Paper Menu >>
Journal Menu >>
 Vol.2, No.6, 589-594 (2010) Health doi:10.4236/health.2010.26087 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Crithidia deanei infection in normal and dexamethasone–immunosuppressed Balb/c mice Dilvani Oliveira Santos1*, Saulo C. Bourguignon1, Helena Carla Castro1, Alice Miranda2,3, Rodrigo Tonioni Vieira1, Suzana Corte-Real4, Otílio Machado Pereira Bastos5 1Department of Cellular and Molecular Biology, Federal Fluminense University (UFF), Rio de Janeiro, Brazil; *Corresponding Author: santosdilvani@gmail.com; lacelauff@yahoo.com.br 2Department of Mycobacteriosis, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil 3Department of Pathology, FCM, University of State of Rio de Janeiro, Rio de Janeiro, Brazil 4Laboratory of Biology Structural, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil 5Department of Microbiology and Parasitology, Federal Fluminense University (UFF), Rio de Janeiro, Brazil Received 18 October 2009; revised 21 December 2009; accepted 24 December 2009. ABSTRACT Monoxenous trypanossomatids protozoa are not believed to cause in vivo infection in verte- brate hosts throughout their life cycle. However, there are reports mentioning some cases of HIV- positive patients who have presented oppor- tunistic infections caused by these protozoa. Recently, we have demonstrated the in vitro in- fection of mouse dermal fibroblasts by these protozoa. The aim of the present work is to in- vestigate the possibility of Crithidia deanei, a endosymbiont-bearing monoxenous trypanos- somatid, infect BALB/c mice under or not Dexamethasone treatment. To attend it, distinct gro- ups of adult BALB/c mice were immuno- suppressed with 50 mg/kg of Dexamethasone. This immunosuppressor was administered 24 hours before infection and daily, for 15 days after C. deanei inoculation. Control groups: C. deanei–inoculated animals but non-immuno- suppressed and non-inoculated animals but immunosuppressed were also used. Light Mi- croscopy analysis revealed an infection process characterized by the presence of the trypanos- somatid inside dermal cells in the groups stud- ied. The experimental inoculation resulted in a non-lethal infection characterized by the pres- ence of the trypanossomatid inside dermal cells in the normal BALB/c mice, but notably, in the C. deanei–inoculated immunosuppressed group. These preliminary results lead to the following conclusions: 1) C. deanei is able to infect nor- mal BALB/c mice; 2) the immunosupressed mice seemed to be more susceptible to the C. deanei infection compared to the control group. Besides C. deanei in dexamethasone-immuno- suppressed mice provides a useful model for studies of monoxenous trypanosomatids ‘in vivo’ infection, resembling that one presumably occurring in imunodeficient individuals with AIDS. Keywords: Monoxenous Trypanossomatid; ‘In Vivo’ Infection; Immunosuppression 1. INTRODUCTION Trypanossomatids parasitize a diverse range of hosts including animals, plants and protists [1]. Some of them, such as Trypanosoma and Leishmania, are heteroxenous and are ethiological agents of serious diseases in humans and experimental animals. Others are a monoxenous and are mostly found in insects [2]. Monoxenous trypano- somatids had never been