Paper Menu >>
Journal Menu >>
 Vol.2, No.6, 595-602 (2010) Health doi:10.4236/health.2010.26088 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Inhibition of H2O2-induced DNA damage in single cell gel electrophoresis assay (comet assay) by castasterone isolated from leaves of centella asiatica Nishi Sondhi1, Renu Bhardwaj1*, Satwinderjeet Kaur1, Madhu Chandel1, Neeraj Kumar2, Bikram Singh2 1Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, India; *Corresponding Author: renubhardwaj82@gmail.com 2Division of Natural Plant Product, Institute of Himalayan Bioresource Technology, Palampur, India Received 10 December 2009; revised 16 February 2010; accepted 20 February 2010. ABSTRACT Brassinosteroids (BRs) are a large group of polyhydroxy steroids, which regulate numerous aspects of plant growth and development, in- cluding stem elongation, leaf bending, tracheary element differentiation, stress protection and photomorphogenesis. Recent studies indicate antigenotoxic and anticancerous activities of these compounds. The role of natural BRs in H2O2 (hydrogen peroxide) -induced DNA damage in human lymphocytes is still unknown. The present study reports the presence of Cas- tasterone from leaves of Centella asiatica, an important medicinal herb commonly used as a memory enhancer and immunomodulator. CA50 fraction isolated from Centella asiatica was characterized as Castasterone by electrospray ionization mass spectral data with standard Castasterone. An attempt has been made to study antigenotoxic activity of the isolated Castasterone against H2O2 -induced DNA dam- age in human blood lymphocytes using Single cell gel electrophoresis assay (Comet Assay). Castasterone at 10–9 M concentration proved to be effective in diminishing the DNA damage by 89.42 %. Keywords: Brassinosteroids; Castasterone; Comet Assay; Hydrogen Peroxide 1. INTRODUCTION In living system, oxidative stress results in the produc- tion of reactive oxygen species (ROS) like superoxide radical (O2 -), hydroxyl radical (HO–) and hydrogen per- oxide (H2O2). H2O2 in Fenton reaction is spontaneously converted to the highly reactive hydroxyl radicals (HO–). These hydroxyl radicals oxidize proteins, lipids and nu- cleic acids leading to even mutations at the cellular level [1]. Several plant hormones are implicated in modulating the response to oxidative stress like ethylene [2], ab- scisic acid [3], auxins and plant steroids [4]. Brassinos- teroids are a group of naturally occurring plant hormones, which are structurally similar to animal steroid hor- mones. They influence diverse physiological processes by regulating the expression of genes like their animal counterparts [5]. Recent studies indicate antiviral active- ties of BRs against various viruses, like herpes simplex virus type I (HSVI), arena virus, measles virus and ve- sicular stomatitis virus [6-8]. The treatment of BRs to these viruses was 10-18 folds more effective than ri- bavirin towards HSV-I and arenavirus. It has further been reported that 24-epibrassinolide can increase the mitochondrial membrane potential, reduce intercellular antibody levels, increase the proportion of cells in G0/G1 phase, reduce the population of cells in