American Journal of Plant Sciences
Vol.4 No.5(2013), Article ID:32163,7 pages DOI:10.4236/ajps.2013.45139
Maternal Environment Effects on Phenolic Defenses in Abutilon theophrasti Seeds
![]()
1Department of Entomology, Plant Pathology & Weed Science, New Mexico State University, Las Cruces, USA; 2Department of Crop Sciences, University of Illinois, Urbana, USA; 3Department of Agronomy and Horticulture, University of Nebraska, Lincoln, USA; 4United States Department of Agriculture-Agricultural Research Service (USDA-ARS), Global Change and Photosynthesis Research Unit, Urbana, USA.
Email: bschutte@nmsu.edu
Copyright © 2013 Brian J. Schutte et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Seed Defense, Seed Protein
Received February 25th, 2013; revised April 1st, 2013; accepted May 2nd, 2013
Keywords: Environmental Maternal Effect; Growth-Differentiation Balance Hypothesis; ortho-Dihydroxyphenols;
ABSTRACT
A class of phenolic compounds, ortho-dihydroxyphenols (hereafter “o-DHP”), has been implicated with seed survival. Based on expectations of the growth-differentiation balance hypothesis, we predicted that seed o-DHP concentration exhibits a curvilinear response to increasing resource availability in the maternal environment, with maximum o-DHP occurring at moderate resource levels. To test this hypothesis, Abutilon theophrasti seeds were produced under field conditions at two locations. Each location included twelve maternal environments established through factorial combinations of soil compost (+/−), species assemblage (A. theophrasti with and without maize), and soil nitrogen fertilizer (0, 0.5× or 1× local recommendations for maize). Resource availability with respect to A. theophrasti growth was summarized by above-ground biomass at seed harvest (maternal biomass). Results indicated that seed o-DHP concentrations increased then decreased in response to increasing maternal biomass. This relationship was modeled with a unimodal function specific to location (Location 1, y = 1.18 + 0.03xe−0.02x, pseudo-R2 = 0.59, p = 0.003; Location 2, y = 1.40 + 0.006xe−0.005x; pseudo-R2 = 0.34, p = 0.05). Seed protein concentrations remained constant across maternal biomass levels. Because inherent vulnerability to predation and decay is considered a consequence of chemical protection relative to nutritional offering, our results suggest that A. theophrasti seed susceptibility to lethal attack is influenced by resource levels in the maternal environment. More broadly, our results suggest that the growthdifferentiation balance hypothesis can be extended to maternal effects on seed phenolics.
1. Introduction
Seed survival under field conditions involves escape from predation and decay through chemicals with antiherbivory, antifungal, and bactericidal properties [1]. Maternal environments may have considerable influence on seed chemical defenses because these compounds are primarily located in structures derived from maternal tissue (i.e., testa, pericarp) [2]. In vegetative structures, concentrations of phenolic defense compounds can exhibit curvilinear responses to environmental resource levels, with maximum phenolic concentrations occurring at moderate resource levels [3-5]. Such associations are thought to result from inherent constraints on plant metabolic pathways such that: 1) in low resource environments, both growth and secondary metabolite production are inhibited because of limited amounts of photosynthate; 2) in moderate resource environments, secondary metabolite production increases but growth remains modest; and 3) in resource rich environments, growth processes become more efficient, resulting in decreased concentrations of secondary metabolites. Alleged tradeoffs between growth and secondary metabolite production are components of the growthdifferentiation balance hypothesis (first proposed by [6] and elaborated by [7]). Predictions of the growth-differentiation hypothesis have yet to be extended to maternal environment effects on seed traits.
Although specific chemical defenses vary among taxa, a broad class of phenolic compounds that occurs widely among plant species, ortho-dihydroxyphenols (hereafter “o-DHP”), has been implicated with seed survival [8]. Mechanisms of o-DHP protection have yet to be clarified, but, based on plant defense chemistry [9], o-DHP may improve seed longevity by inhibiting pathogenic microorganisms and deterring vertebrate and invertebrate predators. Such claims are supported by a report of low rates of seed predation for species characterized by high concentrations of o-DHP in seeds [10].
