Paper Menu >>
Journal Menu >>
 Vol.2, No.6, 609-612 (2010) Health doi:10.4236/health.2010.26090 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Chondrocyte viability depends on the preservative solution Krzysztof Gawęda1*, Marta Tarczyńska1, Ewa Olender2, Izabela Uhrynowska-Tyszkiewicz2,3, Artur Kamiński2,3 1Department of Orthopaedics and Traumatology, Medical University of Lublin, Lublin, Poland; *Corresponding Author: krzylub@o2.pl 2National Centre for Tissue and Cell Banking, Warsaw, Poland 3Department of Transplantology and Central Tissue Bank, Centre of Biostructure Research, Medical University of Warsaw, Warsaw, Poland Received 11 February 2010; revised 15 March 2010; accepted 18 March 2010. ABSTRACT Fresh osteochondral grafts find broad applica- tion in the treatment of extensive and focal damages of joint surfaces. The maintenance of chondrocyte viability of the collected grafts is of key importance. Aim: The evaluation of chon- drocyte survivability in a stable temperature of 40C in different preservative solutions is the aim of this work. Method: Chondrocyte survivability has been evaluated in saline solution (group I), Ringer solution (group II), saline with an addi- tion of hyaluronic acid (group III) and saline en- riched with glucosamine sulphate (group IV). The amount of live chondrocytes was examined on the day of collection and subsequently after 1, 2, 3,6,12, and 21 days using the Promega MTT test. Results: The highest number of live chon- drocytes as calculated for 1g of hyaline carti- lage after 21 days was ascertained in group IV (saline with glucosamine sulphate). The lowest number of live chondral cells was observed in group II (saline with hyaluronic acid). Chon- drocyte survivability in saline (group I) was higher than in the Ringer solution (group II). Conclusions: The enrichment of saline solution with glucosamine sulphate protracts the viabil- ity of chondrocytes in fresh osteochondral gra- fts prepared for chondral transplantation. Keywords: Chondrocyte; Preservative Solution; Osteochondral Grafts; Fresh Grafts 1. INTRODUCTION The use of fresh allogeneic osteochondral grafts to repair extensive damage to joint surfaces spans more than 100 years [1]. Grafts of many different types and sizes have been used, from various cylindrical osteochondral grafts with radii ranging from several millimeters to larger than 1 cm, through shell grafts, to the transplantation of whole joint surfaces of tibial or femoral condyles [2]. The size of fresh allogeneic osteochondral grafts not- withstanding, the determination and maintenance of safe conditions for the donor joint surfaces remains an ongo- ing issue. It is crucial to maintain strict sanitary stan- dards for grafts until their implantation. Although rules regarding such standards are determined by laws, and thus differ by country, it is important for hospitals to organize a specialized transplant service with procedural guidelines, which will mitigate difficulties encountered while performing this repair method. The tasks of such a service should include recruiting donors and preparing potential recipients for transplantation. Efficient use of fresh allogeneic grafts of joint surfaces is essential be- cause the chondrocytes of transplanted cartilage atrophy rapidly. Tissue survival can be extended by cooling col- lected fragments. However, freezing typically destroys live chondral cells and disintegrates the intercellular matrix structure [3]. Thus, maintaining the viability of collected chondrocytes is a key issue to be addressed at centers that use this method of joint cartilage repair. Sig- nificant chondrocyte survival can be