Visual Allesthesia in a Patient with Glioblastoma Multiforme 101
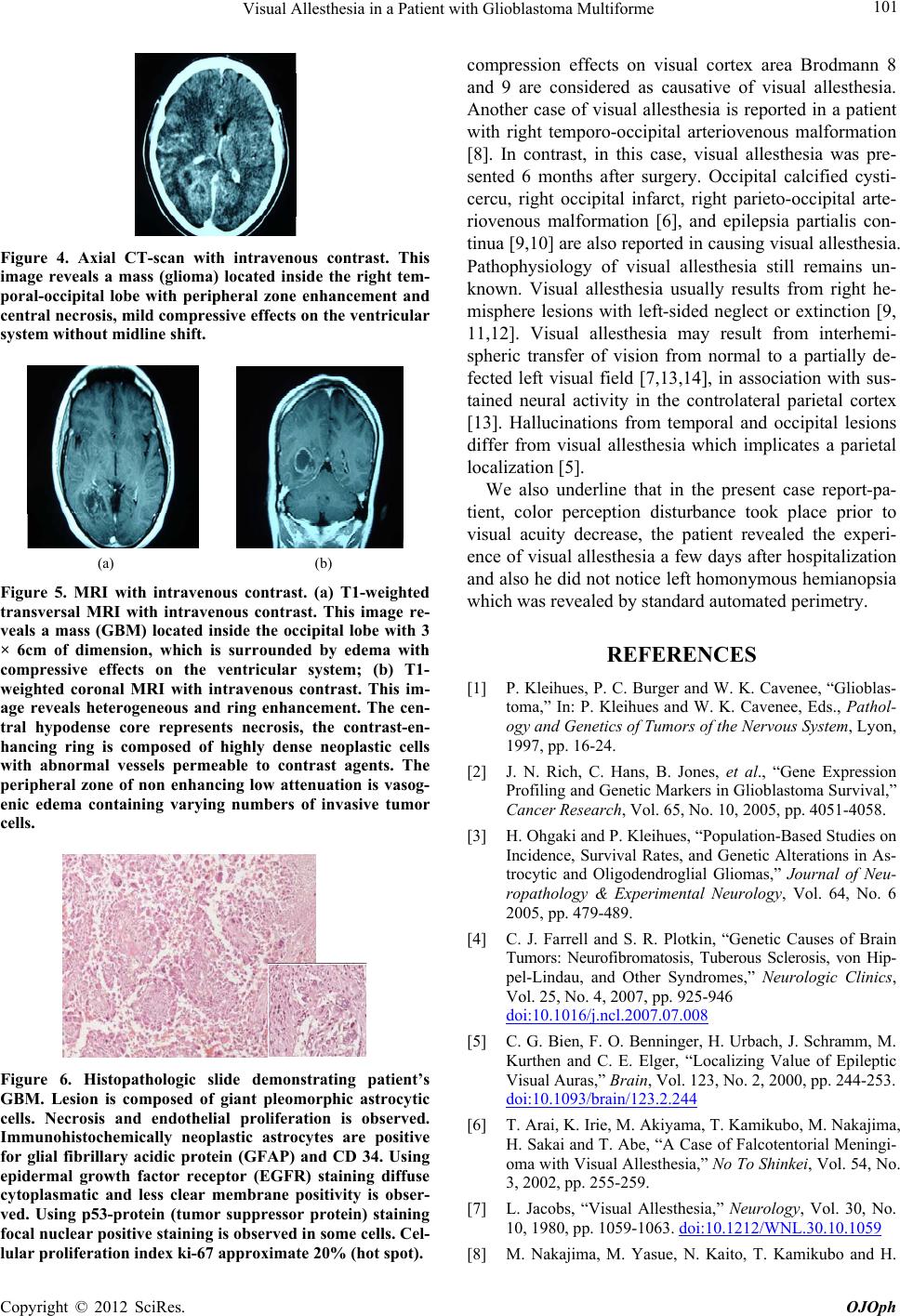
Figure 4. Axial CT-scan with intravenous contrast. This
image reveals a mass (glioma) located inside the right tem-
poral-occipital lobe with peripheral zone enhancement and
central necrosis, mild compressive effects on the ventricular
system without midline shift.