confirmed as pathogenic in vertebrate host. However, there is one report of trypano- somatid, other than Trypanosoma and Leishmania, in some opportunistic cutaneous infections in immuno- compromised individuals [3] or those without any pre- vious history of immunodepression [4]. In addition, our group was pioneer in proving the infection of mouse dermal fibroblasts by two different monoxenous try- panosomatid species—Crithidia deanei and Herpetomo- nas roitmani [5]. Although some of these trypanosomat- ids were classified as a divergent member of the Leishmania genus [6], a visceral leishmaniasis—like in- fection was described in an HIV-positive patient as caused by Leptomonas pulexsimulantis, a monoxenous trypanosomatid found in dog’s flea [3], suggesting that monoxenous protozoa can be considered opportunistic agents in immunocompromised individuals. Therefore,  D. O. Santos et al. / HEALTH 2 (2010) 589-594 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 590 we investigated the ability of C. deanei to infect verte- brate host. For that purpose, we have used BALB/c mice under or not Dexamethasone treatment as an experi- menttal model, based on a previous report of mouse dermal fibroblasts infection by C. deanei and H. roit- mani [5]. 2. MATERIALS AND METHODS Parasite culture. Crithidia deanei was kindly provided by Dr. M. Auxiliadora de Souza (Trypanosomatids Colll- etion of the Oswaldo Cruz Institute, Rio de Janeiro, Brasil). The monoxenous were kept at 28°C with serial passages at 48 h intervals in Warrens’ medium [7] con- taining 10% fetal calf serum. Experimental animal infection. Female 8-week old BALB/c mice (Nau, Instituto de Biologia /UFF) were used. Animals housed in standard conditions were treated with Dexamethasone (Aziume) [8] 24 hours be- fore infection with C. deanei. After infection with 107 2-day-old promastigotes C. deanei by subcutaneous route (hind foot pad) —day 0, dexameth-asone 50 mg/kg was administered daily, for 15 days. Four BALB/c mice group were used: control without dexamethasone; con- trol with dexamethasone; C. deanei–inoculated with dexamethasone and C. deanei–inoculated without dexa- methasone (Table 1). A determined number of mice from each group were euthanasiated at 6 h, 1 d, 2 d, 3 d, d 7 and d 15 after C. deanei inoculation. At each control point, mice were weighted and parasite burdens were determined in foot pad by histological analysis. Histological analysis. Specimens of foot pad were fixed in 10% buffered formalin. After dehydration in graded ethanol, the tissues were embedded in paraffin and, then, processed routinely as previously reported [9]. 5 μm thick sections were obtained with a Leica micro- tome. After that, they were collected on glass slides for Hematoxilin-Eosin (HE) staining. The tissues samples infected or not were observed at least 400 randomly se- lected cells at 1000 × magnification, using a Zeiss photomicroscope. 