maintained for only 1-2 weeks by preserving osteochondral grafts in normal saline with antibiotics, buffered normal saline, or Ringer solution cooled to 4°C, although this period can be ex- tended [4-6]. Thus there is a continuing need for meth- ods that prolong the safe preservation and transport of graft materials with live chondrocytes. Pharmaceuticals and dietary supplements that pur- portedly improve chondrocyte vitality and the ability of damaged chondral surfaces to self-repair have gained 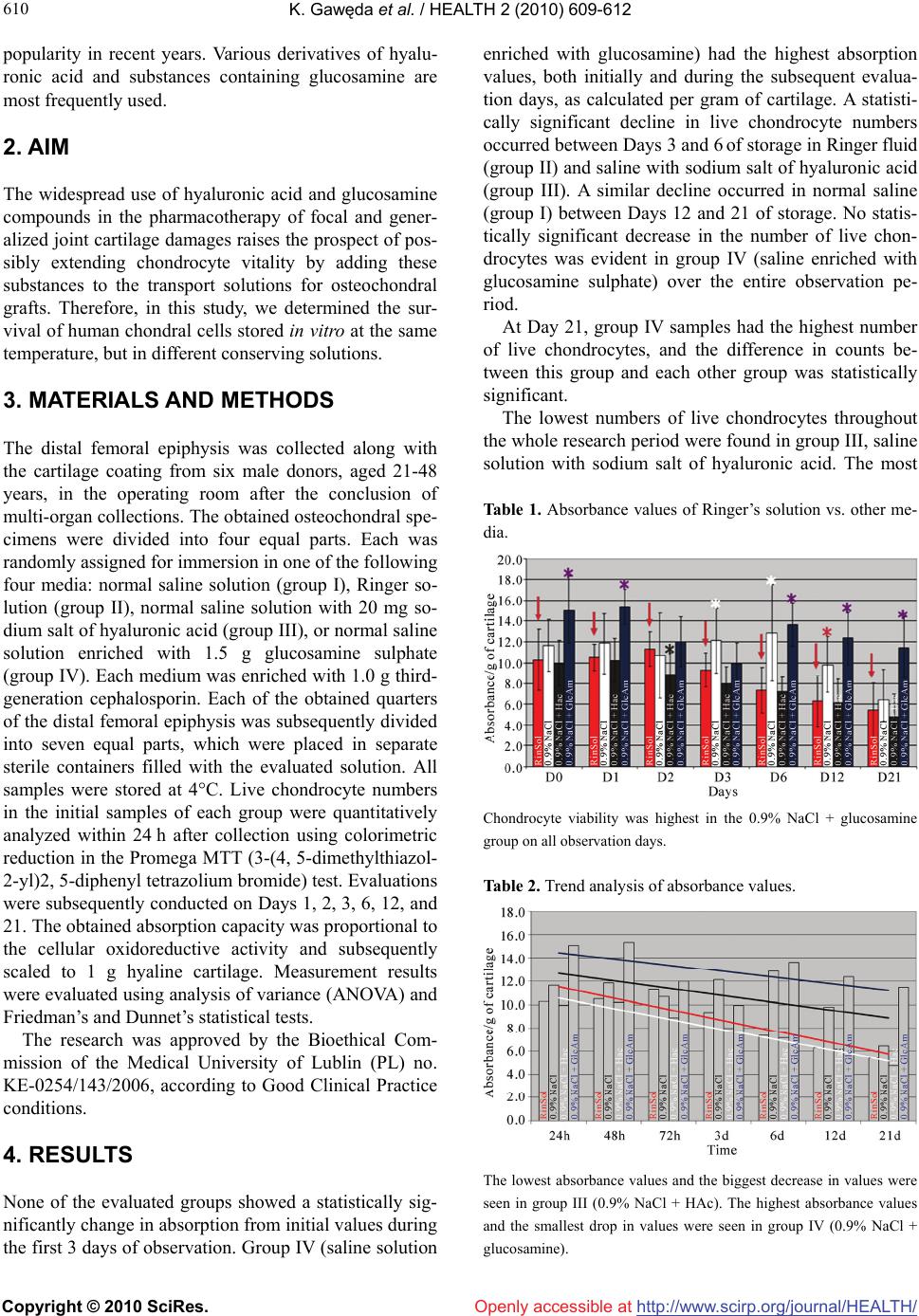 K. Gawęda et al. / HEALTH 2 (2010) 609-612 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 610 popularity in recent years. Various derivatives of hyalu- ronic acid and substances containing glucosamine are most frequently used. 2. AIM The widespread use of hyaluronic acid and glucosamine compounds in the pharmacotherapy of focal and gener- alized joint cartilage damages raises the prospect of pos- sibly extending chondrocyte vitality by adding these substances to the transport solutions for osteochondral grafts. Therefore, in this study, we determined the sur- vival of human chondral cells stored in vitro at the same temperature, but in different conserving solutions. 3. MATERIALS AND METHODS The distal femoral epiphysis was collected along with the cartilage coating from six male donors, aged 21-48 years, in the operating room after the conclusion of multi-organ collections. The obtained osteochondral spe- cimens were divided into four equal parts. Each was randomly assigned for immersion in one of the following four media: normal saline solution (group I), Ringer so- lution (group II), normal saline solution with 20 mg so- dium salt of hyaluronic acid (group III), or normal saline solution enriched with 1.5 g glucosamine sulphate (group IV). Each medium was enriched with 1.0 g third- generation cephalosporin. Each of the obtained quarters of the distal femoral epiphysis was subsequently divided into seven equal parts, which were placed in separate sterile containers filled with the evaluated solution. All samples were stored at 4°C. Live chondrocyte numbers in the initial samples of each group were quantitatively analyzed within 24 h after collection using colorimetric reduction in the Promega MTT (3-(4, 5-dimethylthiazol- 2-yl)2, 5-diphenyl tetrazolium bromide) test. Evaluations were subsequently conducted on Days 1, 2, 3, 6, 12, and 21. The obtained absorption capacity was proportional to the cellular oxidoreductive activity and subsequently scaled to 1 g hyaline cartilage. Measurement results were evaluated using analysis of variance (ANOVA) and Friedman’s and Dunnet’s statistical tests. The research was approved by the Bioethical Com- mission of the Medical University of Lublin (PL) no. KE-0254/143/2006, according to Good Clinical Practice conditions. 4. RESULTS None of the evaluated groups showed a statistically sig- nificantly change in absorption from initial values during the first 3 days of observation. Group IV (saline solution enriched with glucosamine) had the highest absorption values, both initially and during the subsequent evalua- tion days, as calculated per gram of cartilage. A statisti- cally significant decline in live chondrocyte numbers occurred between Days 3 and 6 of storage in Ringer fluid (group II) and saline with sodium salt of hyaluronic acid (group III). A similar decline occurred in normal saline (group I) between Days 12 and 21 of storage. No statis- tically significant decrease in the number of live chon- drocytes was evident in group IV (saline enriched with glucosamine sulphate) over the entire observation pe- riod. At Day 21, group IV