A. Zeb et al. / Natural Science 2 (2010) 626-630
Copyright © 2010 SciRes. OPEN ACCESS
630
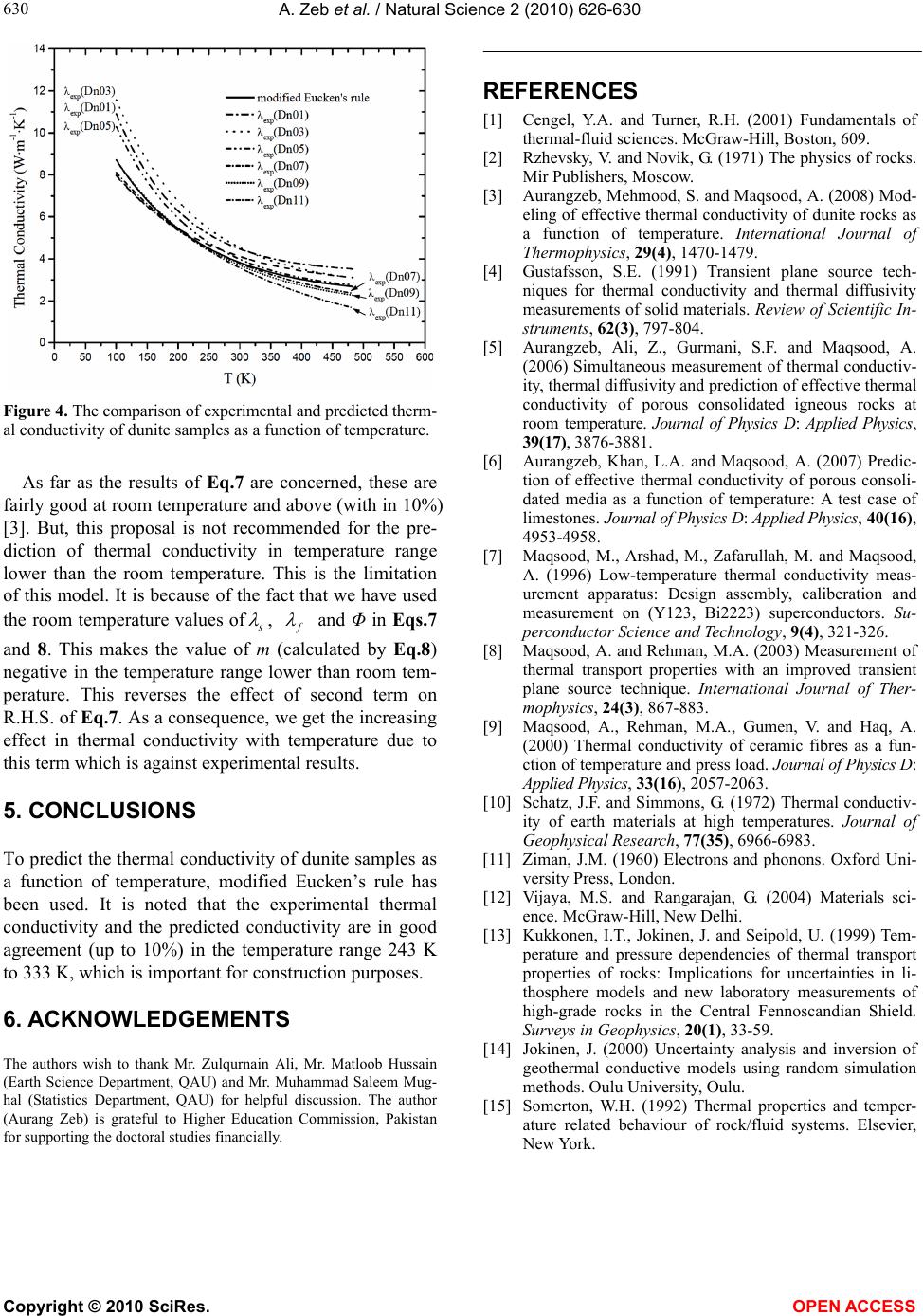
Figure 4. The comparison of experimental and predicted therm-
al conductivity of dunite samples as a function of temperature.