3. RESULTS Clinical finding’s. No mortality, weight loss or clinical signs were observed in mice infected with either dexam- ethasone or not. Macroscopy findings. Both groups C.deanei—inocu- lated immunosuppressed mice and not inoculated immu- nosuppressed mice displayed splenomegaly and heap- tomegaly. Histological analysis. Through light microscopy the morphological analysis just of the foot pad was done. At necropsy, parasites were found in the foot pad from the mice inoculated with C. deanei, regardless immunosup- ressed or not. In the Dexamethasone treated-controls groups (in the absence of C. deanei inoculation), no hist- ological and inflammatory reations of the foot pad were observed until d15 (Figure 2(e1)). Surprisingly, in both experimental design-in the pres- ence or not of dexamethasone, C. deanei was infective to BALB/c mice (Figure 1 and 2), but, notably, in the imm- unosupressed BALB/c mice (Figure 2). Using light microscopy, it observed C. deanei–infected mouse dermal cells after 24 h infection (Figures 1(a1) and (a2)). On the 2nd post infection day, C. deanei was also observed within mice dermal cells (Figures 1(b1) and (b2)). and, between whiles, ex- tracellular parasites were seen (Figure 1(b1)). A large numbers of parasites were clearly present in the dermal cells after the third post-infection day (Figures 1(c1) and (c2)). Although it was possible to observe C. deanei within the dermal cells, their mechanism of entrance is still not clear as it can involve phagocytosis, penetration in the cell or inducing membrane invagination. Anyway, one mechanism of the C. deanei–infection might be through sincicious formation from the host cells as the image of the Figures 1(c1) and (c2) suggest. After 7 days of infection it still can observe parasites present in dermal cells C. deanei–infected mice (Figures 1(d1) and (d2)). At this time, some extracellular parasites were still seen (Figures 1(d1) and (d2)). After 15 days of infection, the light microscopy still revealed intracellular forms of C. dean ei as well as some extracellular forms of this parasite attached to the dermal cells surface (Figures 1(e1) and (e2)). In the controls groups (in the absence of C. deanei in- oculation and presence of dexamethasone) no histology- cal and inflammatory reactions of the foot pat were obs- erved until day 15 (Figure 2(e1)). Interestingly, the kinetics of infection in foot pad from C. deanei–inoculated Dexamethasone immunosuppressed mice showed parasites as early as 6h in the subcutaneous tissues (Figures 2(a1) and (a2)). Notably, the most exu- berant C. deanei–infection was observed in the presence of Dexamethasone on the first day of infection (Figures 2(b1) and (b2). In the meanwhile, it can clearly observe a C. deanei within a vacuole (Figure 2(b2)). On the foll- owing day, it can still observe a large numbers of C. deanei inside the cells (Figures 2(c1) and (c2)). In this time of infection, similar to the findings on the previous day, it can see that each parasite occupies its own vacu- ole (Figures 2(c1) and (c2)). The image of C. deanei inside 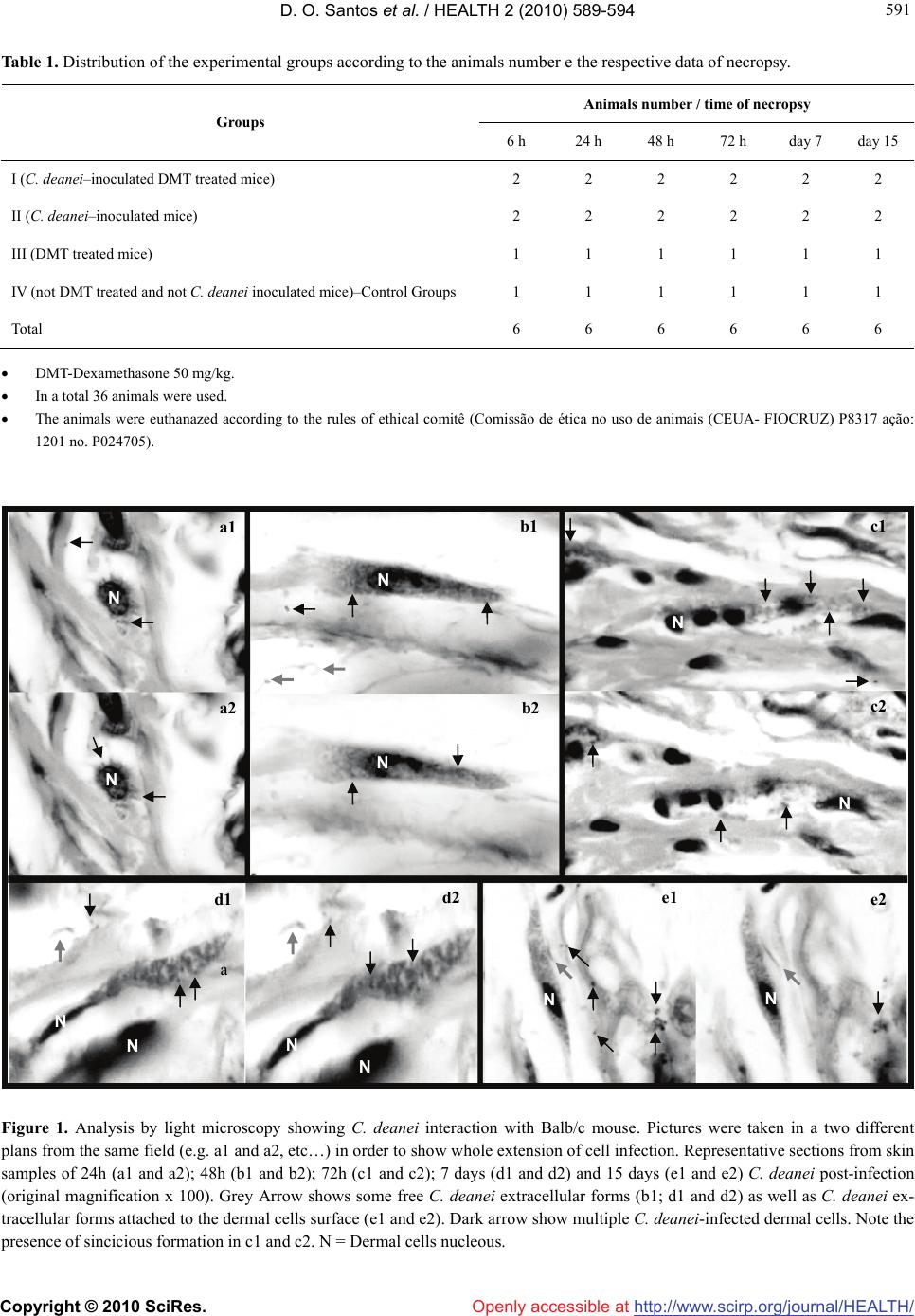 D. O. Santos et al. / HEALTH 2 (2010) 589-594 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 591 591 Table 1. Distribution of the experimental groups according to the animals number e the respective data of necropsy. Animals number / time of necropsy Groups 6 h 24 h 48 h 72 h day 7 day 15 I (C. deanei–inoculated DMT treated mice) 2 2 2 2 2 2 II (C. deanei–inoculated mice) 2 2 2 2 2 2 III (DMT treated mice) 1 1 1 1 1 1 IV (not DMT treated and not C. deanei