s-phase and in- crease the population of viable hybridoma mouse cells at subnanomolar concentrations [9]. Anticancerous active- ties of 28-homocastasterone and 24-epibrassinolide were studied in several normal and cancer cell lines. The anticancer and antiproliferative activities have been documented very recently [10]. The BRs used showed high cytotoxic activity in breast (MCF-7/MDA-MB-468) and prostate cancer cell lines (LNCaP/DU-145) [11]. Centella asiatica (L.) (Family Apiaceae) commonly known as urban herb regarded as rasayana or rejuvenat- ing herb reputed to increase intelligence and memory in Ayurvedic medicines. The methanol extracts of whole plants of Centella asiatica showed a significant increase in the phagocytic index and total WBC count thereby showing immunomodulatory activity. [12] isolated a  N. Sondhi et al. / HEALTH 2 (2010) 595-602 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 596 water soluble arabinogalactan, HBN and traced remark- able immunoenhancing activities on T-and B-lymphocytes in vitro and vivo tests. The antioxidative properties of Centella asiatica were evaluated by [13,14]. The C. asi- atica extract has a chemopreventive effect [15]. [16] studied the healing effects of C. asiatica when orally administered to rats with acetic acid induced gastric ulcers, It reduced the size of ulcers in dose-dependent manner. Chemical studies reveal that triterpene saponins Asiaticoside and Madecassoside are the main active constituents of Centella asiatica. The other saponins and triterpene acids present in this plant are brahmoside, brahmnoside, brahmic acid, isobrahmic acid, betulic acid, centelloside and cetillic etc. The presence and role of brassinosteroids in this plant is yet to be studied. The present study was therefore planned to study the pres- ence of BRs and inhibition of H2O2-induced DNA dam- age by Castasterone isolated from Centella asiatica which is the first report in this direction. 2. MATERIALS AND METHODS 2.1. Extraction and Purification of Brassinosteroids Study material for the present investigation included leaves of Centella asiatica procured from Dehradun (M/s Gautam globals, Dehradun, India). Fresh leaves of Centella asiatica (2 kg) were homogenized and percolated with 80% methanol (3 × 1000 ml). The combined metha- nol extract was dried under vacuum using rotary evapo- rator (Strike 202, Stereoglass, Italy). 80% methanol ex- tract (449.6 g) was partitioned between chloroform and water. Chloroform extract was then partitioned between 80% methanol and hexane. The resulting 80% methanol extract (28.3 g) was partitioned between ethyl acetate and distilled water. The ethyl acetate fraction (20 g) was dried and subjected to silica gel (60-120 mesh) column chromatography with step-gradient elution from 0, 1, 2, 3, 4, 5, 6, 7, 10, 15, 20, 50, 100% (each 500-1000 ml). All the fractions were subjected to radish hypocotyl bio- assay with the aim to find the bioactive fraction. Four fractions CA5, CA10, CA50 and CA60 were found to be active (Figure 1). CA50 fraction was directly subjected to ESI-MS and MS/MS analysis (Figures 2(a), 3(a)). 2.2. Radish Hypocotyl Bioassay The bioactivity of isolated fractions was determined us- ing intact plants of Raphanus sativus as described by [17]. 