The overall objective of this study was to determine the effects of increasing maternal environment resource availability on o-DHP concentrations in Abutilon theophrasti Medik. seeds produced under agronomic field conditions. Abutilon theophrasti is a common, summer annual weed of economic importance in agricultural fields of North America [11]. This species was chosen because previous research indicated that many seed traits, including susceptibility to mortality, are affected by the maternal environment [12-14]. We hypothesized that the response of seed o-DHP concentration to increasing maternal environment resource availability can be modeled with a unimodal function, i.e., a function that projects maximum o-DHP concentration at moderate resource levels. We also assessed the association between maternal environment resource levels and concentrations of seed protein, which is a sizeable constituent of A. theophrasti seeds [15] and a high-quality protein for vertebrate seed predators [16]. Specific predictions regarding the effects of maternal environment resource availability on seed protein concentrations were not made, rather, protein measurements were used to calculate o-DHP: protein ratios. Such ratios are valuable because inherent vulnerability to seed predation and decay is considered a consequence of chemical protection relative to nutritional offering [17].
2.1.2. Materials and Methods
Maternal Environments
Resource gradients with respect to A. theophrasti growth were established under field conditions for maize (Zea mays L.) production at two locations: Mead, NE (latitude 41.229˚, longitude 96.489˚, elevation 352 m) and Havana, IL (latitude 40.300˚, longitude 90.061˚, elevation 140 m). In the year seeds were produced (2008), Mead, NE received 63.3 cm of precipitation and monthly average air temperatures ranged from 14.8˚C to 23.6˚C during the maize growing season (May through September). Havana, IL received 76.4 cm of precipitation and monthly average temperatures ranged from 14.7˚C to 23.3˚C during the maize growing season. Soil at the Mead, NE location was a Sharpsburg silty clay loam (fine, smectitic, mesic typic Argiudoll) with 3.3% soil organic matter. Soil at the Havana, IL location was a Plainfield sand (Typic Udipsamment, 94% sand, 4% silt, 2% clay) with 0.7% soil organic matter.
At each location, twelve distinct maternal environments were established with a full-factorial combination of soil compost amendment (two levels, amended or not amended), species assemblage (two levels, A. theophrasti with or without maize), and soil nitrogen fertilizer (three levels; 0, 0.5× or 1× local recommendations for maize). Twelve levels of a gradient were more than twice the minimum number of levels (five) previously recommended for detecting predictions of the growth-differentiation hypothesis [3]. Factors were arranged in a split-split plot design with four replications. The main plot (36.6 m by 9.2 m) factor was soil compost amendment, the subplot (9.2 m by 3.1 m) factor was species combination and the sub-subplot (3.1 m by 3.1 m) factor was soil nitrogen fertilizer level. Three weeks prior to planting, 10 (Mead, NE) to 30 (Havana, IL) soil cores (2.5 cm diameter by 20 cm deep) were taken from each treatment plot. Composite soil samples and compost materials were analyzed for total N (ammonium N and organic N) and amino sugar N by an analytical laboratory (15N Analysis Service at the University of Illinois, Urbana, IL, USA).
Amino sugar N levels were used to calculate soil compost amendment rates necessary to achieve soil amino sugar N levels of 280 parts per million (ppm). An amino sugar N level of at least 230 ppm represents a critical value for high N mineralization potential soils in which additional fertilizer N does less to promote maize growth than soils with low N mineralization potentials [18]. In this experiment, compost was derived from local beef manure and was applied and incorporated according to regional standards. Specifically, at Mead, NE, compost was applied at 32.5 Mg/ha with a manure spreader and incorporated to a depth of 8 cm using a field disk. At Havana, IL, compost was applied at 29.7 Mg/ha with a manure spreader and incorporated to a depth of 20 cm using a soil finisher. Nutrient analyses indicated that compost at Mead, NE was 0.51% N (dry weight basis) and 0.53% P. Compost at Havana, IL was 1.33% N and 0.25% P.
Soil nitrogen fertilizer treatments were imposed with applications of urea ammonium nitrate at planting. Soil nitrogen fertilizer rates were calculated to meet yield goals consistent with regional standards considering background soil N levels. At Mead, NE, nitrogen fertilizer was applied at a rate of 134 kg·N·ha−1, and at Havana, IL, nitrogen fertilizer was applied at a rate of 201 kg·N·ha−1.
Plots were seeded with both maize (Dekalb‘6166RR’ in Mead, NE; Pioneer ‘33Y45 in Havana, IL) and local A. theophrasti accessions on April 30 at Mead, NE and May 7 at Havana, IL. Throughout the study, all other vegetation was controlled with selective pre-emergence herbicides and hand labor. Plots consisted of 4 plant-rows spaced 0.76 m apart. Within rows, plants were equidistant, with 10 A. theophrasti plants/m (equivalent to 13 A. theophrasti plants/m−2) and, where appropriate, 6 additional maize plants/m (equivalent to 7.7 maize plants/m−2). From each plot, 5 A. theophrasti plants from 2 interior rows were randomly selected for determination of maternal biomass, which was the sum dry weight of vegetative (stems and leaves) and reproductive (fruits and seeds) structures. Determination of maternal biomass took place on July 22-23. On August 8, mature A. theophrasti capsules were harvested by hand from five plants of interior rows. Collected capsules were dried in paper bags under ambient conditions in the laboratory for 14 d. Dried capsules were then carefully crushed by hand to expel seeds that were separated from chaff by aspiration. Seeds were stored in moisture-proof bottles at 4˚C until needed.