inoculated mice)–Control Groups 1 1 1 1 1 1 Total 6 6 6 6 6 6 DMT-Dexamethasone 50 mg/kg. In a total 36 animals were used. The animals were euthanazed according to the rules of ethical comitê (Comissão de ética no uso de animais (CEUA- FIOCRUZ) P8317 ação: 1201 no. P024705). Figure 1. Analysis by light microscopy showing C. deanei interaction with Balb/c mouse. Pictures were taken in a two different plans from the same field (e.g. a1 and a2, etc…) in order to show whole extension of cell infection. Representative sections from skin samples of 24h (a1 and a2); 48h (b1 and b2); 72h (c1 and c2); 7 days (d1 and d2) and 15 days (e1 and e2) C. deanei post-infection (original magnification x 100). Grey Arrow shows some free C. deanei extracellular forms (b1; d1 and d2) as well as C. deanei ex- tracellular forms attached to the dermal cells surface (e1 and e2). Dark arrow show multiple C. deanei-infected dermal cells. Note the presence of sincicious formation in c1 and c2. N = Dermal cells nucleous. N N N N N N N N N N N N a a1 b1 b2 c1 c2 a2 d1 d2 e1 e2 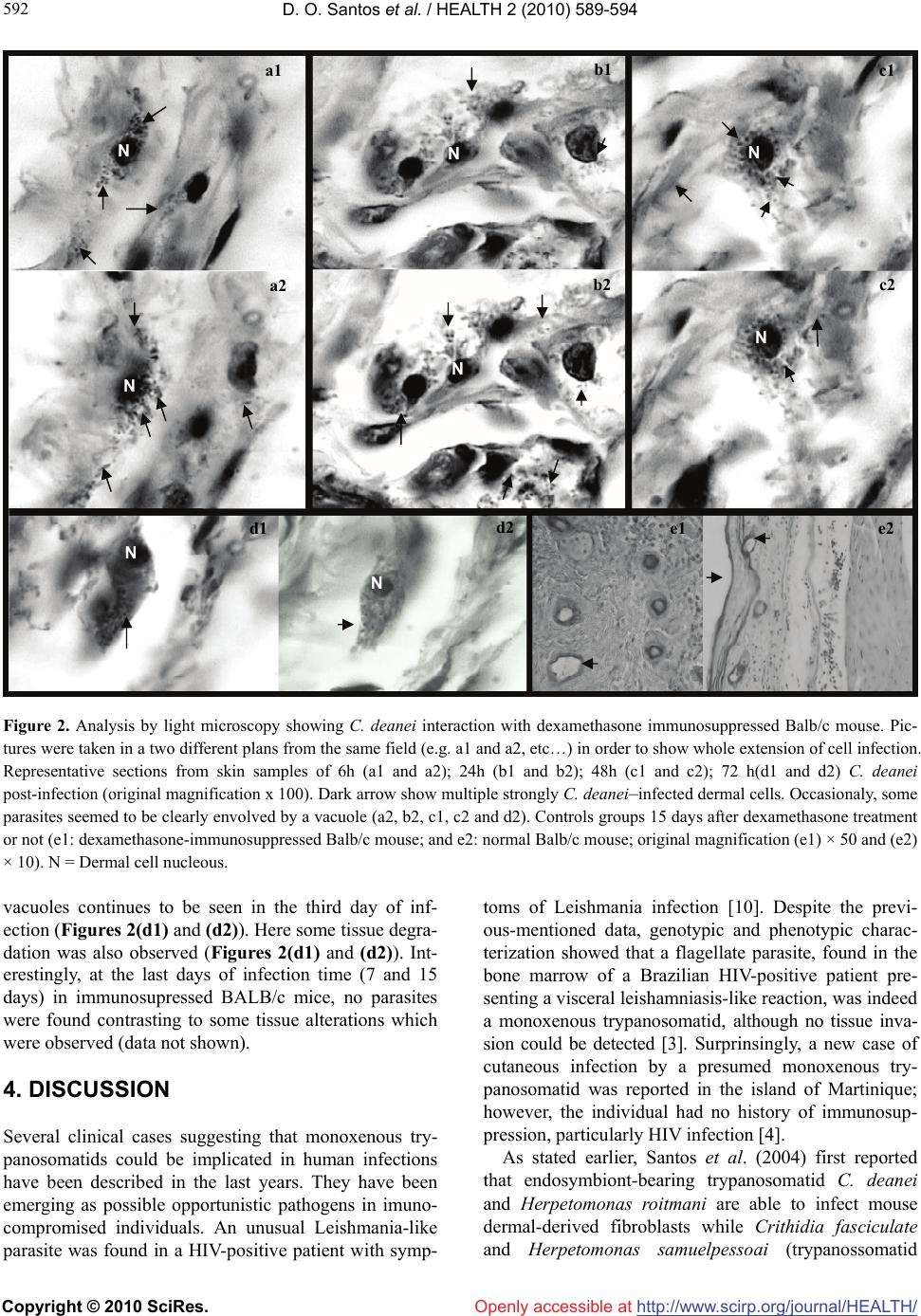 D. O. Santos et al. / HEALTH 2 (2010) 589-594 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 592 Figure 2. Analysis by light microscopy showing C. deanei interaction with dexamethasone immunosuppressed Balb/c mouse. Pic- tures were taken in a two different plans from the same field (e.g. a1 and a2, etc…) in order to show whole extension of cell infection. Representative sections from skin samples of 6h (a1 and a2); 24h (b1 and b2); 48h (c1 and c2); 72 h(d1 and d2) C. deanei post-infection (original magnification x 100). Dark arrow show multiple strongly C. deanei–infected dermal cells. Occasionaly, some parasites seemed to be clearly envolved by a vacuole (a2, b2, c1, c2 and d2). Controls groups 15 days after dexamethasone treatment or not (e1: dexamethasone-immunosuppressed Balb/c mouse; and e2: normal Balb/c mouse; original magnification (e1) × 50 and (e2) × 10). N = Dermal cell nucleous. vacuoles continues to be seen in the third day of inf- ection (Figures 2(d1) and (d2)). Here some tissue degra- dation was also observed (Figures 2(d1) and (d2)). Int- erestingly, at the last days of infection time (7 and 15 days) in immunosupressed BALB/c mice, no parasites were found contrasting to some tissue alterations which were observed (data not shown). 4. DISCUSSION Several clinical cases suggesting that monoxenous try- panosomatids could be implicated in human infections have been described in the last years. They have been emerging as possible opportunistic pathogens in imuno- compromised individuals. An unusual Leishmania-like parasite was found in a HIV-positive patient with symp- toms of Leishmania infection [10]. Despite the previ- ous-mentioned data, genotypic and phenotypic charac- terization showed that a flagellate parasite, found in the bone marrow of a Brazilian HIV-positive patient pre- senting a visceral leishamniasis-like reaction, was indeed a monoxenous trypanosomatid, although no tissue inva- sion could be detected [3]. Surprinsingly, a new case of cutaneous infection by a presumed monoxenous try- panosomatid was reported in the island of Martinique; however, the individual had no history of immunosup- pression, particularly