5 days-old seedlings were placed into the test solu- tions (0.03 ml of fraction diluted with distilled water to get the final volume 3 ml). 3 ml solution was poured in each petriplate containing radish seedlings and kept in 0 50 100 150 200 250 01256710 15 20 50 60 70100 percent increase Figure 1. Biological activity of fractions of Centella asiatica in Radish hypocotyls bioassay after silica gel column chroma- tography. the dark for 24 h at 25 ± 2˚C. After 24 h, the length of hypocotyls were measured and compared to control. Percent increase over control was calculated. 2.3. Electrospray Ionization Mass Spectrometry of CA 50 Fraction ESI-MS analysis of CA50 and standard Castasterone was carried out by the addition of 10 μl of concentrated aqueous formic acid solution to the sample mixture to a total volume of 1000 μl making 0.1% as final concentra- tion. ESI-QTOF-MS was performed in positive ioniza- tion mode in QTOF Mass Spectrometer (Micromass, Manchester, UK). The general conditions were: Source temperature of 280˚C, capillary voltage of 2.1 kV and cone voltage of 23 V. ESI-MS was performed by direct infusion with a flow rate of 10 μl/min using a syringe pump and mass spectra were acquired and accumulated over 60 s. MassLynx 4.0 (Waters, Manchester, UK) was used for data analysis. Tandem mass spectrometry of single molecular ion in the mass spectra was performed by mass-selecting the ion of interest, which was in turn submitted to 15-35 eV collisions with argon in the colli- sion quadrupole. 2.4. Comet Assay DNA damage was determined by alkaline single cell microgel electrophoresis (comet assay) assay following the method proposed by [18] with minor modifications as suggested by [19]. Heparinized blood samples were obtained by venipuncture from a non-smoking, healthy male donor aged 30-40 years. Lymphocytes were iso- lated by the method of [20] and mixed with equal vol- ume of Phosphate Buffer Saline (PBS) pH 7.2. This mixture was then overlayed to double volume of Histo- paque 1077 and centrifuged at 1500 rpm for 20 minutes. The layer containing lymphocytes was aspirated very carefully with the help of pasture pipette. The lympho- cytes were diluted in PBS and centrifuged at 2000 rpm for 15 min. The supernatant was discarded and pellet was again suspended in PBS and centrifuged at 2000 rpm  N. Sondhi et al. / HEALTH 2 (2010) 595-602 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 597 597 (a) (b) Figure 2. (a) ESI-QTOF-MS analysis of Castasterone fraction (CA50) isolated from Centella asiatica; (b) ESI-QTOF-MS of Stan- dard Castasterone. 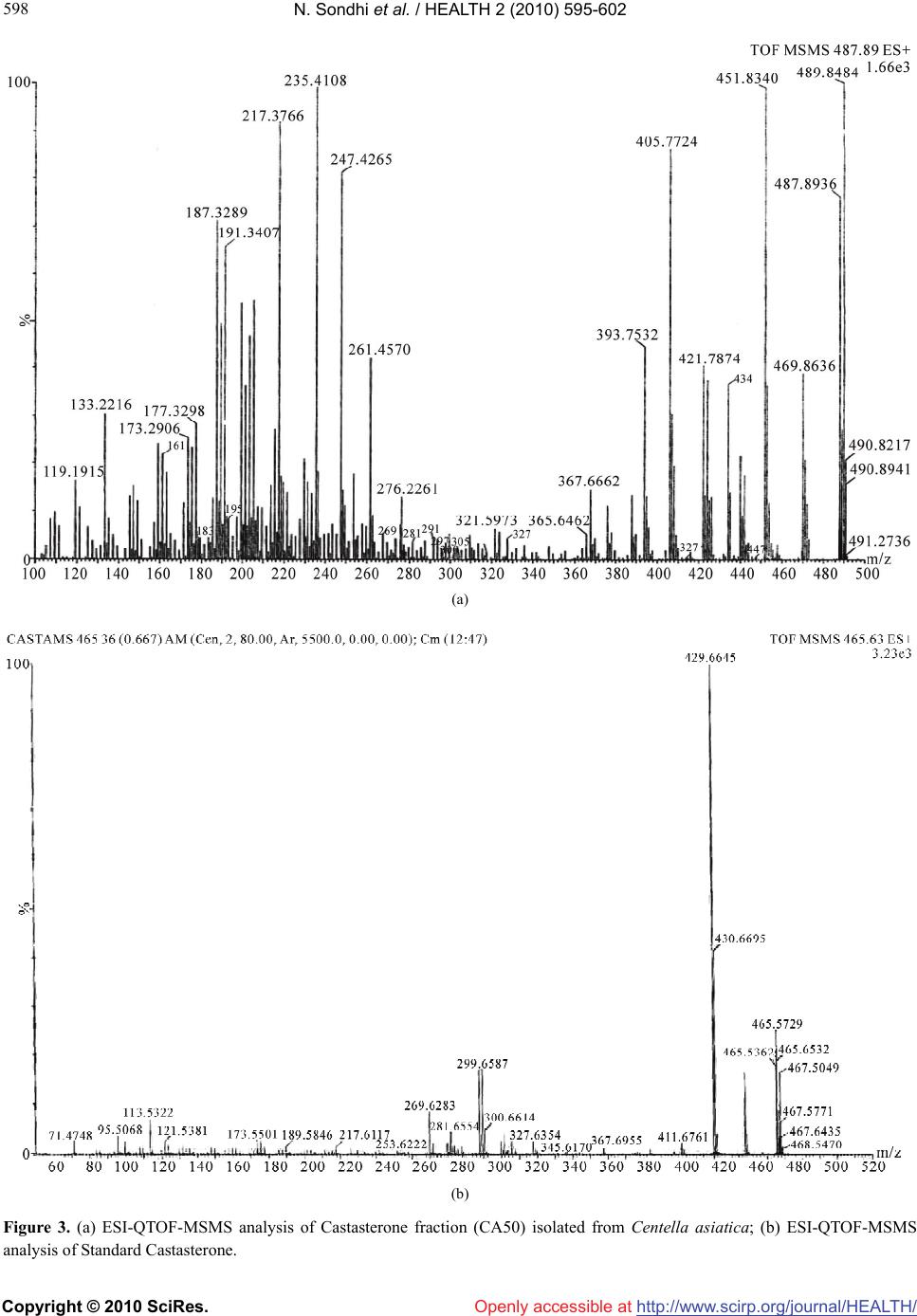 N. Sondhi et al. / HEALTH 2 (2010) 595-602 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 598 (a) (b) Figure 3. (a) ESI-QTOF-MSMS analysis of Castasterone fraction (CA50) isolated from Centella asiatica; (b) ESI-QTOF-MSMS analysis of Standard Castasterone. 