As far as the results of Eq.7 are concerned, these are
fairly good at room temperature and above (with in 10%)
[3]. But, this proposal is not recommended for the pre-
diction of thermal conductivity in temperature range
lower than the room temperature. This is the limitation
of this model. It is because of the fact that we have used
the room temperature values ofs
, f
and Φ in Eqs.7
and 8. This makes the value of m (calculated by Eq.8)
negative in the temperature range lower than room tem-
perature. This reverses the effect of second term on
R.H.S. of Eq.7. As a consequence, we get the increasing
effect in thermal conductivity with temperature due to
this term which is against experimental results.
5. CONCLUSIONS
To predict the thermal conductivity of dunite samples as
a function of temperature, modified Eucken’s rule has
been used. It is noted that the experimental thermal
conductivity and the predicted conductivity are in good
agreement (up to 10%) in the temperature range 243 K
to 333 K, which is important for construction purposes.
6. ACKNOWLEDGEMENTS
The authors wish to thank Mr. Zulqurnain Ali, Mr. Matloob Hussain
(Earth Science Department, QAU) and Mr. Muhammad Saleem Mug-
hal (Statistics Department, QAU) for helpful discussion. The author
(Aurang Zeb) is grateful to Higher Education Commission, Pakistan
for supporting the doctoral studies financially.
REFERENCES
[1] Cengel, Y.A. and Turner, R.H. (2001) Fundamentals of
thermal-fluid sciences. McGraw-Hill, Boston, 609.
[2] Rzhevsky, V. and Novik, G. (1971) The physics of rocks.
Mir Publishers, Moscow.
[3] Aurangzeb, Mehmood, S. and Maqsood, A. (2008) Mod-
eling of effective thermal conductivity of dunite rocks as
a function of temperature. International Journal of
Thermophysics, 29(4), 1470-1479.
[4] Gustafsson, S.E. (1991) Transient plane source tech-
niques for thermal conductivity and thermal diffusivity
measurements of solid materials. Review of Scientific In-
struments, 62(3), 797-804.
[5] Aurangzeb, Ali, Z., Gurmani, S.F. and Maqsood, A.
(2006) Simultaneous measurement of thermal conductiv-
ity, thermal diffusivity and prediction of effective thermal
conductivity of porous consolidated igneous rocks at
room temperature. Journal of Physics D: Applied Physics,
39(17), 3876-3881.
[6] Aurangzeb, Khan, L.A. and Maqsood, A. (2007) Predic-
tion of effective thermal conductivity of porous consoli-
dated media as a function of temperature: A test case of
limestones. Journal of Physics D: Applied Physics, 40(16),
4953-4958.
[7] Maqsood, M., Arshad, M., Zafarullah, M. and Maqsood,
A. (1996) Low-temperature thermal conductivity meas-
urement apparatus: Design assembly, caliberation and
measurement on (Y123, Bi2223) superconductors. Su-
perconductor Science and Technology, 9(4), 321-326.
[8] Maqsood, A. and Rehman, M.A. (2003) Measurement of
thermal transport properties with an improved transient
plane source technique. International Journal of Ther-
mophysics, 24(3), 867-883.
[9] Maqsood, A., Rehman, M.A., Gumen, V. and Haq, A.
(2000) Thermal conductivity of ceramic fibres as a fun-
ction of temperature and press load. Journal of Physics D:
Applied Physics, 33(16), 2057-2063.
[10] Schatz, J.F. and Simmons, G. (1972) Thermal conductiv-
ity of earth materials at high temperatures. Journal of
Geophysical Research, 77(35), 6966-6983.
[11] Ziman, J.M. (1960) Electrons and phonons. Oxford Uni-
versity Press, London.
[12] Vijaya, M.S. and Rangarajan, G. (2004) Materials sci-
ence. McGraw-Hill, New Delhi.
[13] Kukkonen, I.T., Jokinen, J. and Seipold, U. (1999) Tem-
perature and pressure dependencies of thermal transport
properties of rocks: Implications for uncertainties in li-
thosphere models and new laboratory measurements of
high-grade rocks in the Central Fennoscandian Shield.
Surveys in Geophysics, 20(1), 33-59.
[14] Jokinen, J. (2000) Uncertainty analysis and inversion of
geothermal conductive models using random simulation
methods. Oulu University, Oulu.
[15] Somerton, W.H. (1992) Thermal properties and temper-
ature related behaviour of rock/fluid systems. Elsevier,
New York.