Paper Menu >>
Journal Menu >>
 Journal of Minerals and Materials Characterization and Engineering, 2012, 11, 769-773 Published Online August 2012 (http://www.SciRP.org/journal/jmmce) The Influence of Benzophenone Substitution on the Physico-Chemical Characterizations of 8-HydroxyQuinoline NLO Single Crystals M. J. Jarald Brigit Gilda1, S. Anbarasu2, Y. Samson3, Prem Anand Devarajan2* 1Department of Physics, Ponjesly College of Engineering, Alamparai, India 2Deparment of Physics, St. Xavier’s College, Palayamkottai, India 3Departmentof Physics, Annai Velankanni College, Tholayavattam, India Email: *d.prenanand2010@gmail.com Received February 12, 2012; revised March 20, 2012; accepted April 7, 2012 ABSTRACT Single crystals of 8-HydroxyQuinoline (8-HQ) and Benzophenone substituted 8-HydroxyQuinoline (B8-HQ) are grown by slow evaporation of acetone at room temperature. Coloured crystals of 8-HQ and B8-HQ with good optical quality of dimensions 54 × 3 × 1.5 mm3 and 27 × 3 × 1 mm3 are harvested. Single crystal X-ray diffractometer was utilized to measure the unit cell parameters and to confirm the crystal structure. The presence of various functional groups in the molecule was ascertained by FTIR spectral analysis. The cut off wavelength of 8-HQ and B8-HQ was centered at 350 and 356 nm. The functional groups in the molecule are elucidated by 1H and 13C-NMR spectral analyses. Kurtz Perry test confirms the SHG in8-HQ andB8-HQ single crystals. Keywords: Crystal Growth; X-Ray Diffraction (XRD); FTIR; UV-Vis-NIR; 1H and 13C-NMRe 1. Introduction Nonlinear optical materials (NLO) have wide applica- tions in the area of laser technology, optical communica- tion and in storage devices. The high non linearity makes it possible for organic crystals to double the frequency of Ga-Al-As diode lasers for generating blue light which is an important coherent source [1]. One of the advantages in working with organic materials is that they allow one to fine-tune the chemical structures and properties for the desired nonlinear optical properties. In addition, organic crystals have large optical susceptibility, inherent ul- trafast response and high optical threshold for laser power as compared with inorganic materials. Moreover, their lower cutoff wavelength and a wide transparency window in the visible region makes the candidate mate- rials subject for extensive investigation [2]. In this context 