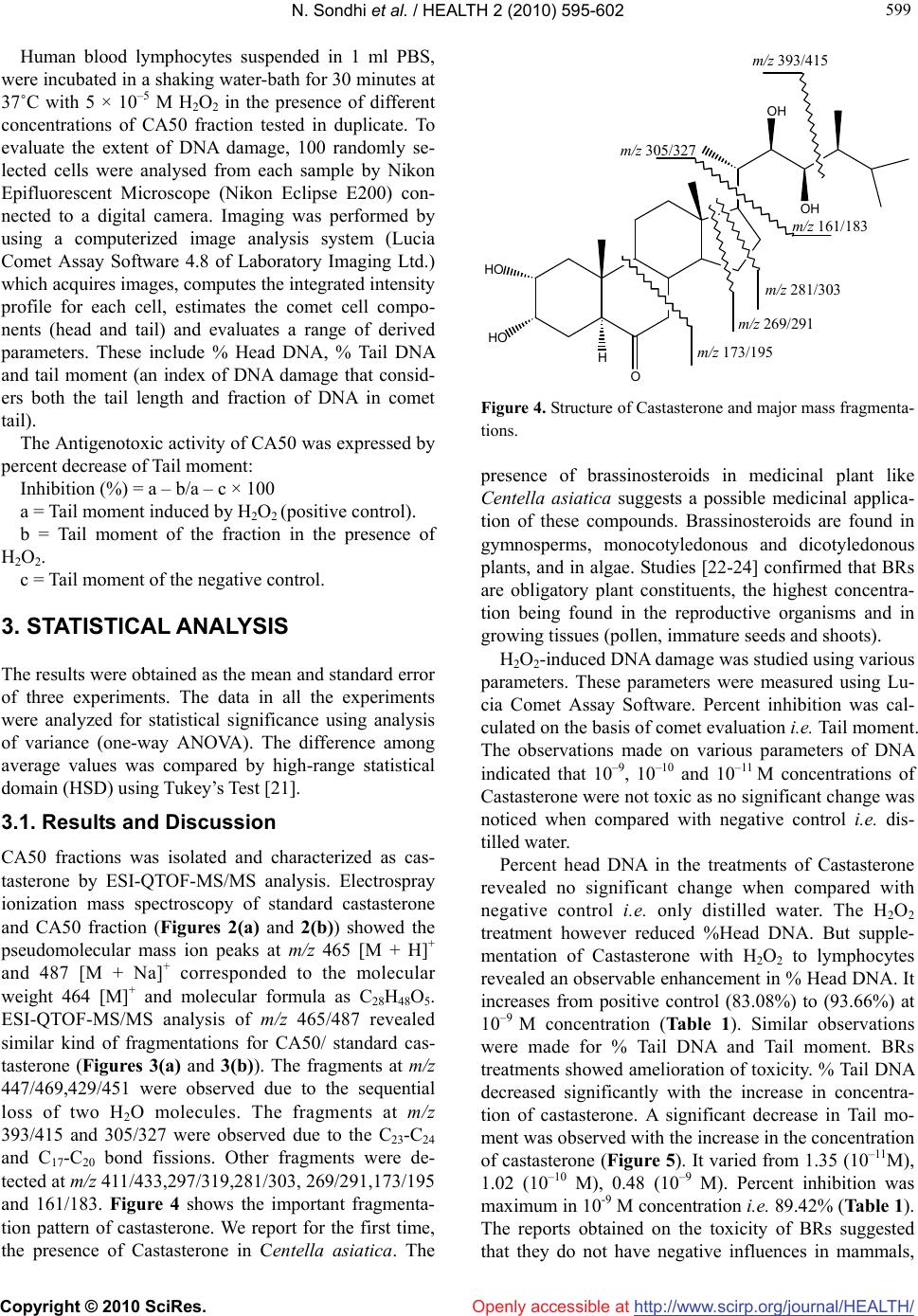 N. Sondhi et al. / HEALTH 2 (2010) 595-602 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 599 599 Human blood lymphocytes suspended in 1 ml PBS, were incubated in a shaking water-bath for 30 minutes at 37˚C with 5 × 10–5 M H2O2 in the presence of different concentrations of CA50 fraction tested in duplicate. To evaluate the extent of DNA damage, 100 randomly se- lected cells were analysed from each sample by Nikon Epifluorescent Microscope (Nikon Eclipse E200) con- nected to a digital camera. Imaging was performed by using a computerized image analysis system (Lucia Comet Assay Software 4.8 of Laboratory Imaging Ltd.) which acquires images, computes the integrated intensity profile for each cell, estimates the comet cell compo- nents (head and tail) and evaluates a range of derived parameters. These include % Head DNA, % Tail DNA and tail moment (an index of DNA damage that consid- ers both the tail length and fraction of DNA in comet tail). The Antigenotoxic activity of CA50 was expressed by percent decrease of Tail moment: Inhibition (%) = a – b/a – c × 100 a = Tail moment induced by H2O2 (positive control). b = Tail moment of the fraction in the presence of H2O2. c = Tail moment of the negative control. 3. STATISTICAL ANALYSIS The results were obtained as the mean and standard error of three experiments. The data in all the experiments were analyzed for statistical significance using analysis of variance (one-way ANOVA). The difference among average values was compared by high-range statistical domain (HSD) using Tukey’s Test [21]. 3.1. Results and Discussion CA50 fractions was isolated and characterized as cas- tasterone by ESI-QTOF-MS/MS analysis. Electrospray ionization mass spectroscopy of standard castasterone and CA50 fraction (Figures 2(a) and 2(b)) showed the pseudomolecular mass ion peaks at m/z 465 [M + H]+ and 487 [M + Na]+ corresponded to the molecular weight 464 [M]+ and molecular formula as C28H48O5. ESI-QTOF-MS/MS analysis of m/z 465/487 revealed similar kind of fragmentations for CA50/ standard cas- tasterone (Figures 3(a) and 3(b)). The fragments at m/z 447/469,429/451 were observed due to the sequential loss of two H2O molecules. The fragments at m/z 393/415 and 305/327 were observed due to the C23-C24 and C17-C20 bond fissions. Other fragments were de- tected at m/z 411/433,297/319,281/303, 269/291,173/195 and 161/183. Figure 4 shows the important fragmenta- tion pattern of castasterone. We report for the first time, the presence of Castasterone in Centella asiatica. The OH OH HO HO H O m/z 393/415 m/z 305/327 m/z 281/303 m/z 269/291 m/z 161/183 m/z 173/195 Figure 4. Structure of Castasterone and major mass fragmenta- tions. presence of brassinosteroids in medicinal plant like Centella asiatica suggests a possible medicinal applica- tion of these compounds. Brassinosteroids are found in gymnosperms, monocotyledonous and dicotyledonous plants, and in algae. Studies [22-24] confirmed that BRs are obligatory plant constituents, the highest concentra- tion being found in the reproductive organisms and in growing tissues (pollen, immature seeds and shoots). H2O2-induced DNA damage was studied using various parameters. These parameters were measured using Lu- cia Comet Assay Software. Percent inhibition was cal- culated on the basis of comet evaluation i.e. Tail moment. The observations made on various parameters of DNA indicated that 10–9, 