HIV infection [4]. As stated earlier, Santos et al. (2004) first reported that endosymbiont-bearing trypanosomatid C. deanei and Herpetomonas roitmani are able to infect mouse dermal-derived fibroblasts while Crithidia fasciculate and Herpetomonas samuelpessoai (trypanossomatid N NN N N N N N a1 b1 c1 a2 b2 c2 d1 d2 e1 e2  D. O. Santos et al. / HEALTH 2 (2010) 589-594 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 593 593 endosimbiont free) did not infect. It is also of interest to observe that both C. deanei and H. roitmani can be res- istant to lysis mediated by the complement system. In contrast, H. samuelpessoai and C. fasciculate displayed 100% of lysis after incubation with the complement sys- tem [5]. The symbionts of C. deanei can influence the phagocytosis of these parasites by macrophages as have been presented by [11]. And, most recently, [12], re- ported the infection of HIV-1-infected prim- ary human macrophages by Blastocrithidia culicis (another endo- symbiont-bearing monoxenous trypanosomatid). Our present data further emphasize the large capacity of C. deanei to infect vertebrate host and reinforce the idea that monoxenous trypanosomatids present low host specifity [2,13,14]. As demonstrated by our work, C. deanei can readily infect normal BALB/c mice by sub- cutaneous route and infection persist in the dermal cells for 15 days. These are very interestingly results, since we have previously reported the “in vitro” C. deanei– infection of dermal cells obtained from a different spe- cie of mouse-the Swiss mouse [5]. Besides, as observed in our present work, extracellular forms of C. deanei are displayed in dermal tissue of the BALB/c mice (Figures 1(b1), (d1) and (d2)). This fact is interesting to be men- tioned since it might suggest that, after intracellular C. deanei cycle, these parasites leave the host cell and, after that, appear in the extracellular medium (in a flagellate form) to re-infect others dermal cells. Taken together, these evidences reinforce the idea that monoxenous try- panosomatids are able to infect and to survive once reaching the vertebrate host. Over and again, we demon- strated the infection of BALB/c mice, but, a much more pronounced C. deanei–infection in a different experi- mental design: in Dexamethasone-immunodepressed mice (Figure 2). Through its limphopenic activity, spe- cially about T cell production [15], the dexamethasone can reduce the mechanisms of anti-parasite effect of immune system and it might explain the increase of sus- ceptibily to C. deanei infection observed in all immuno- suppressed animals. The important survival of the para- site in the murine experimental host contrast strikingly with the weak clinical-pathological effects observed with absence of lymphocytic infiltrates in parasitized foot pad. This can be paralleled to that observed during human visceral leishmaniasis where patent infections with para- site dissemination are frequently associated with T cell unresponsiveness to Leishmania antigen [16], while cure is accompanied with restoration of the cellular response [17,18]. Although monoxenous trypanosomatids in hu- mans are more correlated to opportunistic parasites, our work is pioneer in demonstrating that C. deanei is able