8-HydroxyQuinoline (8-HQ) has been identified as an important material for NLO applications in the visible and ultraviolet regions. M. Rajasekaran has reported the growth of 8-HQ single crystal by slow evaporation technique using chloroform as a solvent. The cut off wavelength is below 400 nm [3]. Recently K. Aravinth has reported that the cut off wavelength of 8-HQ is around 390 nm and the lattice parameters are a = 3.85 Å, b = 24.93 Å, c = 28.72 Å, V = 2754 Å. The grown 8-HQ crystal belongs to orthorhombic system with non-centro-symmetric space group Fdd2 [4]. Elena M. Filip et al. have performed quantum chemical semi empirical PM3 calculations optimization of 8-Hydroxy- Quinoline (8-HQ) structure, dipolemoment, polarizability and other physical parameters [5]. The sonochemical fabrication of 8-HydroxyQuinoline (8-HQ) aluminium (Alq3) nanoflowers with high electro generated chemiluminescence was reported by Chang-Jie Mao et al. [6]. Due to the continuation of interest in the supramolecular arrangements, a new crystal structure of hydrated 8-HydroxyQuinoline chloride was synthesized from a reaction mixture consisting of 2-chloro nicotinoyl chloride and 8-HydroxyQuinoline (8-HQ) [7]. Addition- ally the tunable photoluminescence from tris (8-HQ) al- uminium (Alq3) blended thin films of Alq3 embedded in poly methyl methacrylate matrix at different concentra- tions are also investigated by J. G. Mahakhode et al. [8]. The interaction between donor 8-HQ and π acceptor P-Nitrophenol has been investigated spectroscopically. It was shown by 1H-NMR that 8-HQ with π PNP forms complex of 1:1 stoichiometry. Infrared spectral data in- dicates a charge transfer interaction between donor and acceptor due to n – π* transitions by the formation of radical ion pairs [9]. *Corresponding author. Copyright © 2012 SciRes. JMMCE  M. J. J. B. GILDA ET AL. 770 In this paper, we report the results of our work on the influence of physico chemical characterization studies of benzophenone substituted 8-HydroxyQuinoline (B8-HQ) NLO crystals along with characterization by single crys- tal X-ray diffraction (XRD), Fourier transform infrared (FTIR), UV-Visible-NIR and 1H & 13C-Nuclear magnetic resonance (NMR) spectral measurements and NLO test for the first time. 2. Experimental Details The starting materials are commercially available (E- Merck). The solvent evaporation technique was used to grow single crystals of 8-HQ. The