 Vol.1, No.1, 5-10 (2012) Advances in Parkinson’s Disease doi:10.4236/apd.2012.11002 Association of adverse effects with monoamine oxidase type B inhibitor and catechol-o-methyl transferase inhibitor combination therapy in Parkinson’s disease patients Rui Zhang, Danielle C. Spengler, Marie-Helene Saint-Hilaire*, Anna D. Hohler Department of Neurology, Boston University School of Medicine, Boston, USA; *Corresponding Author: Marie.Saint-Hilaire@bmc.org Received 1 July 2012; revised 24 July 2012; accepted 22 August 2012 ABSTRACT Currently, levodopa is the most effective and commonly used medication to control motor symptoms in Parkinson’s disease (PD). However, its long-term use is associated with adverse effects (AEs). Combination therapy of a monoamine oxidase type B inhibitor (MAOBI) with levodopa or a catechol-O-methyl transfer ase inhibitor (COMTI) with levodopa provides benefits to PD patients. Direct comparison of efficacy and side effect profiles is complex. The aim of this study is to investigate the different AE profiles of MAOBI and COMTI combination therapies. Data used to analyze the AEs of different PD medications were retrieved from “The Boston University Me- dical Center’s Parkinson’s Disease and Move- ment Disorder Database”. Ten categories of AEs were compared between patients receiving MAOBI and COMTI combination treatment. In total, 87 subjects were included in the analysis. Out of ten AEs, the presence of dementia was signifi- cantly different between the MAOBI and COMTI groups with an OR of 6.9 (COMTI vs MAOBI, 95% CI 1.3 - 37.0). Motor fluctuations were also found to be differently distributed in the two medication groups with an OR of 3.1 (COMTI vs MAOBI, 95% CI 1.0 - 9.8). In this ret- rospective database analysis of patients treated with combination treatment for PD, combination therapy of a COMTI with levodopa was more likely to be associated with dementia and motor fluctuations than a MAOBI with levodop a. Keywords: Catechol-O-Methyl Transferase Inhibitors; Dementia; Monoamine Oxidase Type B Inhibitors; Motor Fluctuations; Parkinson ’s Disease 1. INTRODUCTION Parkinson’s disease (PD) is a neurodegenerative disorder [1], characterized by resting tremor, rigidity or stiffness, bradykinesia and postural instability [2]. While the eti- ology of PD remains uncertain [3,4], the progressive loss of dopaminergic neurons in the substantia nigra (SN) is considered a marker of the motor disorder of PD [5]. Currently, medication therapy is the most widely used treatment for PD. Several types of medications have been approved by the US Food and Drug Administration (FDA) for the treatment of PD, including levodopa (L-Dopa)/ carbidopa (dopa-decarboxylase inhibitor), rasagiline and selegiline (monoamine oxidase type B inhibitors, MAOBIs), entacapone and tolcapone (catechol-o-methyl transferase inhibitors, COMTIs), ropinirole and pramipexole (non- ergolin dopamine agonists), and amantadine (which has both anticholinergic and antiglutaminergic properties) [6-11]. Levodopa is the most effective and commonly used medication to control motor symptoms in PD [12]. The long-term treatment of PD using levodopa, however, has been shown to induce several adverse effects (AEs). The most common AEs include motor fluctuations (“on-off” phenomenon), dyskinesia, and psychosis [8,13]. As a result, other classes of medication, including COMTIs, MAOBIs, and dopamine agonists, have been widely used as alternatives or adjuncts in treating PD [14]. Combination therapy of a MAOBI with levodopa has been proven to decrease the required dosage of levodopa, improve symptom relief, and reduce “off” time [8,14-17]. However, the