samples had the highest number of live chondrocytes, and the difference in counts be- tween this group and each other group was statistically significant. The lowest numbers of live chondrocytes throughout the whole research period were found in group III, saline solution with sodium salt of hyaluronic acid. The most Table 1. Absorbance values of Ringer’s solution vs. other me- dia. Chondrocyte viability was highest in the 0.9% NaCl + glucosamine group on all observation days. Table 2. Trend analysis of absorbance values. The lowest absorbance values and the biggest decrease in values were seen in group III (0.9% NaCl + HAc). The highest absorbance values and the smallest drop in values were seen in group IV (0.9% NaCl + glucosamine).  K. Gawęda et al. / HEALTH 2 (2010) 609-612 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 611 611 rapid drops in numbers of live chondral cells were also observed in this group. The number of live chondral cells in group II (Ringer solution) was lower than in group I (normal saline solution). 5. DISCUSSION The storage period of fresh osteochondral grafts greatly affects their clinical value, where longer storage reduces the number of live chondral cells [7].Allen et al. re- ported a significant decline in live chondral cell numbers in osteochondral grafts within 3 weeks of collection [4]. Previous research has focused on protecting chondrocyte vitality in allogeneic osteochondral grafts primarily by changing the temperature. Voss et al. demonstrated that storing cartilage at room temperature accelerated cell death [8], and a critical increase occurred between 50°C and 55°C. In contrast, lowering the temperature is gen- erally favorable to chondrocyte survival. Pearsall re- ported that the number of live chondrocytes dropped to 67% of the initial value after a storage period of 44 days at 4°C [9]. Judas et al. showed that adding protective substances to the agents in which osteochondral grafts were stored before freezing extended chondrocyte sur- vival [10]. Adding tetracycline-type antibiotics may im- prove the vitality of chondral graft cells [11]. Teng et al. examined the impact of IGF-1(insulin-like growth factor 1) and the apoptosis inhibitor zVAD-fmk in Ringer fluid and Dulbecco’s modified Eagle’s medium (DMEM) on the survival of bovine chondrocytes [7]. Chondrocytes atrophied most rapidly in Ringer fluid; the process was slower in DMEM. Adding either IGF-1 or zVAD-fmk to the DMEM significantly extended the survival of chondral cells. Pennock analyzed the survivability of human chondrocytes in osteochondral grafts suspended in normal saline, glucose, amino acid solution, and 10% fetal bovine serum solution [12]. Chondrocyte survival was significantly lower in solutions without bovine se- rum. However, storing tissue with bovine serum requires further research on the possibility of the transmission of infections as well as immune reactions to proteins from foreign species. The results of our research demonstrate that chondro- cyte cell death was quickest in saline solution enriched with sodium salt of hyaluronic acid. We are not in a po- sition to determine why adding hyaluronic acid deriva- tives did not improve chondrocyte graft vitality. Samples of human joint cartilage stored for 3 weeks in saline so- lution enriched with glucosamine sulphate retained their vitality to the greatest degree compared to samples pre- served in normal saline alone, Ringer solution, and nor- mal saline solution plus sodium salt of hyaluronic acid. Thus, chondrocyte vitality can be increased by enriching the fluid environment of graft preservation with gluco- samine sulphate. The lowest