to infect normal mice (whithout dexamethasone treat- ment). Our findings corroborate to the reports of [4], who also found monoxenous tripanosomatids in a non- immunocompromised individual though in a localized skin lesion. Besides, our previous report demonstrated the monoxenous trypanosomatid infection by dermal cells isolated from skin of normal Swiss mice [5]. Nev- erthless, our data shows that the infection of C. deanei by dexamethasone-treated mice, although earlier proemi- nent at the beginning of the time of infection (Figures 2(a),(b)), could not be followed longer, since the dermal cells seemed to be degenerated (data not shown). These results suggest that C. deanei might induce dermal cells degeneration. Most recently, [19] reported that C. deanei was able to induce fibroblasts lysis. Besides the interaction of monoxenous trypanossom- atids with vertebrate cells, the literature have also men- tioned some results obtained from the interaction of these trypanossomatids with invertebrate cells. Then, [20,21], reported the colonization of Aedes aegypti mid- gut by the endosymbiont-bearing trypanosomatid Blas- tocrithidia culicis and C. deanei respectively. Considering the colonization of hematophagous in- sects by monoxenous trypanosomatids and their low host specificity, human cases of infection with lower tryp- anosomatids could have been largely underestimated until now due to their morphological similarity with Leishmania species. This emphasizes the relevance of enzymatic characterization, whenever possible, of all Leishmania-like parasites isolated from skin or visceral lesions of patients with or not immunosuppression his- tory. Taken together, these reports reinforce the idea of the urgent need of elucidating the epidemiology of these lower trypanosomatids that so far remains poorly known. 5. ACKNOWLEDGEMENTS We thank FAPERJ, CNPq, UFF and FIOCRUZ for the finantial sup- port. REFERENCES [1] Vickermank, K. (1994) The evolutionary expansion of the trypanossomatid flagellates. International Journal for Parasitology, 24(8), 1317-1331. [2] Wallace, F. G. (1966) The trypanosomatid parasites of insects and arachnids. Experimental Parasitology, 18(1), 124-193. [3] Pacheco, R.S., Marzochi, M. and Pires, M. (1998) Para- site genotypically related to a monoxenous trypnosoam- tid of dog’s flea causing opportunistic infection in HIV- positive patient. Memórias do Instituto Oswaldo Cruz, 93 (4), 531-537. [4] Boisseau–Garsaud, A.M., Cales-Quist, D. and Desbois, N. (2000) A new case of cutaneous infection by a pre- sumed monoxenous trypanosomatid in the island of Mar-  D. O. Santos et al. / HEALTH 2 (2010) 589-594 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 594 tinique (French WEst Indies). Transactions of the Royal Society of Tropical Medicine and Hygiene, 94(1), 51-52. [5] Santos, D.O., Bourguignon, S.C. and Castro, H. C. (2004) Infection of mouse dermal fibroblasts by the mono- xenous trypanosomatid Protozoa Crithidia deanei and Her- petomonas roitmani. Journal of Eukaryotic Micro- biology, 51, 570-574. [6] Noyes, P., Pratlong, F. and Chance, M. (2002) A previ- ously unclassified trypnosomatid responsible for human cutaneous lesions in Martinique (French West Indies) is the most divergent member of the genus Leishmania ss. Parasitology, 124(Pt 1), 17-24. [7] Warren, L.G. (1960) Metabolism of schizotrypanumcruzi Chagas. 