corresponding 8-HQ salt was dissolved in acetone to prepare a saturated solu- tion and the solution was filtered using Whatmann filter paper. The filtered solution was then taken in a beaker which was hermetically sealed to avoid the evaporation of the solvent. Optically defect free good quality single crystals of 8-HQ with reasonable dimensions were har- vested in a month. Benzophenone (5 M) was substituted with the solution containing 8-HQ and acetone. The concentration of the growth solution was fixed at 3 M. Within a month, seed crystals are formed due to sponta- neous nucleation. In the present work, good optical qual- ity crystals of pure 8-HQ and Benzophenone substituted 8-HQ crystals (B8-HQ) are harvested. Figures 1 and 2 show the photographs of asgrown pure 8-HQ and B8-HQ crystals. 3. Single Crystal XRD The X-ray diffraction data was collected using an auto- matic X-ray diffractometer, EnrafNonius CAD 4-MV 31 Bruker kappa APE XIII. The structure was solved by the direct method using SHELXL program. It is observed from the X-ray diffraction data that both pure 8-HQ and Figure 1. Photograph of as grown pure 8-HQ single crystal. Figure 2. Photograph of as grown B8-HQ single crystal. Bf Fdd2. The calculated lattice parameter values of pure 8-HQ and B8-HQ are presented in Table 1. The results of the present work are in good agreement with the re- ported values [4]. It is evident from Table 1, that the doped sample has a slight variation in the cell volume. The variation in the cell volume may be due to the in- corporation of Benzophenone into 8-HQ crystal lattice. 4. Ftir Spectral Analysis The FTIR spectroscopy study is effectively used to iden- tify the functional groups present in the crystal and to determine the molecular structure. In order to analyze qualitatively the presence of the functional groups in 8-HQ and B8-HQ, FTIR was recorded using the Perkin Elmer Model spectrum-100 in the range 450 - 4000 cm–1 and the corresponding spectra obtained are shown in Figures 3 and 4 respectively. The FTIR spectrum of pure 8-HQ is presented in Figure 5. The aromatic C-H stretching appears at 3029 cm–1. The band assigned at 1576 cm–1 is attributed to C=C ring stretching vibrations. The C=N stretching vibrations appears at the region of 1694 cm–1. The peak at 3743 cm–1 is designated to O-H stretching. The peaks at 1271 and 1222 cm–1 are assigned to C-O stretching vibration. The