Determination of o-DHP and Protein
For each seed lot (i.e., seed population from a specific maternal environment and production location), two 1-g seed samples were ground and passed through a forty mesh screen with a benchtop precision mill. To prevent cross-contamination of seed lots, dried maize kernels were milled prior to each A. theophrasti seed sample. Using powdered seed, o-DHP concentrations were determined according to the protocol of [17], modified by [19]. This spectrophotometric procedure provided accurate measurements of seed o-DHP concentrations, as evidenced by a previous investigation in which resulting concentrations correlated well with seed o-DHP concentrations determined with gas chromatography-mass spectrometry (r = 0.80, p < 0.05, N = 6) [19]. In the current investigation, the spectrophotometric procedure for seed o-DHP concentration was repeated ten times for each seed lot. Seed protein concentration was determined according to the spectrophotometric procedure of [20], using powdered seed from above and six repetitions per seed lot. For both o-DHP and protein, concentrations were expressed in units of both seed weight and individual seed. This enabled comparisons with previous research and facilitated improved understanding of seed characteristics at a scale pertinent to seed fate. Weightbased concentrations were converted to units based on individual seeds through division by mean individual seed weights, which were determined with 50 seeds per lot.
2.3. Data Analysis
The effects of increasing maternal environment resource availability on seed o-DHP concentrations and o-DHP: protein ratios were assessed with nonlinear least squares regression using spectrophotometric assay means and calibrated maternal biomass values that accounted for classical measurement error in the dependent variable [21]. Anticipating skewness in maternal biomass data resulting from rarity of low resource environments under agronomic conditions [5], regression analyses were performed with both original maternal biomass values and log-transformed maternal biomass values. Regression analyses were executed using the nlme library of the open source statistical software program R v.2.6.2 (R Foundation for Statistical Computing, http://www.r-project.org). Data for each location were analyzed separately. The F-test for coincident regression was used to test the null hypothesis that location-specific regressions were estimates of the same population [23].
Responses of seed o-DHP concentration to increasing maternal biomass were modeled with a unimodal function that included:
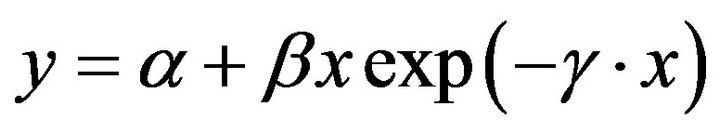 (1)
(1)
where y is seed o-DHP concentration, x is maternal biomass, α is the y-intercept parameter, β is the initial slope at low maternal biomass, and γ is the rate of decline in seed o-DHP concentration as maternal biomass increased. Responses of o-DHP:protein ratios to increasing maternal biomass were modeled with a negative exponential function. Model fits to data were evaluated with pseudo-R2 values [22].
3. Results
The twelve maternal environments produced by combinations of agronomic treatments formed a resource gradient with respect to A. theophrasti growth, as indicated by the significant differences in maternal biomass among maternal environments at Mead, NE (F11,36 = 12.03, p < 0.001) and at Havana, IL (F11,36 = 10.41, p < 0.001). Maternal biomass at Mead, NE ranged from 38.6 to 414.1 g/m−2, and at Havana, IL, maternal biomass ranged from 94.5 to 837.9 g/m−2. Vegetative biomass was positively correlated with reproductive biomass at Mead, NE (r = 0.95, p < 0.001) and Havana, IL (r = 0.90, p < 0.001).
Seed o-DHP concentrations were influenced by location (t = 2.47, df = 11, p = 0.03). Overall mean seed o-DHP concentration was greater for Havana, IL (17.9 ± s.e. 0.5 µmol o-DHP/g seed) than for Mead, NE (15.3 ± s.e. 1.0 µmol o-DHP/g seed). Despite location effects, minimum o-DHP concentrations consistently occurred in seeds from A. theophrasti monocultures, amended with compost and fertilized with nitrogen at a 1.0× rate (Table 1).