(a) (b)
Figure 5. MRI with intravenous contrast. (a) T1-weighted
transversal MRI with intravenous contrast. This image re-
veals a mass (GBM) located inside the occipital lobe with 3
× 6cm of dimension, which is surrounded by edema with
compressive effects on the ventricular system; (b) T1-
weighted coronal MRI with intravenous contrast. This im-
age reveals heterogeneous and ring enhancement. The cen-
tral hypodense core represents necrosis, the contrast-en-
hancing ring is composed of highly dense neoplastic cells
with abnormal vessels permeable to contrast agents. The
peripheral zone of non enhancing low attenuation is vasog-
enic edema containing varying numbers of invasive tumor
cells.
Figure 6. Histopathologic slide demonstrating patient’s
GBM. Lesion is composed of giant pleomorphic astrocytic
cells. Necrosis and endothelial proliferation is observed.
Immunohistochemically neoplastic astrocytes are positive
for glial fibrillary acidic protein (GFAP) and CD 34. Using
epidermal growth factor receptor (EGFR) staining diffuse
cytoplasmatic and less clear membrane positivity is obser-
ved. Using p53-protein (tumor suppressor protein) staining
focal nuclear positive staining is observed in some cells. Cel-
lular proliferation index ki-67 approximate 20% (hot spot).
compression effects on visual cortex area Brodmann 8
and 9 are considered as causative of visual allesthesia.
Another case of visual allesthesia is reported in a patient
with right temporo-occipital arteriovenous malformation
[8]. In contrast, in this case, visual allesthesia was pre-
sented 6 months after surgery. Occipital calcified cysti-
cercu, right occipital infarct, right parieto-occipital arte-
riovenous malformation [6], and epilepsia partialis con-
tinua [9,10] are also reported in causing visual allesthesia.
Pathophysiology of visual allesthesia still remains un-
known. Visual allesthesia usually results from right he-
misphere lesions with left-sided neglect or extinction [9,
11,12]. Visual allesthesia may result from interhemi-
spheric transfer of vision from normal to a partially de-
fected left visual field [7,13,14], in association with sus-
tained neural activity in the controlateral parietal cortex
[13]. Hallucinations from temporal and occipital lesions
differ from visual allesthesia which implicates a parietal
localization [5].
We also underline that in the present case report-pa-
tient, color perception disturbance took place prior to
visual acuity decrease, the patient revealed the experi-
ence of visual allesthesia a few days after hospitalization
and also he did not notice left homonymous hemianopsia
which was revealed by standard automated perimetry.
REFERENCES
[1] P. Kleihues, P. C. Burger and W. K. Cavenee, “Glioblas-
toma,” In: P. Kleihues and W. K. Cavenee, Eds., Pathol-
ogy and Genetics of Tumors of the Nervous System, Lyon,
1997, pp. 16-24.
[2] J. N. Rich, C. Hans, B. Jones, et al., “Gene Expression
Profiling and Genetic Markers in Glioblastoma Survival,”
Cancer Research, Vol. 65, No. 10, 2005, pp. 4051-4058.
[3] H. Ohgaki and P. Kleihues, “Population-Based Studies on
Incidence, Survival Rates, and Genetic Alt erations in As-
trocytic and Oligodendroglial Gliomas,” Journal of Neu-
ropathology & Experimental Neurology, Vol. 64, No. 6
2005, pp. 479-489.
[4] C. J. Farrell and S. R. Plotkin, “Genetic Causes of Brain
Tumors: Neurofibromatosis, Tuberous Sclerosis, von Hip-
pel-Lindau, and Other Syndromes,” Neurologic Clinics,
Vol. 25, No. 4, 2007, pp. 925-946
doi:10.1016/j.ncl.2007.07.008
[5] C. G. Bien, F. O. Benninger, H. Urbach, J. Schramm, M.
Kurthen and C. E. Elger, “Localizing Value of Epileptic
Visual Auras,” Brain, Vol. 123, No. 2, 2000, pp. 244-253.
doi:10.1093/brain/123.2.244
[6] T. Arai, K. Irie, M. Akiyama, T. Kamikubo, M. Nakajima,
H. Sakai and T. Abe, “A Case of Falcotentorial Meningi-
oma with Visual Allesthesia,” No To Shinkei, Vol. 54, No.
3, 2002, pp. 255-259.
[7] L. Jacobs, “Visual Allesthesia,” Neurology, Vol. 30, No.
10, 1980, pp. 1059-1063. doi:10.1212/WNL.30.10.1059
[8] M. Nakajima, M. Yasue, N. Kaito, T. Kamikubo and H.
Copyright © 2012 SciRes. OJOph