decline in live chondrocyte numbers over a 3-week period decreased the biological value of the graft only slightly. Our results show that the inevitable process of in vitro chondral cell atrophy dur- ing the storage period for surgical transplantation of joint surfaces may be slowed to maintain high biological and mechanical value of the graft. Otsuki et al. showed that a decrease in glycosaminoglycan concentration did not lead directly to the intensification of chondral joint cell atrophy [13]. Based on our analyses, increasing the amount may slow these processes. REFERENCES [1] Lexer, E. (1908) Substitution of whole or half joints from freshly amputated extremities by free plastic operations. Surgery, Gynecology and Obstetrics, 6, 601-607. [2] Gross, A.E., Silverstein, E.A., Falk, J., Falk, R. and Langer, F. (1975) The allotransplantation of partial joints in the treatment of osteoarthritis of the knee. Clinical Orthopaedics and Related Research, 108, 7-14. [3] Csönge, L., Bravo, D., Newman-Gage, H., Rigley, T., Conrad, E.U., Bakay, A., Strong, D.M. and Pellet, S. (2002) Banking of osteochondral allografts, Part II. Pres- ervation of Chondrocyte Viability during Long-Term Storage. Cell and Tissue Banking, 3(3), 161-168. [4] Allen, T.R., Robertson, C.M., Pennock, A.T., Bugbee, W.D., Harwood, F.L., Wong, W., Chen, A.C., Sah, R.L. and Amiel, D. (2005) Analysis of stored osteochondral allografts at the time of surgical implantation. America Journal of Sports Medicine, 33(10), 1479-1484. [5] LaPrade, R.F., Botker, J., Herzog, M. and Agel, J. (2009) Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. Journal of Bone and Joint Surgery American, 91(4), 805-811. [6] Williams III, R.J., Dreese, J.C., Chen and C.-T. (2004) Chondrocyte survival and material properties of hy- pothermically stored cartilage. An evaluation of tissue used for osteochondral allograft transplantation. Ameri- can Journal of Sports Medicine, 32(1), 132-139. [7] Teng, M.S., Yuen, A.S. and Kim, H.T. (2008) Enhancing osteochondral allograft viability: effects of storage media composition. Clinical Orthopaedics and Related Re- search, 466(8), 1804-1809. [8] Voss, J.R., Lu, Y., Edwards, R.B., Bogdanske, J.J., Markel, M.M. (2006) Effects of thermal energy on chon- drocyte viability. American Journal of Veterinary Re- search, 67(10), 1708-1712. [9] Pearsall, A.W., Tucker, J.A., Hester, R.B. and Heitman, R.J. (2004) Chondrocyte viability in refrigerated osteo- chondral allografts used for transplantation within the knee. American Journal of Sports Medicine, 32(1), 125- 131. [10] Judas, F., Rosa, S., Teixeira, L., Lopes, C. and Ferreira Mendes, A. (2007) Chondrocyte viability in fresh frozen large human osteochondral allografts: effect of cryopro- tective agents. Transplantations Proceedings, 39(8), 2531-2534.  K. Gawęda et al. / HEALTH 2 (2010) 609-612 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 612 [11] Snasshall, J.T., Teng, M.S., Yuen, A.S. and Kim, H.T. (2006) Minocycline increases articular chondrocyte vi- ability during storage of osteochondral allografts. 52nd Annual Meeting of the Orthopedic Research Society, Chicago, paper 0267. [12] Pennock, A.T., Wagner, F., Robertson, C.M., Harwood, F.L., Bugbee, W.D., Amiel, D. (2006) Prolonged storage of osteochondral allografts: Does the addition of fetal bovine erum improve chondrocyte viability? The Journal of Knee Surgery, 19, 265-272. [13] Otsuki, S., Brinson, D.C., Creighton, L., Kinoshita, M., Sah, R.L., D’Lima, D., Lotz, M. (2008) The effect of glycosaminoglycan loss on chondrocyte viability: A study on porcine cartilage explants. Arthritis and Rheu- matism, 58(4), 1076-1085. |