combination of a MAOBI with levodopa has been observed to cause more frequent AEs than levodopa monotherapy, including dyskinesia, hypotension, nausea, dizziness, dry mouth, psychiatric abnormalities, and bal- ance difficulties [7,8,16,18-20]. The combination therapy of levodopa with a COMTI can increase levodopa duration of action [21], regulate the Copyright © 2012 SciRes. Openly accessible at http://www.scirp.org/journal/apd/  R. Zhang et al. / Advances in Parkinson’s Disease 1 (2012) 5-10 6 motor fluctuations, and reduce levodopa dose [8,14,22-26]. Adjunct therapy with a COMTI is also associated with increased frequency of dopaminergic AEs, including dyskinesia, nausea, postural hypotension, and hallucina- tions [8,20,24]. There have been some comparative studies involving MAOBIs and COMTIs. One randomized, double-blinded trial involving 687 subjects on levodopa compared the efficacy and safety between the additions of rasagiline (MAOBI), entacapone (COMTI), or placebo (levodopa monotherapy), and found that the overall relative fre- quency of dopaminergic AEs was similar among the three groups. Rasagiline was, however, better tolerated with less early-discontinuation than entacapone and pla- cebo [27]. A meta-analysis study evaluated the efficacy and safety of MAOBIs or COMTIs as adjunct therapies with levodopa. It concluded that the efficacy of combina- tion therapy was superior to monotherapy with levodopa, but the incidence of dyskinesia was observed to be higher in both combination therapy groups than with monotherapy. However, no direct comparison between the two adjunct therapy groups was reported [20]. A meta-analysis review study of 44 clinical trials demon- strated, through indirect comparison of the drugs, more dyskinesia and higher overall incidence of AEs with COMTIs than with MAOBIs [14]. Although these studies have been helpful in elucidat- ing the differences in the safety profiles of these combi- nation therapies (MAOBI vs COMTI), available data are too limited to directly compare them. The aim of this study is to investigate and compare the difference be- tween the AE profiles of MAOBIs and COMTIs when used in combination with levodopa for the treatment of PD. 2. METHODS Data were taken from “The Boston University Medical Center’s Parkinson’s Disease and Movement Disorder Database”, a collection of information acquired from movement disorder patients seen at the Boston Univer- sity Neurological Associates Clinic. The data collection form contains three major sections: demographic infor- mation, relevant disease information and medications. The relevant disease information section includes the neurological diagnosis with onset and diagnosis dates, family history of related disease, and common complica- tions of disease/therapy. These include compulsive be- havior, dyskinesia, dementia, depression, freezing, hallu- cinations, motor fluctuations, orthostatic hypotension, psychosis, and other autonomic dysfunction. Subjects were asked about their current and previous medication use. In addition, subjects were asked about surgical in- terventions such as Deep Brain Stimulation (DBS) and pallidotomy to treat their movement disorders. To be included in the analysis for this study, subjects had to meet the following inclusion and exclusion crite- ria. The subject must have a diagnosis of PD in “The Boston University Medical Center’s Parkinson’s Disease and Movement Disorder Database”, and have been be- tween the ages of 18 and 89. (Subjects who were over 89 years old were excluded in order to meet the privacy regulation for de-identified data research.) Furthermore, the subject must have been able to