aromatic C-H plane bending appears at 1163 cm–1. The C-H out of plane bending oc- curs at 707 and 738 cm–1. The peak at 1433 cm–1 is as- signed to O-H plane bending. The spectrum of B8-HQ is shown in Figure 4. The spectrum carries nearly similar features of pure 8-HQ spectra. An additional peak at 3057 cm–1 is observed which is due to the C=O stretch- ing of Benzophenone. An interesting observation to be mentioned here is all the peaks of Figure 4 are shifted to a higher frequencies of 1 cm–1, and hence it can be stated that there is harmonious existence of dopant Benzophe- none into the crystal lattice of 8-HQ. Higher resolution of peaks and enhancement of the bands between the region 3400 - 1500 cm–1 are also observed. Table 1. XRD data for 8-HQ and B8-HQ single crystal. Data 8-HQ B8-HQ 8-HQ crystals are orthorhombic with a space group o a (Å) 3.84 3.85 b (Å) 24.97 24.98 c (Å) 28.68 28.71 α˚ 90˚ 90˚ β˚ 90˚ 90˚ γ˚ 90˚ 90˚ Crystal system Orthorhombic Orthorhombic Volume (Å) 2749.97 2761.12 Space group Fdd2 Fdd2 Copyright © 2012 SciRes. JMMCE 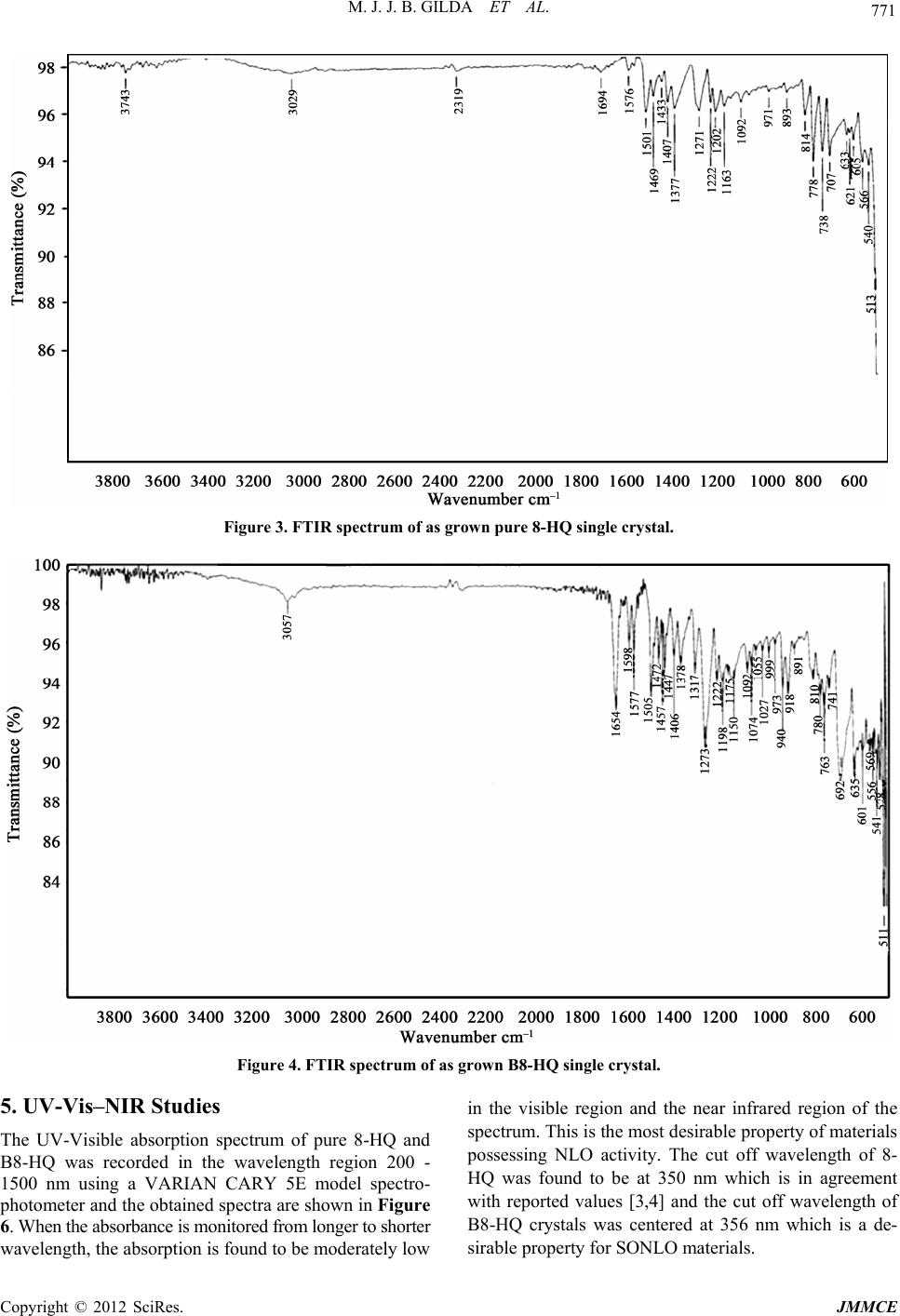 M. J. J. B. GILDA ET AL. Copyright © 2012 SciRes. JMMCE 771 grown pure 8-HQ single crystFigure 3. FTIR spectrum of as al. Figure 4. FTIR spectrum of as grown B8-HQ single crystal. 5. UV-Vis–NIR Studies The UV-Visible absorption s 8-HQ was recorded in the wavelength region 200 - 1500 nm using a VARIAN CARY 5E model spectro- photometer and the obtained spectra are shown in Figure 6. When the absorbance is monitored from longer to shorter wavelength, the absorption is found to be moderately low in the visible region and the near infrared region of th sirable property of materials t off wavelength of 8- HQ was found to be at 350 nm which is in agreement with reported values [3,4] and the cut off wavelength of B8-HQ crystals was centered at 356 nm which is a de- sirable property for SONLO materials. e pectrum of pure 8-HQ and spectrum. This is the most de possessing NLO activity. The cu B 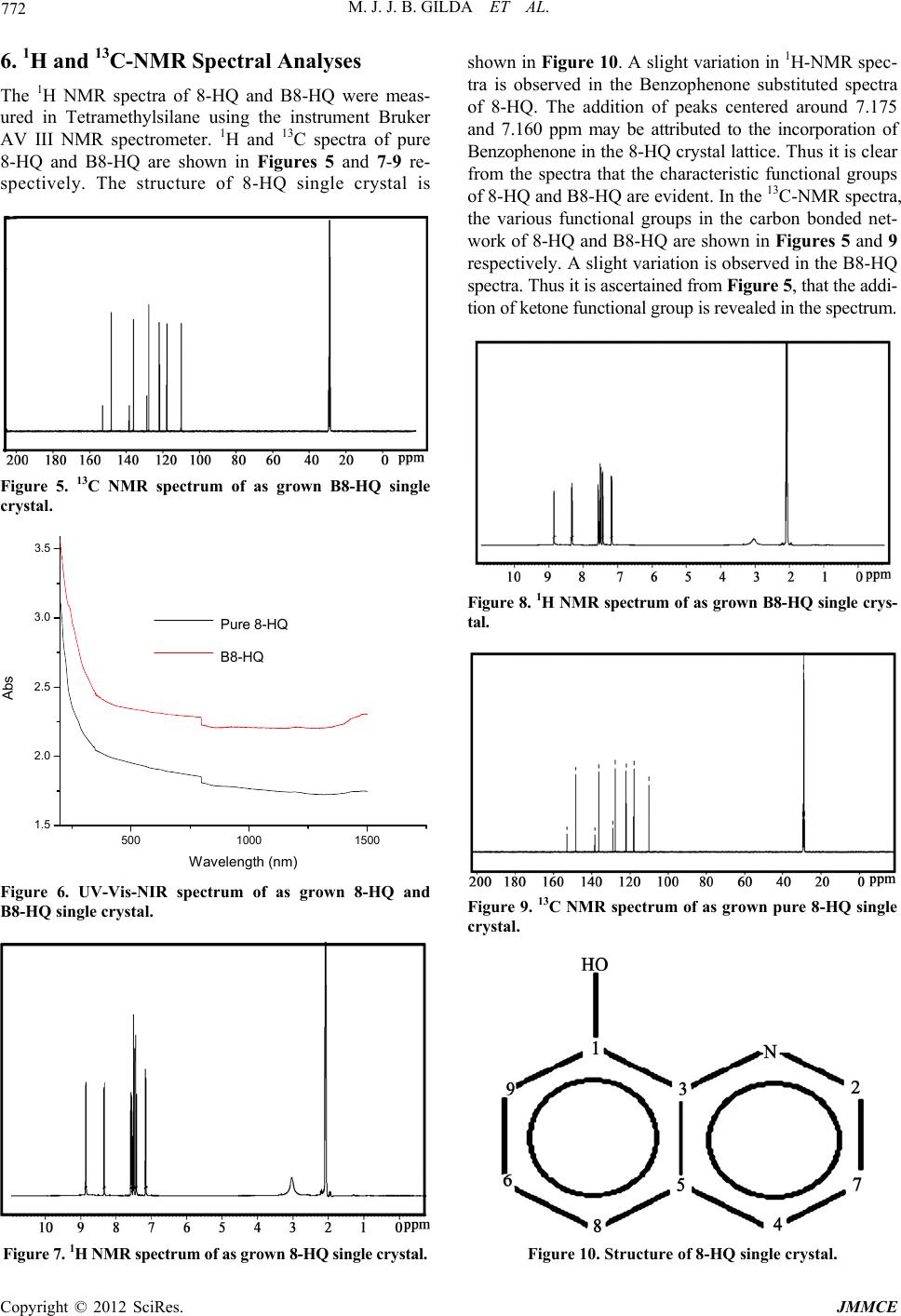 M. J. J. B. GILDA ET AL. 772 6. 1H and 13C-NMR Spectral Analyses The 1H NMR spectra of 8-HQ and B8-HQ were meas- ured in Tetramethylsilane using the instrument Bruker AV III NMR spectrometer. 1H and 13C spectra of pure 8-HQ and B8-HQ are shown in Figures 5 and 7-9 re- spectively. The structure of 8-HQ single crystal is B8-HQ single 1500 3.5 Figure 5. 13C NMR spectrum of as grown crystal. 