10–10 and 10–11 M concentrations of Castasterone were not toxic as no significant change was noticed when compared with negative control i.e. dis- tilled water. Percent head DNA in the treatments of Castasterone revealed no significant change when compared with negative control i.e. only distilled water. The H2O2 treatment however reduced %Head DNA. But supple- mentation of Castasterone with H2O2 to lymphocytes revealed an observable enhancement in % Head DNA. It increases from positive control (83.08%) to (93.66%) at 10–9 M concentration (Table 1). Similar observations were made for % Tail DNA and Tail moment. BRs treatments showed amelioration of toxicity. % Tail DNA decreased significantly with the increase in concentra- tion of castasterone. A significant decrease in Tail mo- ment was observed with the increase in the concentration of castasterone (Figure 5). It varied from 1.35 (10–11M), 1.02 (10–10 M), 0.48 (10–9 M). Percent inhibition was maximum in 10-9 M concentration i.e. 89.42% (Table 1). The reports obtained on the toxicity of BRs suggested that they do not have negative influences in mammals,  N. Sondhi et al. / HEALTH 2 (2010) 595-602 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 600 Table 1. Inhibition of H2O2-induced DNA damage in Human blood lymphocytes by CA50 fraction isolated from Centella asiatica using Comet assay. Treatment Dose concentration %Head DNA (Mean ± SE)% Tail DNA (Mean ± SE)Tail moment (Mean ± SE) % Inhibition Negative control D.W 96 ± 0.32 4.0 ± 0.32 0.30 ± 0.02 10–11M 92.28 ± 2.06 7.72 ± 0.40 0.24 ± 0.03 10–10M 96.09 ± 0.41 3.91 ± 0.41 0.26 ± 0.03 Castasterone 10–9M 95.82 ± 0.56 4.22 ± 0.58 0.26 ± 0.03 Positive control H2O2 (50 M) 83.02 ± 0.72 16.98 ± 0.72 2.34 ± 0.13 10–11M 88.68 ± 1.06* 11.32 ± 1.06* 1.35 ± 0.13* 47.14% 10–10M 91.69 ± 0.8* 8.28 ± 0.81* 1.02 ± 0.11* 63.46% Castasterone + H2O2 (50M) 10–9M 93.66 ± 0.98* 6.34 ± 0.98* 0.48 ± 0.07* 89.42% *indicates significant values at p ≤ 0.05. 0 0.5 1 1.5 2 2.5 3 1234 Tail moment Concentrations Figure 5. Effect of Castasterone isolated from Centella asiat- ica on the genotoxicity induced by Hydrogen peroxide (5 × 10–5 M) in human lymphocytes using comet assay. 1 = treat- ment with H2O2. 2-4 = treatment with different concentrations of Castasterone. (a) (b) (c) Digital images illustrating the inhibition of DNA damage by Castasterone in the Comet assay (a) +ve control i.e. H2O2 (b) –ve control i.e. Castasterone only (c) Castasterone + H2O2. water organisms, soil microbiological processes and plants [25]. Mutagenic studies carried out at the Scien- tific Research Center of Toxicological and Hygienic Regulation of Biopreparations of Russia showed that Ames test, with or without metabolic activitation, was negative with the tester strains of Salmonella typhi- murium TA1534, TA1537, TA1950, TA98 and TA100 [25]. Antigenotoxic properties of EBL isolated from A.marmelos had also been studied by [26]. Reactive oxygen species can damage the normal cel- lular functions and can cause atherosclerosis in vessels or malignant growth in other tissues and ageing proc- esses [27]. The lymphocytes when treated with H2O2 showed the significant DNA damage. However this damage was ameliorated