provide medication information, and have had history of current or prior use of levodopa combined with a MAOBI but never used a COMTI, or vice versa. Potential subjects were excluded if they were diagnosed with any movement disorder other than PD. Subjects who fit the criteria and who were entered in the database between January 2007 and Janu- ary 2011 were included in the final analysis. In addition to complications and medication use, other variables were included in the analysis as covariates, including gender, family history, occupation, ethnicity, education level, other PD medication use, age, and disease dura- tion. Fisher’s exact test was applied to test whether different AEs were associated with the different PD medications (MAOBI vs COMTI). Multivariate logistic regression models were applied to test whether discovered associa- tions would still exist after controlling for other possible confounding factors, such as age, disease duration, edu- cation level, and other PD medication use. Two models of multivariate logistic regression were applied: general model (including all the potential covariates) and step- wise model (only including those covariates with statis- tical significance with entry criterion p ≤ 0.1 and removal criterion p > 0.1). Odds ratio (OR) and 95% confidence interval of OR were calculated to show the strength of association. To account for occasional missing data in the database, relative frequency, mean and interquartile range (IQR) were used to compare distributions of vari- ous variables in non-missing cohort and sample cohort. SAS (Statistical Analysis System) 9.1.3, provided by SAS Institute Inc., was employed to conduct all the data analysis. Results were considered significant if the two- tailed p value was < 0.05. This study (H-31069) was de- termined to be exempt from review by the IRB of Bos- ton University Medical Campus and Boston Medical Center. 3. RESULTS Of the 577 subjects in the database, 87 met all inclu- sion and exclusion criteria and were included in the data analysis (Table 1). The reasons for excluding subjects included: never used a MAOBI or a COMTI (140, 51%), used both a MAOBI and a COMTI (78, 28%), and were prescribed a MAOBI or a COMTI without levodopa (58, 21%). In those 87 subjects, 39 took a MAOBI with Copyright © 2012 SciRes. Openly accessible at http://www.scirp.org/journal/apd/ 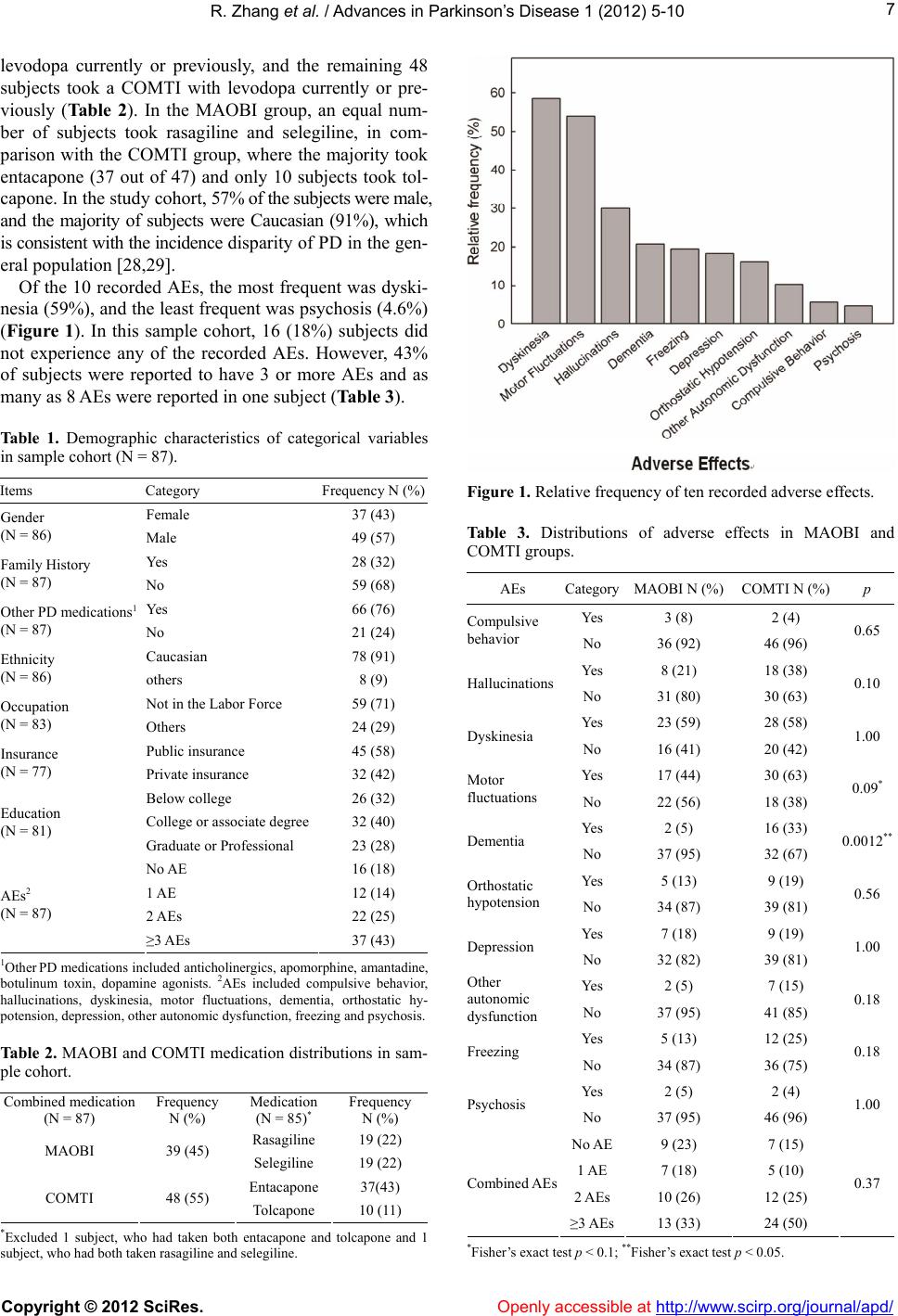 R. Zhang et al. / Advances in Parkinson’s Disease 1 (2012) 5-10 7 levodopa currently or previously, and the remaining 48 subjects took a COMTI with levodopa currently or pre- viously (Ta b l e 2 ). In the MAOBI group, an equal num- ber of subjects took rasagiline and selegiline, in com- parison with the COMTI group, where the majority took entacapone (37 out of 47) and only 10 subjects took tol- capone. In the study cohort, 57% of the subjects were male, and the majority of subjects were Caucasian (91%), which is consistent with the incidence disparity of PD in the gen- eral population [28,29]. Of the 10 recorded AEs, the most frequent was dyski- nesia (59%), and the least frequent was psychosis (4.6%) (Figure 1). In this sample cohort, 16 (18%) subjects did not experience any of the recorded AEs. However, 43% of subjects were reported to have 3 or more AEs and as many as 8 AEs were reported in one subject (Table 3). Table 1. Demographic characteristics of categorical variables in sample cohort (N = 87). Items Category Frequency N (%) Female 37 (43) Gender (N = 86) Male 49 (57) Yes 28 (32) Family History (N = 87) No 59 (68) Yes 66 (76) Other PD medications1 (N = 87) No 21 (24) Caucasian 78 (91) Ethnicity (N = 86) others 8 (9) Not in the Labor Force 59 (71) Occupation (N = 83) Others 24 (29) Public insurance 45 (58) Insurance (N = 77) Private insurance 32 (42) Below college 26 (32) College or associate degree 32 (40) Education (N = 81) Graduate or Professional 23 (28) No AE 16 (18) 1 AE 12 (14) 2 AEs 22 (25) AEs2 (N = 87) ≥3 AEs 37 (43) 1Other PD medications included anticholinergics, apomorphine, amantadine, botulinum toxin, dopamine agonists. 