At Mead, NE, maximum o-DHP concentration occurred in seeds from A. theophrasti-maize bicultures, not amended with compost and fertilized with nitrogen at a
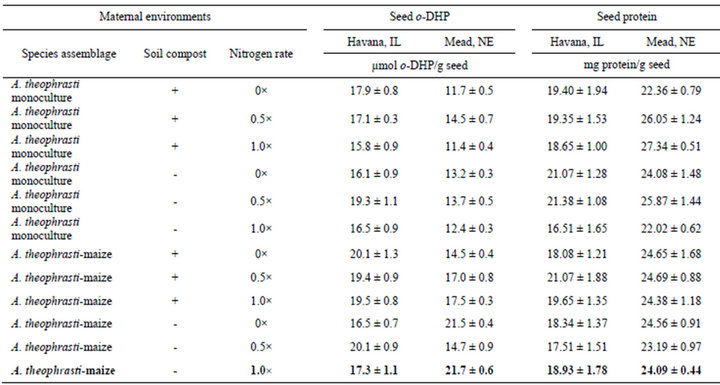
Table 1. ortho-dihydroxyphenol (o-DHP) and protein concentrations for seeds from different maternal environments. Data are means with standard errors for ten (o-DHP) and six (protein) assay replicates.
1.0× rate. At Havana, IL, maximum o-DHP concentration occurred in seeds originating from A. theophrastiaize bicultures, amended with compost and fertilized with nitrogen at a 1.0× rate.
Responses of seed o-DHP concentrations to increasing maternal biomass were modeled with Equation (1) (Figures 1 and 2); however, location-specific regressions did not estimate the same population regression (F2,20 = 9.77, p = 0.001). As expected, log transformation created more uniform spread in maternal biomass compared to the original data, but transformation of the dependent variable did not improve model fit (Figures 1(b) and 2(b)). Thus, compression of maternal biomass values at the low end of the data range did not affect modeling the responses of o-DHP concentrations to increasing maternal biomass.
Seed protein concentrations were influenced by location (t = −10.96, df = 11, p < 0.001). Overall mean seed protein concentration was greater for Mead, NE (24.44 ± s.e. 0.45 mg protein/g seed) than for Havana, IL (19.16 ± s.e. 0.43 mg protein/g seed). At Mead, NE, minimum seed protein concentration occurred in seeds from A. theophrasti monocultures, amended with compost and fertilized with nitrogen at a 0x rate; and maximum seed protein concentration occurred in seeds from A. theophrasti monocultures, amended with compost and fertilized with nitrogen at a 1× rate. At Havana, IL, minimum seed protein concentration occurred in seeds from A. theophrasti-maize bicultures, not amended with compost and fertilized with nitrogen at a 0.5× rate; and maximum seed protein concentration occurred in seeds from A. theophrasti monocultures, not amended with compost and fertilized with nitrogen at a 0× rate.
A negative exponential function was found to describe the response of o-DHP:protein ratios to increasing maternal biomass at Mead, NE (Figure 3). At Havana, IL, the negative exponential function indicated only 0.14 of the variance in o-DHP:protein ratio was in common with variance in maternal biomass (Figure 4).
4. Discussion
The growth-differentiation balance hypothesis provides a framework for understanding phenotypic differences in secondary metabolite concentrations that arise from plastic responses to environmental gradients [3-5]. The unimodal function fit to the response of seed o-DHP concentration to increasing maternal biomass indicated that seed o-DHP concentrations were, in part, influenced by maternal environment resource availability in manners consistent with the growth-differentiation balance hypothesis. Thus, building on a rich understanding of maternal effects on seed traits [24], we extended predictions of the growth-differentiation balance hypothesis to include maternal environment effects on concentrations of phenolic compounds in seeds.