significantly by the simultane- ous application of different concentrations of this BR. The H2O2 stress protective properties of BRs in human lymphocytes are the first such study carried out with plant steroids. In the present study, the protective effect observed against the ROS may in part be responsible to the anticancer activity of brassinosteroids reported by some workers [11,28]. In the studies carried out by [29] three types of 5α-androstane and ergostane analogues of brassinolide, containing a fluorine atom in either the 3α or the 5α positions or in 3α or the 5α positions, were prepared using standard operations. The 5α fluorine was found to effect chemical reactivity as well as physical properties of the products. Cytotoxicity of the products was studied using human normal and cancer cell lines with 28-homocastasterone as positive control and their brassinolide type activity was established using the bean second-internode test with 24-epibrassinolide as stan- dard. The equivalence of F and OH groups was observed in some of the active compounds. Ergostane derivatives were most active in the anticancer activity while andro- stane derivatives were active in brassinolide type activity. Brassinolide was found to induce a time and concentra- tion dependent cytotoxicity in androgen–independent human prostate cancer in PC-3 cells. The mode of cell death appeared to be predominately apoptosis. Western blot studies indicated that treatment with brassinolide triggered a time dependent decrease in the expression of  N. Sondhi et al. / HEALTH 2 (2010) 595-602 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 601 601 antiapoptotic protein Bcl-2. 24-epibrassinolide and 28- homoCS were found to inhibit the growth, at micro- molar concentrations, of several human cancer cell lines without affecting the growth of normal cells [10]. Stud- ies carried out by Malikova et al. (2008) indicate that BRs may prove to be promising leads for the develop- ment of new generation of anticancer drugs. Varoius animal steroids have been found to exhibit antioxidant properties [30]. BRs have also been reported to regulate antioxidative defence system of plants under stress con- ditions [31]. The reduction in DNA damage indicates amelioration of oxidative stress generation by H2O2 in lymphocytes. The tissues are protected from oxidative damage by variety of mechanism including antioxidants and antioxidative enzymes, repair enzymes and growth regulators. Further studies are needed to understand the mechanism of protective effect of these steroids in ani- mal system which opens a field of study on possible medical applications of these plant steroids. 4. ACKNOWLEDGEMENTS This work is supported by grants from University Grants Commission, Government of India. Dr. P.S. Ahuja, Director, Institute of Himalyan Bioresource Technology (IHBT) Palampur (HP) is duly acknowledged for providing necessary lab facilities. Dr. Chalev Pachthong, Depart- ment of Chemistry, Faculty of Science and Technology Kanchanaburi Rajabhat University, Kanchanaburi, Thailand is also acknowledged for his kind gift of Castasterone. REFERENCES [1] Halliwell, B. and Gutteridge, J.M. (2007) Free Radicals in Biology and Medicine. 