2AEs included compulsive behavior, hallucinations, dyskinesia, motor fluctuations, dementia, orthostatic hy- potension, depression, other autonomic dysfunction, freezing and psychosis. Table 2. MAOBI and COMTI medication distributions in sam- ple cohort. Combined medication (N = 87) Frequency N (%) Medication (N = 85)* Frequency N (%) Rasagiline 19 (22) MAOBI 39 (45) Selegiline 19 (22) Entacapone 37(43) COMTI 48 (55) Tolcapone 10 (11) *Excluded 1 subject, who had taken both entacapone and tolcapone and 1 subject, who had both taken rasagiline and selegiline. Figure 1. Relative frequency of ten recorded adverse effects. Table 3. Distributions of adverse effects in MAOBI and COMTI groups. AEs CategoryMAOBI N (%) COMTI N (%)p Yes 3 (8) 2 (4) Compulsive behavior No 36 (92) 46 (96) 0.65 Yes 8 (21) 18 (38) Hallucinations No 31 (80) 30 (63) 0.10 Yes 23 (59) 28 (58) Dyskinesia No 16 (41) 20 (42) 1.00 Yes 17 (44) 30 (63) Motor fluctuations No 22 (56) 18 (38) 0.09* Yes 2 (5) 16 (33) Dementia No 37 (95) 32 (67) 0.0012** Yes 5 (13) 9 (19) Orthostatic hypotension No 34 (87) 39 (81) 0.56 Yes 7 (18) 9 (19) Depression No 32 (82) 39 (81) 1.00 Yes 2 (5) 7 (15) Other autonomic dysfunction No 37 (95) 41 (85) 0.18 Yes 5 (13) 12 (25) Freezing No 34 (87) 36 (75) 0.18 Yes 2 (5) 2 (4) Psychosis No 37 (95) 46 (96) 1.00 No AE9 (23) 7 (15) 1 AE 7 (18) 5 (10) 2 AEs10 (26) 12 (25) Combined AEs ≥3 AEs13 (33) 24 (50) 0.37 *Fisher’s exact test p < 0.1; **Fisher’s exact test p < 0.05. Copyright © 2012 SciRes. Openly accessible at http://www.scirp.org/journal/apd/  R. Zhang et al. / Advances in Parkinson’s Disease 1 (2012) 5-10 8 Using Fisher’s exact test to compare the AE profiles of the different combination treatments, only the frequency of dementia was found to be statistically significantly different between the MAOBI and COMTI groups (p = 0.0012, Table 3): subjects who took a COMTI with levodopa had a higher relative frequency of dementia compared to subjects who took a MAOBI (33% versus 5%). Motor fluctuations also showed a different distribu- tion between the two medication groups (p = 0.09): the COMTI subjects had a higher relative frequency of mo- tor fluctuations than the MAOBI subjects (63% versus 44%). The differences of relative frequency of other AEs between the two groups were not statistically significant in the sample cohort (Table 3). As subjects could not be randomized as part of this study design, the distributions of other potential con- founding factors were compared with available informa- tion in the database and were included in multivariable logistic regressions. Two multivariable logistic models were applied to test the association between dementia and PD medication use. Only those subjects with complete data were re- tained in the analysis (n = 61). The stepwise model only contained those variables with at least p = 0.1 signifi- cance level. Variables of treatment (MAOBI or COMTI) and age were retained in the stepwise logistic regression model. The OR of developing dementia was 6.9 (COMTI vs MAOBI, 95% CI: 1.3 - 37.0). Based on the general logistic regression model, which included all of the potential confounding factors with recorded information, the OR of developing dementia was 8.0 (COMTI vs MAOBI, 95% CI: 