We emphasize that maternal environment resource availability was one of several factors influencing o-DHP
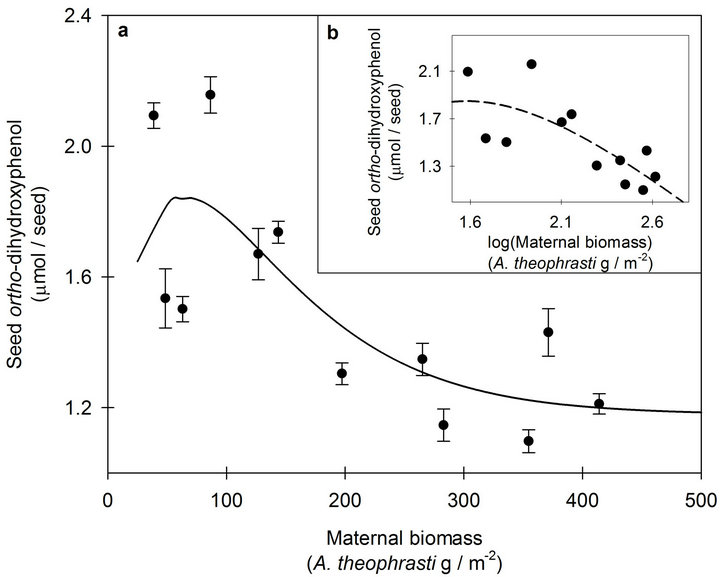
Figure 1. (a) The response of seed ortho-dihydroxyphenol (o-DHP) concentrations to increasing maternal biomass for Abutilon theophrasti seed lots originating from a maternal environment resource gradient at Mead, NE, USA. Data points represent means ± s.e. of ten o-DHP assay replicates. The solid line (y = 1.18 + 0.03xe−0.02x, pseudo-R2 = 0.59, p = 0.003) represents the model corresponding with the hypothesized response. (b) The response of seed o-DHP to increasing maternal biomass following log transformation of the dependent variable. The dashed line (y = −3.03 + 8.40xe−0.63x; pseudo-R2 = 0.57, p = 0.02) represents the model corresponding with the hypothesized response.
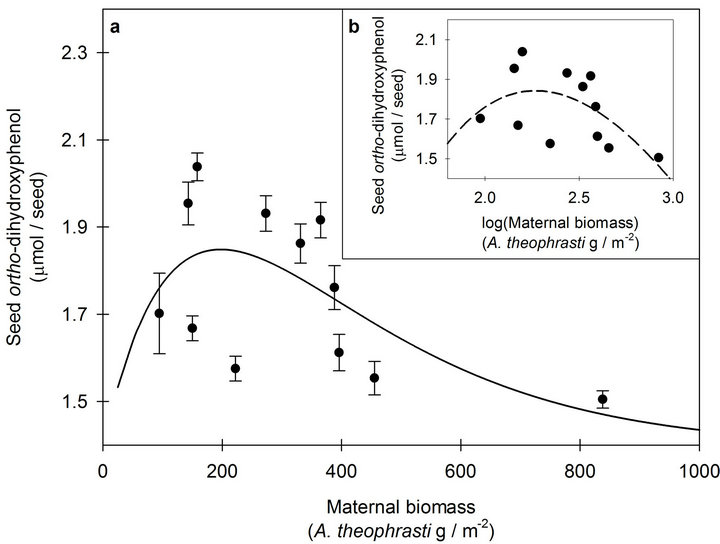
Figure 2. (a) The response of seed ortho-dihydroxyphenol (o-DHP) concentrations to increasing maternal biomass for Abutilon theophrasti seed lots originating from maternal environment resource gradients at Havana, IL. Data points represent means ± s.e. of ten o-DHP assay replicates. The solid line (y = 1.40 + 0.006xe−0.005x; pseudo -R2 = 0.34, p = 0.05) represents the model corresponding with the hypothesized response. b) The response of seed o-DHP to increasing maternal biomass following log transformation of the dependent variable. The dashed line (y = −9.09 + 13.1xe−0.44x; pseudo-R2 = 0.33, p = 0.05) represents the model corresponding with the hypothesized response.
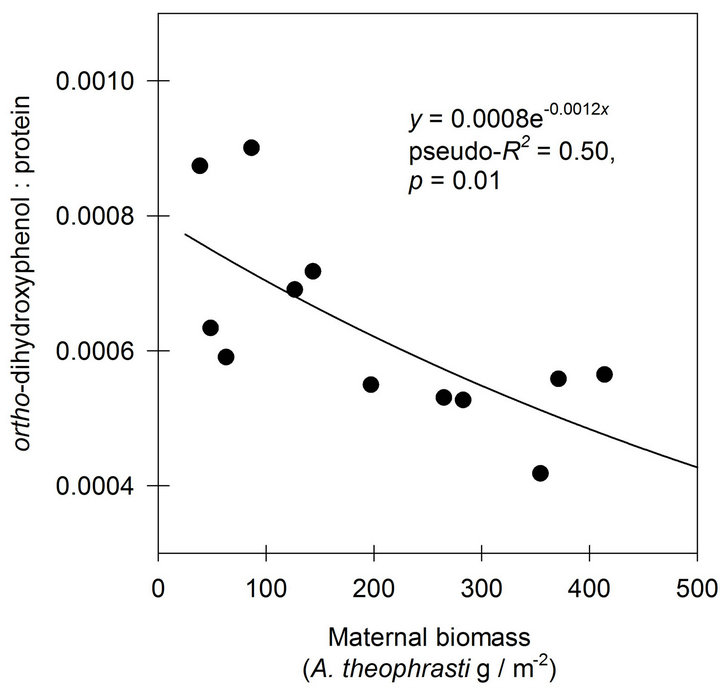
Figure 3. The effects of increasing maternal biomass on o-DHP: protein concentration ratios for Abutilon theophrasti seed lots originating from a maternal environment resource gradient at Mead, NE.