4th Edition, Oxford University Press, USA. [2] Vahala, J., Keinanen, M., Schutzendubel, A., Polle, A. and Kangasjarvi, J. (2003) Differential effects of elevated ozone on two hybrid aspen genotypes predisposed to chronic ozone fumigation. Role of ethylene and salicylic acid. Plant Physiology, 132(1), 196-205. [3] Kovtun, Y., Chiu, W.L., Tena, G. and Sheen, J. (2000) Functional analysis of oxidative stress-activated mitogen- activated protein kinase cascade in plants. Proceedings of National Academy of Sciences, USA, 97(6), 2940-2945. [4] Cao, S., Xu, Q., Cao, Y., Qian, K., An, K., Zhu, Y., Binzeng, H., Zhao, H. and Kuai, B. (2005) Loss-of- function mutation in DET2 gene lead to an enhanced re- sistance to oxidative stress in Arabidopsis. Physiologia Plantarum, 123(1), 57-66. [5] Sasse, J.M. (2003) Physiological actions of brassinoster- oids: An update. Journal of Plant Growth Regulation, 22 (4), 276-288. [6] Wachsman, M.B., Ramirez, J.A., Galagovsky, L.R. and Coto, C.E. (2002) Antiviral activity of brassinosteroids derivatives against measles virus in cell cultures. Antivi- ral Chemistry and Chemotherapy, 13(1), 61-66. [7] Wachsman, M.B., Castilla, V., Talarico, L.B., Ramirez, J.A., Galagovsky, L.R. and Coto, C.E. (2004) Antiher- patic mode of action of (22S, 23S)-3beta-Bromo-5alpha, 22, 23-trihydroxystigmastan-6-one in vitro. International Journal of Antimicrobial Agents, 23(5), 524-526. [8] Romanutti, C., Castilla, V., Coto, C.E. and Wachsman, M.B. (2007) Antiviral effect of a synthetic brassinoster- oid on the replication of vesicular stomatitis virus in Vero cells. International Journal of Antimicrobial Agents, 29 (3), 311-316. [9] Franek, F., Eckschlager, T. and Kohout, L. (2003) 24-epibrassinolide at subnanomolar concentrations mod- ulates growth and production characteristics of a mouse hybridoma. Collection of Czechoslovak Chemical Com- munications, 68(11), 2190-2200. [10] Malıkova, J., Swaczynova, J., Kolar, Z. and Strnad, M. (2008) Anticancer and antiproliferative activity of natural brassinosteroids. Phytochemistry, 69(2), 418-426. [11] Swaczynova, J., Malikova, J., Hoffmannova, L., Kohout, L. and Strnad, M. (2006) Anticancer properties of brass- inosteroids. Planta Medica, 72. [12] Wang, X.S., Zheng, Y., Zuo, J. and Fang, J. (2005) Structural features of an immunoactive acidic arabinoga- lactan from Centalla asiatica. Carbohydrate Polymers, 59(3), 281-288. [13] Zainol, M.K., Abd-Hamid, A., Yusof, S. and Muse, R. (2003) Antioxidant activity and total phenolic com- pounds of leaf, root and petiole of four accessions of Centella asiatica (L) Urban. Food Chemistry, 81(4), 575- 581. [14] Gnanapragasam, A., Ebenezar, K.K., Satish, V., govinda- raju, P. and Devaki, T. (2004) Protective effects of Cen- tella asiatica on antioxidant tissue defence system against adriamycin induced cardiomyopathy in rats. Life Sci- ences, 76(5), 585-597. [15] Bunpo, P., Kataoka, K. and Arimochi, H. (2004) Inhibi- tory effect of Centella asiatica on azoxymethane-induced aberrant crypt focus formation and carcinogenesis in the intestines of F344 rats. Food and Chemical Toxicology, 42(12), 1987-1997. [16] Cheng, C.L., Guo, J.S., Luk, J. and Koo, M.W.L. (2004) The