1.2 - 52.6), marking an increase from 6.9 in the stepwise model. This indi- cated that after controlling for the potential confounding factors, the association between dementia and COMTI was still statistically significant. Multivariable logistic models were also employed to test the association between PD medication use and mo- tor fluctuations. In the stepwise model, with at least p = 0.1 significance level, 2 variables, treatment of MAOBI or COMTI, and disease duration, were retained in step- wise logistic regression model. The OR of developing motor fluctuations was 3.1 (COMTI vs MAOBI, 95% CI: 1.0 - 9.8, p = 0.05). The stepwise statistical model also suggested that, for a PD subject treated with MAOBI combination therapy with an average disease duration of 10 years, the probability of experiencing motor fluctua- tions was 44.8% compared to 71.1% in a similar subject treated with COMTI combination therapy. After taking into account all of the potential con- founding factors with available information in general regression analysis, the OR of developing motor fluctua- tions was 3.7 (COMTI vs MAOBI, 95% CI: 1.01 - 13.8), indicating that the association between motor fluctua- tions and COMTI became stronger and statistically sig- nificant after controlling for the potential confounding factors. Twenty-six subjects were removed from the lo- gistic regression models because data were incomplete. Upon examination of the distribution of all testing vari- ables, no major differences were observed in the two groups, and all the variables were similarly distributed between non-missing data cohort and sample cohort. 4. DISCUSSION Results from other studies have indicated that com- pared to levodopa monotherapy, MAOBI and COMTI adjunct therapies increase therapeutic effects, reduce levodopa dose, and are more effective in controlling mo- tor fluctuations [8,14,16,21-23]. However, the combined therapies have also been associated with more frequent AEs, especially dyskinesia [8,14,20]. Despite the wide use of these medication regimens, few studies have di- rectly and systematically compared their AE profiles, as this one has. The results are interesting and incite further questions and ideas for future prospective comparative trials. Based on the results of Fisher’s exact test, out of the 10 recorded complications analyzed, only dementia showed a statistically significant difference among pa- tients on MAOBI and COMTI combination therapies with levodopa. Patients on COMTI had a higher relative frequency of dementia than patients on MAOBI (33% vs 5%) (Table 3). There are various estimates of the prevalence of de- mentia (8% - 93%) in PD patients. Cognitive difficulties can greatly impact the PD treatment plan and influence the quality of life of PD subjects [30]. In the current sample cohort, the subjects on COMTI were slightly older than the MAOBI group; the middle 50% percent of subjects were 60 - 77 years old in the COMTI group, versus 59 - 74 years old in the MAOBI group. Since age is known to be an important risk factor for dementia [31,32], this difference could slightly impact the associa- tion of the medications with dementia found in the cur- rent study. The logistic regression model took the sub- ject’s age into account. The OR age was 1.1 in the step- wise logistic regression model, which indicated that, with an increase of 1 year in age, the risk of dementia would increase 10%, regardless of the treatment group. Severity of motor symptoms is also considered to be a risk factor for dementia [30,31]. This, however, cannot be estimated using the current database. The Hoehn and Yahr stage information was only