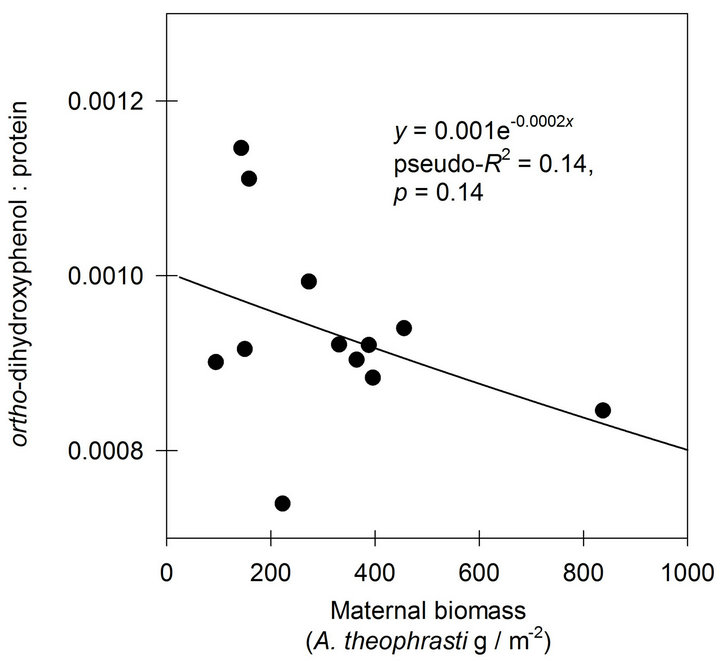
Figure 4. The effects of increasing maternal biomass on o-DHP: protein concentration ratios for Abutilon theophrasti seed lots originating from a maternal environment resource gradient at Havana, IL.
concentrations in A. theophrasti seeds. Previous research determined that concentrations of defense compounds in seeds can be affected by numerous maternal environment variables including incidences of abiotic stress during early life-stages [25] and occurrences of predation during the seed maturation period [26]. These and additional microenvironment factors may have contributed to the unpredictability in seed o-DHP concentrations observed in this study.
Differences in model fit between locations indicated that the negative association between seed o-DHP concentration and maternal biomass was conditioned by the local environment. A major difference between locations was soil physiochemical properties, which can influence spatial distributions of environmental factors that impact the production of phenolics in vegetative tissue [27]. Assuming that endogenous physiological conditions of maternal plants are replicated in seeds [28], extension of the growth-differentiation balance hypothesis to seed traits requires maternal environments free of microenvvironments that differentially influence phenolic compound synthesis. The results of this study suggest that such environments are more likely at locations characterized by loam-type soils with relatively high percent organic matter (as seen at Mead, NE) compared to locations with sandy soils that are low in percent organic matter (as seen at Havana, IL).
Abutilon theophrasti seeds feature water-impermeable coats that preserve viability through physical exclusion of microorganisms [29]. In general, phenolic compounds in water impermeable seed coats enhance capacities for seed survival by mitigating the harmful effects of surface injury [19,29]. Understanding the impacts of variability in seed o-DHP on A. theophrasti seed fate will improve knowledge of this weed’s reproductive ecology and may be important for establishing more ecologically-based weed management strategies. Along these lines, it is important to note that the differences in o-DHP concentration were detected in A. theophrasti seeds produced under field conditions and were consequences of changes in crop production practices.
Although not the primary focus of this investigation, we detected differences in seed o-DHP concentrations between locations, with o-DHP concentrations reduced for seeds from Mead, NE compared to seeds from Havana, IL. Seed protein concentration also varied between locations, with seeds from Mead, NE enriched in protein compared to seeds from Havana, IL. These location differences in seed protein and o-DHP suggested that seeds from Mead, NE were inherently more susceptible to predation and decay than seeds from Havana, IL. Understanding sources of variation in seed o-DHP and protein concentration among locations is important because local adaptation in seed defense chemistry can prompt reciprocal evolutionary changes between plants and predators [30], potentially influencing trophic interactions across the ecosystem.