healing effets of Centella asiatica extrats and asiati- coside on acetic acid induced gastric ulcers in rats. Life Sciences, 74(18), 2237-2249. [17] Takatsuto, S., Yazawa, N., Ikekawa, N., Takematsu, T., Takeuchi, Y. and Koguchi, M. (1983) Structural-activity relationship of Brassinosteroids. Phytochemistry, 22(11), 2437-2441. [18] Singh, N.P., McCoy, M.T., Tice, R.R. and Schneider, E.L. (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research, 175(1), 184-191. [19] Anderson, D., Dobrzynska, M.M., Basaran, N., Basaran, A. and Yu, T.W. (1998) Flavonoids modulate Comet as- say response to food mutagens in human lymphocytes and sperm. Mutation Research, 402(1-2), 269-277. [20] Boyum, A. (1968) Separation of leucocytes from blood and bone marrow. Scandinavian Journal of Clinical and Laboratory Investigation, 21(Suppl 97), 78-89 [21] Mayers, L.S. and Grossen, N.E. (1974) Analysis of inde- pendent group designs. In. Meyers, L.S. and Grossen, N.E. Eds., Behavioural Research, Theory, Procedure and  N. Sondhi et al. / HEALTH 2 (2010) 595-602 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 602 Design, W.H. Freeman, San Francisco. [22] Fujioka, S., Noguchi, T., Yokota, T., Takatsuto, S. and Yoshida, S. (1998) Brassinosteroids in Arabidopsis thaliana. Phytochemistry, 48(4), 595-599 [23] Schmidt, J., Porzel, A. and Adam, G. (1998) Brassinos- teroids and a pregnane glucoside from Daucus carota. Phytochemical Analysis, 9(1), 14-20. [24] Yokota, T., Higuchi, K., Takahashi, N., Kamuro, Y., Wa- tanabe, T. and Takatsuto, S. (1998) Identification of brassinosteroids with epimerized substituents and/or the 23-Oxo group in pollen and anthers of Japanese Cedar. Bioscience Biotechnology and Bichemistry, 62(3), 526- 531 [25] Onatskiy, N.M., Marchenko, A.I. and Mikhina, L.V. (1997) Technical Report: Evaluation of mutagenic active- ity of epibrassinolide (active ingredient of Epin) in Ames test, chromosome aberrations and in micronuclear tests. Scientific Research Centre of Toxicologie and Hygienic Regulation of Biopreparations of Russia, Serpukhov. [26] Sondhi, N.P., Bhardwaj, R., Kaur, S., Kumar, N. and Singh, B. (2008) Isolation of 24-epibrassinolide from leaves of Aegle marmelos and evaluation of its anti- genotoxicity employing Allium cepa chromosomal aber- ration assay. Plant Growth Regulation, 54(3), 217-224. [27] Merzenich, U.G., Zeitler, H., Vetter, H. and Kraft, K. (2008) Synergy research: Vitamins and secondary plant components in the maintenance of the redox homeostasis and cell signaling. Phytomedicine, 16(1), 2-16. [28] Wu, Y.D. and Lou, Y.J. (2007) Brassinolide, a plant sterol from pollen of Brassica napus L., induces apoptosis in human prostrate cancer PC-3 cells. Die Pharmazie, 62(5), 392-395. [29] Slavikova, B., Kohout, L., Budesinsky, M., Swaczynova, J. and Kasal, A. (2008) Brassinosteroids: Synthesis and activity of some fluoro analogues. Journal of Medicinal Chemistry, 51(13), 3979-3984 [30] Mooradian, A.D. (1993) Antioxidant properties of ster- oids. Journal of steroid Biochemistry and Molecular Bi- ology, 45(6), 509-511. [31] Krishna, P. (2003) Brassinosteroid-mediated stress re- sponses. Journal of Plant Growth Regulation, 22(4), 289- 297. |

