menten and double reciprocal plots for veratryl alcohol as the variable substrate.The reaction 1 mL solution contained 250 μM concen- tration of H2O2, 2 mM of veratryl alcohol in 100 mM phosphate buffer pH 2.5 at 25˚C and the reaction was initiated by addition of 5 μL of the crude enzyme.
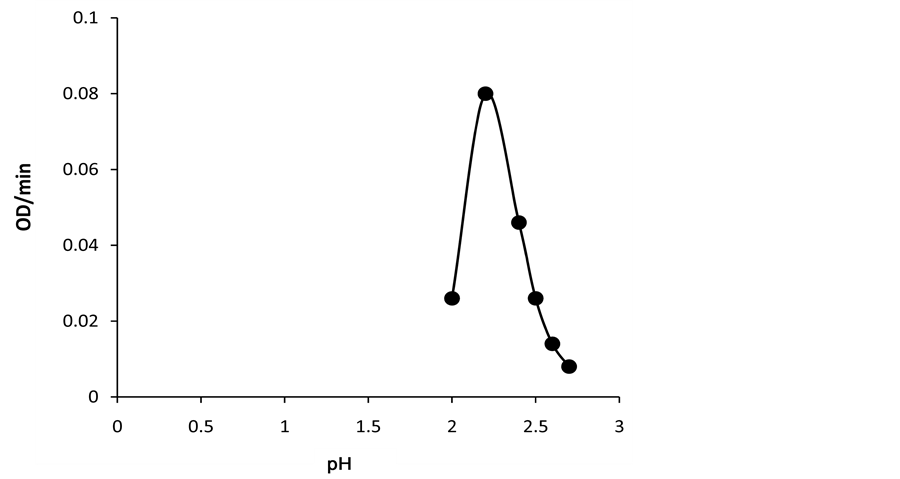
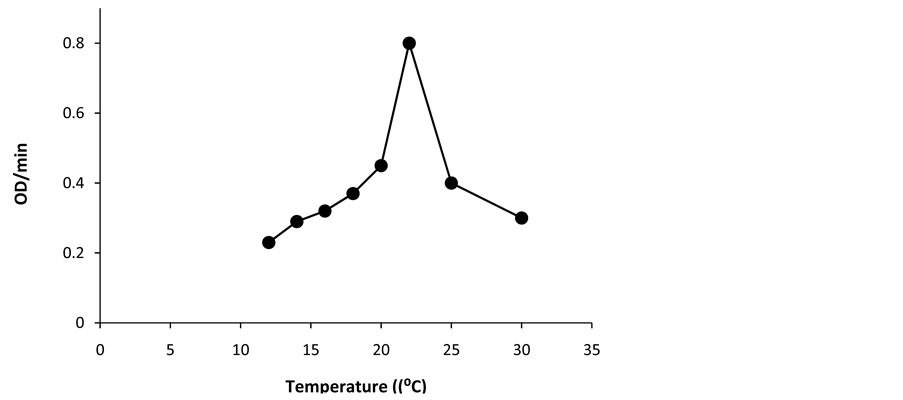
Km value using the above substrate for the lignin peroxidase of Phanarochaete chrysosporium have been reported to be 60 μM [21] respectively indicating that Km value of lignin peroxidase of Luffa aegyptiaca is less compared to lignin peroxidase of Phanarochaete chrysosporium. Specificity of enzyme from Luffa aegyptiaca is more for substrate compared to LiP from Phanarochaete chrysosporium. The activity-pH and activity-tempera- ture profiles of lignin peroxidase of Luffa aegyptiaca are shown in Figure 6 and Figure 7 respectively. It is obvious from Figure 6 and Figure 7 that the pH and temperature optima of this enzyme are 2.4˚C and 22˚C respectively. These values of pH and temperature optima are in the same order as reported [21] for lignin peroxidase of Phanarochaete chrysosporium, the values being 2.5˚C and 26˚C respectively.
Figure 6. Activity-pH profile diagram. The reaction solution contained 5 μL of the crude enzyme in 100 mM phosphate buffer pH 2.5 at 25˚C at the fixed 250 μM concentration of H2O2 and 2 mM of veratryl alcohol. pH of the buffer was varied at the fixed temperature of 25˚C.
Figure 7. Activity-temperature profile diagram. The reaction solution contained 5 μL of the crude enzyme in 100 mM phosphate buffer pH 2.5 at 25˚C at the fixed 250 μM concentration of H2O2 and 2 mM of veratryl alcohol. The tem- perature of the reaction solution was varied at fixed pH of 2.5.