In general, the effects of o-DHP on seed survival are understood from studies that identified correlations between seed o-DHP concentration and seed longevity [8, 19]. To our knowledge, specific mechanisms for o-DHP protection of seeds have yet to be clarified. Such studies would benefit from seed lots that differ in o-DHP concentration but show limited variability in other traits related to seed survival (e.g., size, morphology and composition of nutritional reserves). Our results suggest a study system conducive to improving knowledge of the mechanisms in which o-DHP protects seeds. Specifically, we propose that maternal environment resource levels can be manipulated to produce seed lots that differ in o-DHP concentration but exhibit little variation in other seed traits. These seed lots can then be subjected to mortality assays, thereby revealing the role of o-DHP in seed survival. Further clarification of seed defense chemistry will increase knowledge of plant-environment interactions involving seeds, which represent a lifecycle stage critical to population dynamics of plant species with annual life histories.
REFERENCES
- M. Fenner and K. Thompson, “The Ecology of Seeds,” Cambridge University Press, Cambridge, 2005. doi:10.1017/CBO9780511614101
- Y. Mohamed-Yasseen, S. A. Barringer, W. E. Splittstoesser and S. Costanza, “The Role of Seed Coats in Seed Viability,” The Botanical Review, Vol. 60, No. 4, 1994, pp. 427-439. doi:10.1007/BF02857926
- R. T. Wilkens, J. M. Spoerke and N. E. Stamp, “Differential Responses of Growth and Two Soluble Phenolics of Tomato to Resource Availability,” Ecology, Vol. 77, No. 1, 1996, pp. 247-258. doi:10.2307/2265674
- C. Glynn, D. A. Herms, C. M. Orians, R. C. Hansen and S. Larsson, “Testing the Growth-Differentiation Balance Hypothesis: Dynamic Responses of Willows to Nutrient Availability,” New Phytologist, Vol. 176, No. 3, 2007, pp. 623-634. doi:10.1111/j.1469-8137.2007.02203.x
- J. Le Bot, C. Benard, C. Robin, F. Bourgaud and S. Adamowicz, “The ‘Trade-Off’ between Synthesis of Primary and Secondary Compounds in Young Tomato Leaves Is Altered by Nitrate Nutrition: Experimental Evidence and Model Consistency,” Journal of Experimental Botany, Vol 60, No. 15, 2009, pp. 4301-4314. doi:10.1093/jxb/erp271
- W. F. Loomis, “Growth-Differentiation Balance vs Carbohydrate-Nitrogen Ratio,” Proceedings of the American Society for Horticultural Science, Vol. 29, 1932, pp. 240- 245.
- D. A. Herms and W. J. Mattson, “The Dilemma of Plants: To Grow or Defend,” Quarterly Review of Biology, Vol. 67, No. 3, 1992, pp. 283-335. doi:10.1086/417659
- G. F. Hendry, K. Thompson, C. J. Moss, E. Edwards and P. C. Thorpe, “Seed Persistence: A Correlation between Seed Longevity in the Soil and Ortho-Dihydroxyphenol Concentration,” Functional Ecology, Vol. 8, No. 5, 1994, pp. 658-664. doi:10.2307/2389929
- R. L. Nicholson and R. Hammerschmidt, “Phenolic Compounds and Their Role in Disease Resistance,” Annual Review of Phytopathology, Vol. 30, 1992, pp. 369-389. doi:10.1146/annurev.py.30.090192.002101
- D. L. Clark and M. V. Wilson, “Post-Dispersal Seed Fates of Four Prairie Species,” American Journal of Botany, Vol. 90, No. 5, 2003, pp. 730-735. doi:10.3732/ajb.90.5.730
- S. I. Warwick and L. D. Black, “The Biology of Canadian Weeds: Abutilon theophrasti,” Canadian Journal of Plant Science, Vol. 68, No. 4, 1988, pp. 1069-1085. doi:10.4141/cjps88-127
- R. E. Nurse and A. Ditommaso, “Corn Competition Alters the Germinability of Velvetleaf (Abutilon theophrasti) Seeds,” Weed Science, Vol. 53, No. 4, 2005, pp. 479-488. doi:10.1614/WS-04-185R1
- J. A. D. Parrish and F. A. Bazzaz, “Nutrient content of Abutilon theophrasti seeds and the competitive ability of the resulting plants,” Oecologia, Vol. 65, No. 2, 1985, pp. 247-251. doi:10.1007/BF00379224