500 1000 1.5 2.0 2.5 3.0 Abs Wavelength (nm) Pure 8-HQ B B8-HQ C Figure 6. UV-Vis-NIR spectrum of as grown 8-HQ and B8-HQ single crystal. n 8-HQ single crystal. shown in Figure 10. A slight variation in 1H-NMR spec- tra is observed in the Benzophenone substituted spectra of 8-HQ. The addition of peaks centered around 7.175 and 7.160 ppm may be attributed to the incorporation of Benzophenone in the 8-HQ crystal lattice. Thus it is clear from the spectra that the characteristic functional groups of 8-HQ and B8-HQ are evident. In the 13C-NMR spectra, the various functional groups in the carbon bonded net- work of 8-HQ and B8-HQ are shown in Figures 5 and 9 respectively. A slight variation is observed in the B8-HQ spectra. Thus it is ascertained from Figure 5, that the addi- tion of ketone functional group is revealed in the spectrum. Figure 7. 1H NMR spectrum of as grow Figure 8. 1H NMR spectrum of as grown B8-HQ single crys- tal. Figure 9. 13C NMR spectrum of as grown pure 8-HQ single crystal. Figure 10. Structure of 8-HQ single crystal. Copyright © 2012 SciRes. JMMCE  M. J. J. B. GILDA ET AL. Copyright © 2012 SciRes. JMMCE 773 . NLO Test The NLO property of pure 8-HQ and B8-HQ were measured by Kurtz Perry’s NLO test. ANd-YAG laser beam of wavelength 1064 nm of pulse width 8ns and repetition rate of 10 Hz was made to incident on the sample. Second harmonic radiation generated by 8-HQ and B8- HQ were focused by a lens and collected by a photomul- tiplier tube. In our study, KDP was taken as a reference crystal. The output power intensity of pure 8-HQ was found to be 0.75 times that of KDP [4] and B8-HQ was found to be 0.85 times that of KDP single crystal. 8. Conclusion Single crystals of pure 8-HQ and B8-HQ were grown successfully by slow evaporation technique at room onth. The non-Centro-symmetric nature of the crystals was confirmed by XRD. The various functional groups assigned to 8-HQ and B8-HQ were confirmed by FTIR spectral analysis. The UV-Vis-NIR studies show that the cut off wavelength of 8-HQ is at 350 nm and that of B8- HQ is at 356 nm. The protons and the carbon bonded network in these samples were elucidated by 1H and 13C- NMR spectral analysis. Studies pertaining to TG/DTA, SEM, Dielectric and Photoconductivity are in progress. 