4. Conclusion
Luffa aegyptiaca is a better source of lignin peroxidase (3.14 U/ml) as compared to Phanarochaete chrysosporium ATCC-24725, the culture filtrate of which has only 0.075 U/ml and can be used for commercial production of lignin peroxidase. The Km value using veratryl alcohol as the substrate has been found to be 50 μM which less than Km value reported for Phanarochaete chrysosporium. LiP from Luffa aegyptiaca has more substrate affinity as compared to LiP from Phanarochaete chrysosporium. The pH and temperature optima for the activity of lignin peroxidase of Luffa aegyptiaca have been found to be 2.4˚C and 22˚C respectively. Thus knowing enzymatic characteristics properties of LiP from Luffa aegyptiaca, it can be used for different biotechnological applications as mentioned above in the introduction section.
Acknowledgements
The financial support of DST, Government of India, New Delhi through its Project no. SR/WOS-A/CS-138/ 2012 and the Department of Chemistry for providing facilities to do this work are thankfully acknowledged.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article and regarding the funding that they have received.
Cite this paper
Nivedita Rai,Meera Yadav,Hardeo Singh Yadav, (2016) Enzymatic Characterisation of Lignin Peroxidase from Luffa aegyptiaca Fruit Juice. American Journal of Plant Sciences,07,649-656. doi: 10.4236/ajps.2016.73057
References
- 1. Alan, F. (1998) Structure and Mechanism in Protein Science. W. H. Freeman and Company, New York.
- 2. Tein, M., Kirk, T.K., Bull, C. and Fee, J.A. (1986) Steady-State and Transient-State Kinetic Studies on the Oxidation of 3,4-Dimethoxybenzyl Alcohol Catalyzed by the Ligninase of Phanerocheate chrysosporium Burds. The Journal of Biological Chemistry, 261, 1687-1693.
- 3. Kang, S., Shin, K.S., Han, Y.H., Youn, H.D. and Hah, Y.C. (1993) Purification and Characterization of an Extracellular Peroxidase from the Culture Filtrate of Pleurotus ostreatus. Bio-chimica et Biophysica Acta, 1163, 158-164.
http://dx.doi.org/10.1016/0167-4838(93)90177-S - 4. Vyas, B.R.M., Vole, J. and Saaek, V. (1994) Ligninolytic Enzymes of Selected White Rot Fungi Cultivated on Wheat Straw. Folia Microbiologica, 39, 235-240.
http://dx.doi.org/10.1007/BF02814655 - 5. Tien, M. and Kirk, T.K. (1998) Legnin Peroxidase of Phanerochaete chrysosporium. Methods in Enzymology, 161, 238-249.
http://dx.doi.org/10.1016/0076-6879(88)61025-1 - 6. Salvachúa, D., Prieto, A., Mattinen, M.L., Tamminen, T., Liitiä, T., Lille, M., Willför, S., Martínez, A.T., Martínez, M.J. and Faulds, C.B. (2013) Versatile Peroxidase as a Valuable Tool for Generating New Biomolecules by Homogeneous and Heterogeneous Cross-Linking. Enzyme and Microbial Technology, 52, 303-311.
http://dx.doi.org/10.1016/j.enzmictec.2013.03.010 - 7. Hiroshi, U. and Shiro, K. (1999) Enzymatic Polymerization Yields Useful Polyphenols. Chemtech, 29, 22-28.
- 8. Cenek, N., Katerina, S., Pavla, E., Tomas, C., Aparna, K., Elke, L. and Vaclav, S. (2004) Ligninolytic Fungi in Bioremediation: Extracellular Enzyme Production and Degradation Rate. Soil Biology and Biochemistry, 36, 1545-1551.
http://dx.doi.org/10.1016/j.soilbio.2004.07.019 - 9. Levin, L., Papinutti, L. and Forchiassin, F. (2004) Evaluation of Argentinean White Rot Fungi for Their Ability to Produce Lignin-Modifying Enzymes and Decolorize Industrial Dyes. Bioresource Technology, 2, 169-176.
http://dx.doi.org/10.1016/j.biortech.2003.12.002 - 10. Satwinder, S.M., Rajesh, G., Chand, P. and John, F.K. (1998) Continuous Biobleaching of Black Liquor from the Pulp and Paper Industry Using an Immobilized Cell System. Journal of Chemical Technology and Biotechnology, 73, 292-296.