collected for some sub- jects to record the severiy of PD, and only 25 out of 87 (29%) subjects had an “off” Hoehn and Yahr score, and therefore provides limited information. Motor fluctuations, also known as deterioration at the Copyright © 2012 SciRes. Openly accessible at http://www.scirp.org/journal/apd/  R. Zhang et al. / Advances in Parkinson’s Disease 1 (2012) 5-10 9 end of a dose or wearing-off, consist of fluctuations be- tween “on” and “off” states. They are prevalent in long- term PD patients and appear to be an inevitable outcome of levodopa treatment [33]. In our sample cohort, 54% of subjects had motor fluctuations. This proportion was higher in the group on combination treatment with a COMTI compared to the group treated with an MAOBI (63% vs 44%, p = 0.09) (Table 3). Because motor fluctuations increase with the progres- sion of PD and long-term usage of levodopa, disease duration should be considered a risk factor for these fluctuations. In this study, disease duration was signifi- cantly associated with motor fluctuations with an OR of 1.2 (p < 0.05). This suggests a 20% increase in the risk of motor fluctuations for every 1 year after the diagnosis of the disease. Both COMTI and MAOBI combination regimens with levodopa have been found to reduce “off” time and mo- tor fluctuations [8,14-17,22-24]. Our study showed that COMTI subjects had a higher relative frequency of mo- tor fluctuations with an OR of 2.2 in the single variable model (95% CI: 0.9 to 5.1). This association became significant and stronger (OR = 3.7) after controlling for confounding factors. These results suggest that compared to COMTI combination therapy, MAOBI combination therapy may be better able to control motor fluctuations. This study relied on available data in the movement disorders database, which occasionally lacked specific data points. The current and previous medication use had to be taken into account as a factor over time. In this study all AEs included both side effects from medication and symptoms/signs of the underlying illness; we were therefore unable to specifically link the causality be- tween a specific AE and PD medication. The study design did not allow subjects to be random- ized into medication groups, which would have ensured that they were comparable at baseline with respect to confounding factors. To control for this, we applied mul- tivariable logistic regression models, which incorporated potential confounding factors. 5. CONCLUSION Based on the available data, combination therapy with a COMTI combined with levodopa was more likely to be associated with dementia and motor fluctuations than a MAOBI combined with levodopa in PD patients. Physi- cians pay close attention to side effect profiles when prescribing medications. These study results may impact decision making related to PD medication choice in pa- tients with concomitant dementia or motor fluctuations. Prospective head to head trials would be useful to further assess the significance of these adverse effects in vul- nerable populations. 6. ACKNOWLEDGEMENTS This work did not receive outside financial support. A. D. Hohler wishes to disclose that she is currently on the speaker’s bureau for TEVA pharmaceuticals. The remainder of the authors has no conflicts of interest to disclose. REFERENCES [1] Dewey Jr., R.B. (2004) Management of motor complica- tions in Parkinson’s disease. Neurology, 62, S3-S7. doi:10.1212/WNL.62.6_suppl_4.S3 [2] Hoehn, M.M. and Yahr, M.D. (1967) Parkinsonism: On- set, progression and mortality. Neurology, 17, 427-442. doi:10.1212/WNL.17.5.427 [3] Schapira, A.H. and Jenner, P. (2011) Etiology and patho- genesis of Parkinson’s