9. Acknowledgements One of the authors (M. J. Jarald Brigit Gilda) is pleased to acknowledge Prof. I. Sebasdiyar, Head of the depart- ment of physics, St. Xavier’s college, Palayamkottai and (ASN),” Rasayan Journal of Chemistry, Vol. 1, No. 4, 2008, pp. 782-787. [2] C. Sekar and R. Parimala Devi, “Effect of Silver Nitrate (AgNO3) on the Growth, Optical, Spectral, Thermal and Mechanical Properties of γ-Glycine Single Crystal,” Jour- nal of Optoelectronics and Biomedical Materials, Vol. 1, No. 2, 2009, pp. 215-225. [3] M. Rajasekaran, P. Anbusrinivasan and S. C. Mojumdar, “Growth, Spectral and Thermal Characterization of 8-HydroxyQuinoline,” Journal of Thermal Analysis and Calorimetry, Vol. 100, No. 3, 2010, pp. 827-830. doi10.1007/s10973-010-0761-5 7 temperature. Defect free, optically transparent single crystals of 8-HQ and B8-HQ were harvested within a m Dr. Maniysundar, Principal, Ponjesly College of Engineer- ing, for their constant help, support and encouragement. REFERENCES [1] S. Palanisamy and O. N. Balasundaram, “Growth, Optical and Mechanical Properties of Alanine Sodium Nitrate “Growth of <201> 8-HydroxyQuinoline Organic Crystal by Czochralski Method and Its Characterizations,” Jour- nal of Thermal Analysis and Calorimetry, 2011, 7 p. [5] E. M. Filip, I. V. Humelnicu and C. I. Ghirve, “Some Aspects of 8-HydroxyQuinoline in Solvents,” Acta Chem- ica Iasi, Vol. 17, 2009, pp. 85-96. [6] C.-J. Mao, D.-C. Wang, H.-C. Pan and J.-J. Zhu, “Sono- chemical Fabrication of 8-HydroxyQuinoline Aluminum (Alq3) Nanoflowers with High Electrogenerated Chem- iluminescence,” Ultrasonics Sonochemistry, Vol. 18, No. 2, 2011, pp. 473-476. [7] J. M. S. Skakle, J. L. Wardell and S. M. S. V. Wardell, “Formation of Ladders from R44(8) and R66(12) Ring in 8-Hydroxyquinoliniumchloride Monohydrate: Com- d Salts,” Acta Crystallographica Section C Crystal Structure Communications, Vol. 62, 2006, pp. 312-314. [8] J. G. Mahakhode, B. M. Bahirwar, S. J. Dhoble and S. V. Moharil, “Tunable Photoluminescence from Tris (8-Hy- droxyquinoline) Aluminum (Alq3),” Proceedings of Asian Symposium on Information Display, New Delhi, 8-12 October 2006, pp. 237-239. [9] I. M. Khan, N. Singh and A. Ahmad, “Spectroscopic Studies of Multiple Charge Transfer Complexes of 8-HydroxyQuinoline with π-Acceptor P-Nitrophenol in Different Solvents at Room Temperature,” Canadian Journal of Analytical sciences and Spectroscopy, Vol. 54, No. 1, 2009, pp. 31-37. [4] K. Aravinth, G. Anandha Babu and P. Ramasamy, s parisons with the Supramolecular Arrangements in Re- late |