- B. J. Schutte, A. S. Davis, K. A. Renner and J. Cardina, “Maternal and Burial Environment Effects on Seed Mortality of Velvetleaf (Abutilon theophrasti) and Giant Foxtail (Setaria faberi),” Weed Science, Vol. 56, No. 6, 2008, pp. 834-840. doi:10.1614/WS-08-031.1
- F. R. Earle and Q. Jones, “Analyses of Seed Samples from 113 Plant Families,” Economic Botany, Vol. 16, No. 4, 1962, pp. 221-250. doi:10.1007/BF02860181
- G. M. Dugan and M. R. Gumbmann, “Toxicological and Nutritional Evaluation of Velvetleaf Seed: Subchronic 90-Day Feeding Study and Protein Efficiency Ratio Assay,” Food and Chemical Toxicology, Vol. 28, No. 2, 1990, pp. 95-99. doi:10.1016/0278-6915(90)90016-G
- G. F. Hendry, “Defense Chemistry (Phenols),” In: G. F. Hendry and J.P. Grime, Eds., Methods in Comparative Plant Ecology: A Laboratory Manual, Chapman & Hall, London, 1993, pp. 180-181. doi:10.1007/978-94-011-1494-3
- R. L. Mulvaney, S. A. Khan and T. R. Ellsworth, “Need for a Soil-Based Approach in Managing Nitrogen Fertilizers for Profitable Corn Production,” Soil Science Society of America Journal, Vol. 70, No. 1, 2006, pp. 172-182. doi:10.2136/sssaj2005.0034
- A. S. Davis, B. J. Schutte, J. Iannuzzi and K. A. Renner, “Chemical and Physical Defense of Weed Seeds in Relation to Soil Seedbank Persistence,” Weed Science, Vol. 56, No. 5, 2008, pp. 676-684. doi:10.1614/WS-07-196.1
- G. F. Hendry and P. C. Thorpe, “Organic Reserves,” In: G. F. Hendry and J. P. Grime, Eds., Methods in Comparative Plant Ecology: A Laboratory Manual, Chapman & Hall, London, 1993, pp. 196-199. doi:10.1007/978-94-011-1494-3
- R. J. Carroll, D. Ruppert, L. A. Stefanski and C. M. Crainiceanu, “Measurement Error in Nonlinear Models: A Modern Perspective,” 2nd Edition, Chapman & Hall/CRC, New York, 2006. doi:10.1201/9781420010138
- O. Schabenberger and F. J. Pierce, “Contemporary Statistical Models for the Plant and Soil Sciences,” CRC Press, New York, 2002.
- J. H. Zar, “Biostatistical Analysis,” 4th Edition, Prentice Hall, Upper Saddle River, 1999.
- Y. Gutterman, “Maternal Effects on Seeds during Development,” In: M. Fenner, Ed., Seeds: The Ecology of Regeneration in Plant Communities, 2nd Edition, CAB International, New York, 2000, pp. 59-84. doi:10.1079/9780851994321.0059
- A. S. Steinbrenner, N. Agerbirk, C. M. Orians and F. S. Chew, “Transient Abiotic Stresses Lead to Latent Defense and Reproductive Responses over the Brassica Rapa Life Cycle,” Chemoecology, Vol. 22, No. 4, 2012, pp. 239- 250 doi:10.1007/s00049-012-0113-y
- D. Kestring, L. R. Menezes, C. A. Tomaz, G. P. Lima and M. N. Rossi, “Relationship among Phenolic Contents, Seed Predation, and Physical Seed Traits in Mimosa bimucronata Plants,” Journal of Plant Biology, Vol. 52, No. 6, 2009, pp. 569-576. doi:10.1007/s12374-009-9073-3
- W. Otten and C. A. Gilligan, “Soil Structure and SoilBorne Diseases: Using Epidemiological Concepts to Scale from Fungal Spread to Plant Epidemics,” European Journal of Soil Science, Vol. 57, No. 1, 2006, pp. 26-37. doi:10.1111/j.1365-2389.2006.00766.x
- K. Donohue and J. Schmitt, “Maternal Environmental Effects in Plants: Adaptive Plasticity?” In: T. A. Mousseau and C. W. Fox, Eds., Maternal Effects as Adaptations, Oxford University Press, New York, 1998, pp. 137-158.
- R. J. Kremer, L. B. Hughes and R. J. Aldrich, “Examination of Microorganisms and Deterioration Resistance Mechanisms Associated with Velvetleaf Seed,” Agronomy Journal, Vol. 76, No. 5, 1984, pp. 745-749. doi:10.2134/agronj1984.00021962007600050009x
- J. W. Dalling, A. S. Davis, B. J. Schutte and A. E. Arnold, “Seed Survival in Soil: Interacting Effects of Predation, Dormancy and the Soil Microbial Community,” Journal of Ecology, Vol. 99, No. 1, 2001, pp. 89-95. doi:10.1111/j.1365-2745.2010.01739.x