http://dx.doi.org/10.1002/(SICI)1097-4660(1998110)73:3<292::AID-JCTB950>3.0.CO;2-C - 11. Kwant, S.S. and Chang, J.K. (1998) Decolorisation of Artificial Dyes by Peroxidase from the White Rot Fungus Pleurotus ostreatus. Biotechnology Letters, 20, 569-572.
http://dx.doi.org/10.1023/A:1005301812253 - 12. Bumpus, J.A., Tien, M., Wright, D. and Aust, S.D. (1985) Oxidation of Persistent Environmental Pollutants by a White Rot Fungus. Science, 228, 1434-1436.
http://dx.doi.org/10.1126/science.3925550 - 13. Eriksson, K.E. and Kirk, T.K. (1994) Biopulping: An Overview of Developments in an Environmentally Safe Paper Making Technology. FEMS Microbiology Reviews, 13, 351-364.
http://dx.doi.org/10.1111/j.1574-6976.1994.tb00054.x - 14. Catcheside, D.E.A. and Ralph, J.P. (1999) Biological Processing of Coal. Applied Microbiology and Biotechnology, 53, 16-24.
http://dx.doi.org/10.1007/s002530051482 - 15. Harley, B.S., Brodo, P.M.A. and Senior, P.J. (1988) Proceedings of Royal Society Discussion Meeting on Utilisation of Lignocellulosic Wastes. Cambridge University Press, Cambridge.
- 16. Yadav, M. and Yadav, K.D.S. (2007) Structural and Functional Aspects of Lignolytic Enzymes. In: Kuhad R.C. and Singh, A. Eds., Lignocellulose Biotechnology: Future Prospects, I. K. International Pvt. Ltd., New Delhi, 63-88.
- 17. Poulos, T.L., Edwards, S.L., Wariishi, H. and Gold, M.H. (1993) Crystallographic Refinement of Lignin Peroxidase at 2 A. The Journal of Biological Chemistry, 268, 4429-4440.
- 18. Renganathan, V. and Gold, M.H. (1986) Spectral Characterization of the Oxidized States of Lignin Peroxidase, an Extracellular Heme Enzyme from the White Rot Basidiomycete Phanerochaete chrysosporium. Biochemistry, 25, 1626-1631.
http://dx.doi.org/10.1021/bi00355a027 - 19. Marcia, J.M.M., Ademir, C.E.S. and Helena, C.T.R. (2010) Industrial and Biotechnological Applications of Ligninolytic Enzymes of the Basidiomycota: A Review. Electronic Journal of Biotechnology, 13, 1-12.
http://dx.doi.org/10.2225/vol13-issue6-fulltext-2 - 20. Fernández-Fueyo, E., Ruiz-Duenas, F.J. and Martinez, A.T. (2014) Engineering a Fungal Peroxidase that Degrades Lignin at Very Acidic pH. Biotechnology for Biofuels, 7, 114.
http://dx.doi.org/10.1186/1754-6834-7-114 - 21. Yadav, M. and Yadav, H.S. (2015) Applications of ligninolytic Enzymes to Pollutants, Wastewater, Dyes, Soil, Coal, Paper and Polymers. Environmental Chemistry Letters, 13, 309-318.
http://dx.doi.org/10.1007/s10311-015-0516-4 - 22. Hatakka, A. (1994) Lignin-Modifying Enzymes from Selected White-Rot Fungi: Production and Role in Lignin Degradation. FEMS Microbiology Reviews, 13, 125-135.
http://dx.doi.org/10.1111/j.1574-6976.1994.tb00039.x - 23. Pollegioni, L., Tonin, F. and Rosini, E. (2015) Lignin-Degrading Enzymes. FEBS Journal, 282, 1190-1213.
http://dx.doi.org/10.1111/febs.13224 - 24. Adler, E.J. (1977) Lignin Chemistry—Past, Present and Future. Wood Science and Technology, 11, 169-218.
http://dx.doi.org/10.1007/BF00365615 - 25. Isroi, M.R., Syamsiah, S., Niklasson, C., Cahyanto, M.N., Lundquist, K. and Taherzadeh, M.J. (2011) Biological Pretreatment of Lignocelluloses with White-Rot Fungi and Its Applications: A Review. BioResources, 6, 5224-5259.
NOTES
*Corresponding author.