1. Effect of culture age and substrate concentra- tion on respiratory rate. Journal of Parasitology, 46, 529- 539. [8] Lallo, M.A., Santos, M.J. and Bondam, E.F. (2002) Ex- perimenatal encephalitozoon cuniculi infection in dexa- methasone-immunosuppressed mice. Revista de Saúde Pública. 36(5), 621-626. [9] Santos, D.O, Castro, H.C. and Bourguignon, S.C. (2007) Expression of B7-1 costimulatory molecule in patients with multibacillary-leprosy and reactional states. Clinical and Experimental Dermatology, 32(1), 75-80. [10] Jiménez, M.L., lópez-Vélez, R. and Molina, R. (1996) HIV coinfection with a currently non-pathogenic flagel- late. Lancet, 347(8996), 264-265. [11] Rozenthal, S., De Carvalho, T.U. and De Souza, W. (1987) Influence of the endosymbiont on the interaction of Chrithidia deanei with macrophages. Microscopy Elec- tron Biology Cell, 11, 167-179. [12] Barreto-de-Souza, V., Xavier Medeiros, T. and Machado Motta, M.C. (2008) HIV-1 infection and HIV-1 Tat pro- tein permit the survival and replication of a non-patho- genic trypanosomatid in macrophages through TGF beta 1 production. Microbes and Infection, 10(6), 642-649. [13] Podlipaev, S.A. (2001) The more insect trypanosomatids under study-the more diverse Trypanosomatidae appears. International Journal for Parasitology, 31(5-6), 648-652. [14] Podlipaev, S.A., Strurm, N.R. and Fiala, I. (2004) Di- versity of insect trypanossomatids assessed from the splice leader RNA and 5S rRNA genes and intergenic re- gion. Journal of Eukaryotic Microbiology, 51(3), 283- 290. [15] Diasio, R.B. and LoBuglio, A.F. (1996) Immunomodula- tors: Immunosuppressive agents and immunostimulants the pharmacological basis of therapeutics. 9th Edition, Mcgraw Hill, London. [16] Ghalib Ghalib, H.W., Piuvezam, M.R. and Skeiky, Y.A.W. (1993) Interleukin 10 production correlates with pa- thology in human Leishmania donovani infections. Jour- nal of Clinical Investigation, 92(1), 324-329. [17] Carvalho, E.M., Bacellar, O. and Brownell, C. (1994) Restoration of the IFN-g production and lymphocyte proliferation in visceral leishmaniasis. Journal of Immu- nology, 152(12), 5949-5956. [18] Mary, C., Lamouroux, D. and Dunan, S. (1992) Western blot analysis of antibodies to Leishmania infantum anti- gens: potencial of the 14-KD and 16–KD antigens for diagnosis and epidemiologic purposes. American Journal of Tropical Medicine and Hygiene, 47(6), 764-771. [19] Matteoli, F.P., D’Avila-Levy, C.M. and Santos, L.O. (2009) Roles of the endosymbiont and leishmanolysin- like molecules expressed by Crithidia deanei in the in- teraction with mammalian fibroblasts. Experimental Pa- rasitology, 121(3), 246-253. [20] Corrêa-da Silva, M., Fampa, P. and Lessa, L.P. (2006) Colonizatin of Aedes aegypti midgut by the endosymbi- ont-bearing trypanosomatid Blastocrithidia culicis. Para- sitology Research, 99(4), 384-391. [21] D’Avila-Levy, C.M., Santos, L.O., Marinho, F.A. and Matteoli, F.P. (2008) Crithidia deanei: Influence of para- site gp63 homologue on the interaction of endosymbi- ont–harbouring and aposymbiotic strains with Aedes ae- gypty midgut. Experimental Parasitology, 118(3), 345- 353. |