disease. Movement Disorders, 26, 1049-1055. doi:10.1002/mds.23732 [4] Wirdefeldt, K., Adami, H.O., Cole, P., Trichopoulos, D. and Mandel, J. (2011) Epidemiology and etiology of Parkinson’s disease: A review of the evidence. European Journal of Epidemiology, 26, S1-S58. doi:10.1007/s10654-011-9581-6 [5] Henchcliffe, C. and Beal, M.F. (2007) Pathogenesis: Oxi- dative stress, mitochodrial dysfunction, and excitotoxicity. In: Factor, S.A. and Weiner, W.J., Eds., Parkinson’s Dis- ease Diagnosis & Clinical Management, 2nd Edition, Demos Medical Publishing, New York, 341-356. [6] Jankovic, J. and Stacy, M. (2007) Medical management of levodopa-associated motor complications in patients with Parkinson’s disease. CNS Drugs, 21, 677-692. doi:10.2165/00023210-200721080-00005 [7] Schwid, S.R., Shoulson, I., Stern, M., Oakes, D., Kieburtz, K., Fahn, S., et al. (2005) A randomized placebo-con- trolled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: The PRESTO study. Archives of Neurology, 62, 241-248. doi:10.1001/archneur.62.2.241 [8] Simuni, T., Hurtig, H., Adler, C.H., Rajput, A., Zesiewicz, T.A., Hauser, R.A., et al. (2007) Drugs. In: Factor, S.A. and Weiner, W.J., Eds., Parkinson’s Disease Diagnosis & Clinical Management, Demos Medical Publishing, New York, 471-571. [9] Smith, L.P. (2003) Steady the course of Parkinson’s dis- ease. Nurs Manage, 34, 35-39. doi:10.1097/00006247-200304000-00011 [10] Solla, P., Cannas, A., Marrosu, F. and Marrosu, M.G. (2010) Therapeutic interventions and adjustments in the management of Parkinson disease: Role of combined car- bidopa/levodopa/entacapone (Stalevo). Journal of Neu- ropsychiatric Disease and Treatment, 6, 483-490. doi:10.2147/NDT.S5190 [11] Tarrants, M.L., Denarie, M.F., Castelli-Haley, J., Millard, J. and Zhang, D. (2010) Drug therapies for Parkinson’s disease: A database analysis of patient compliance and persistence. American Journal of Geriatric Pharmaco- therapy, 8, 374-383. doi:10.1016/j.amjopharm.2010.08.001 Copyright © 2012 SciRes. Openly accessible at http://www.scirp.org/journal/apd/  R. Zhang et al. / Advances in Parkinson’s Disease 1 (2012) 5-10 Copyright © 2012 SciRes. http://www.scirp.org/journal/apd/Openly accessible at 10 [12] Gardian, G. and Vecsei, L. (2010) Medical treatment of Parkinson’s disease: Today and the future. International Journal of Clinical Pharmacology, Therapy, 48, 633-642. [13] Caslake, R., Macleod, A., Ives, N., Stowe, R. and Coun- sell, C. (2009) Monoamine oxidase B inhibitors versus other dopaminergic agents in early Parkinson’s disease. Cochrane Database of Systematic Reviews, 4, CD006661. [14] Stowe, R., Ives, N., Clarke, C. E., Deane, K., Wheatley, K., Gray, R., et al. (2010) Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Park- inson s disease patients with motor complications. Coch- rane Database of Systematic Reviews, 7, CD007166. [15] Myllyla, V.V., Sotaniemi, K.A., Vuorinen, J.A. and Hei- nonen, E.H. (1993) Selegiline in de novo parkinsonian patients: The Finnish study. Movement Disorders, 8, S41- S44. doi:10.1002/mds.870080509 [16] Parkinson Study Group (2005) A randomized placebo- controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: The PRESTO study. Archives of Neurology, 62, 241-248. doi:10.1001/archneur.62.2.241 [17] Sivertsen, B., Dupont, E., Mikkelsen, B., Mogensen, P., Rasmussen, C., Boesen, F., et al. (1989) Selegiline and levodopa in early or moderately advanced Parkinson’s disease: A double-blind controlled short- and long-term study. Acta Neurologica Scandinavica, Supplementum, 126, 147-152. doi:10.1111/j.1600-0404.1989.tb01794.x [18] Boyson, S.J. (1991) Psychiatric effects of selegiline. Ar- chives of Neurology, 48, 902. doi:10.1001/archneur.1991.00530210028017 [19] Kurlan, R and Dimitsopulos, T. (1992) Selegiline and manic behavior in Parkinson’s disease. Archives of Neu- rology, 49, 1231. doi:10.1001/archneur.1992.00530360029012 [20] Talati, R., Reinhart, K., Baker, W., White, C.M. and Coleman, C.I. (2009) Pharmacologic treatment of ad- vanced Parkinson’s disease: A meta-analysis of COMT inhibitors and MAO-B inhibitors. Parkinsonism & Re- lated Disorders, 15, 500-505. doi:10.1016/j.parkreldis.2008.12.007 [21] Kurth, M.C. and Adler, C.H. (1998) COMT inhibition: A new treatment strategy for Parkinson’s disease. Neurology, 50, S3-S14. doi:10.1212/WNL.50.5_Suppl_5.S3 [22] Adler, C.H., Singer, C., O’Brien, C., Hauser, R.A., Lew, M.F., Marek, K.L., et al. (1998) Randomized, placebo- controlled study of tolcapone in patients with fluctuating Parkinson disease treated with levodopa-carbidopa. Tol- capone Fluctuator Study Group III. Archives of Neurology, 55, 1089-1095. doi:10.1001/archneur.55.8.1089 [23] Brooks, D.J., Leinonen, M., Kuoppamaki, M. and Nissi- nen, H. (2008) Five-year efficacy and safety of levodopa/ DDCI and entacapone in patients with Parkinson’s dis- ease. Journal of Neural Transmission, 115, 843-849. doi:10.1007/s00702-008-0025-8 [24] Kurth, M.C., Adler, C.H., Saint-Hilaire, M., Singer, C., Waters, C., LeWitt, P., et al. (1997) Tolcapone improves motor function and reduces levodopa requirement in pa- tients with Parkinson’s disease experiencing motor fluc- tuations: A multicenter, double-blind, randomized, pla- cebo-controlled trial. Tolcapone Fluctuator Study Group I. Neurology, 48, 81-87. doi:10.1212/WNL.48.1.81 [25] Parkinson Study Group (1997) Entacapone improves motor fluctuations in levodopa-treated Parkinson’s dis- ease patients. Parkinson Study Group. Annals of Neurol- ogy, 42, 747-755. [26] Rajput, A.H., Martin, W., Saint-Hilaire, M.H., Dorflinger, E. and Pedder, S. (1997) Tolcapone improves motor func- tion in parkinsonian patients with the “wearing-off” phe- nomenon: A double-blind, placebo-controlled, multicenter trial. Neurology, 49, 1066-1071. doi:10.1212/WNL.49.4.1066 [27] Rascol, O., Brooks, D.J., Melamed, E., Oertel, W., Poewe, W., Stocchi, F., et al. (2005) Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): A randomised, double-blind, parallel-group trial. Lancet, 365, 947-954. doi:10.1016/S0140-6736(05)71083-7 [28] Hemming, J.P., Gruber-Baldini, A.L., Anderson, K.E., Fishman, P.S., Reich, S.G., Weiner, W.J., et al. (2011) Ra- cial and socioeconomic disparities in parkinsonism. Ar- chives of Neurology, 68, 498-503. doi:10.1001/archneurol.2010.326 [29] Van Den Eeden, S.K., Tanner, C.M., Bernstein, A.L., Fross, R.D., Leimpeter, A., Bloch, D.A., et al. (2003) In- cidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. American Journal of Epidemiology, 157, 1015-1022. doi:10.1093/aje/kwg068 [30] Marder, K.S. and Jacobs, D.M. (2007) Dementia. In: Factor, S.A. and Weiner, W.J., Eds., Parkinson’s Disease Diagnosis & Clinical Management, Demos Medical Pub- lishing, New York. [31] Aarsland, D., Andersen, K., Larsen, J.P., Lolk, A., Nielsen, H.and Kragh-Sorensen, P. (2001) Risk of dementia in Parkinson’s disease: A community-based, prospective study. Neurology, 56, 730-736. doi:10.1212/WNL.56.6.730 [32] Hughes, T.A., Ross, H.F., Musa, S., Bhattacherjee, S., Nathan, R.N., Mindham, R.H., et al. (2000) A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurology, 54, 1596-1602. [33] Gunzler, S.A. and Nutt, J.G. (2007) Motor fluctuations and dyskinesia. In: Factor, S.A. and Weiner, W.J., Eds., Parkinson’s Disease Diagnosis & Clinical Management, Demos Medical Publishing, New York.
|