Paper Menu >>
Journal Menu >>
 Vol.2, No.6, 582-588 (2010) Health doi:10.4236/health.2010.26086 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Enumeration of microbial contaminants in sachet water: a public health challenge Narasimhan Banu*, Himabindu Menakuru Department of Biotechnology, Vels University, Chennai, India; *Corresponding Author: banunkl@yahoo.com Received 18 November 2009; revised 8 March 2010; accepted 10 March 2010. ABSTRACT Accessibility and availability of fresh clean wa- ter is a key to sustainable development and es- sential element in health, food production and poverty reduction. In the present study, we have collected water sachet containing CM/L number and they were analysed for physical and bacte- riological nature. The organisms isolated in this study were Proteus mirabilis, Klebsiella pneu- moniae, Pseudomonas vesicularis and Pseu- domonas aeruginosae. The harmful effects of these isolates were evidenced by antibiotic re- sistance, heavy metal tolerance and antibacte- rial activity. They were resistant to the antibiot- ics like amoxiclav, methicillin, chloramphenicol and streptomycin. They showed tolerance to the heavy metals at 5 mM conc. except for lead. For antibacterial activity, they were tested against human pathogens Klebsiella pnemoniae, Pro- teus mirabilis, Micrococcus leuteus and Sal- monella paratyphium. But at the same time these organisms could be exploited for the in- dustrial production of amylase, protease and cellulase. Keywords: Sachet Water; Bacterial Contaminants; Pathogens; Industrially Useful Bacteria 1. INTRODUCTION Bottled water is defined as water that is intended for human consumption and this is sealed in bottles or other container with no added ingredients except that it may contain safe and suitable fluorides. Its price is within the reach of tautology. Small scale entrepreneurs introduced small nylon sachets which are electrically heated and sealed at both ends to the market and is popularly called pure water. It finds patronage from members of low socio-economic class. Besides, majority of the water sachets do not carry the National Food and Drug Administration Control (NAFD AC) approval number. This means that they are either not registered or the procedures have not completed the registration of their products with NAFDAC. It was therefore considered implication of sachet water. Acces- sibility and availability of fresh clean water is a key to sustainable development and essential element in health, food production and poverty reduction. 1.2 billion Peo- ple around the world lack access to safe water and 2.5 billion are not provided with adequate sanitation (Third World water forum on water, 2003). In metropolis as a whole pipe born water is inadequate both in quality and quantity. Consequently water born diseases such as cholera and typhoid often have their epidemic during the dry season. Typhoid remains a great socio-economic problem in developing countries. Proliferation of intestine is associ- ated with high mortality with wound infection occurring in 50-75% of surviours [1]. Controlling wound sepsis or wound infection also affected mortality [2,3]. Since this health problem was largely traceable to unhygienic water supply, an alternative to the seemingly inadequate water supply was found in bottled water. Drinking water including bottled water, may reasona- bly to expected to contain at least small amounts of some contaminants includes microbial pathogens, organic pollutants like heavy metals. The presence of contami- nants does not necessarily indicated that water poses a health risk. Environmental Protection Agency (EPA) sets standards for approximately 90 contaminants in drinking water. EPA standards, along with each contaminant’s likely source and health effects, are available at www. epa.gov/safewater/mcl.html. Microbiological contamination of water has long been a concern to the public. There is a concern in increasing due to outbreak of coliform bacteria, and protozoans like Giardiasis, Cryptosporidiosis. Coliforms are not a single type of bacteria but a group of bacteria that includes Klebsiella, Proteus, E. coli, and Salmonella. Coliform organisms are not necessarily  N. Banu et al. / HEALTH 2 (2010) 582-588 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 583 583 pathogens and are rarely found in bottled water, they serve as an indicator of insanitation or possible con- tamination. Microbial potability of bottled and packaged drinking water hawked in Ilorin metropolis was done by selecting 81 samples containing 11 brands of drinking water packaged and hawked in cellophane bags, did not met drinking water standards. Pseudomonas was frequently recovered as a contaminant of packaged water [4]. Bot- tled mineral water consumption has significantly in- creased in Brazil. Public health determines the parasi- tological and microbiological status of some brands and found occurrence of Cryptosporidial oocysts and Guardia cyst in bottled mineral water [5]. An assessment of the health and social economic implantations of sa- chet water in Ibadam Nigeria [6] selected 78 samples from 20 brands of sachet water from hawkers/vendors. Bacteria obtained include: Klebsiella sp., Streptococcus faecalis and Pseudomonas aeruginosae. By sterile filtra- tion of water, broad diversity of viable bacteria was iso- lated by using 0.2 μ filter for the removal of microor- ganisms and is commonly referred as ‘sterile filtration’. 19 bacterial taxa were isolated by the acclimatization method from 0.2 micron filtered fresh water samples. Cryptosporidium parvum infection in Bergen and Nor- way was found during the large water borne Giardiasis outbreak [7]. The enforcement of the regulation guiding water qual- ity before the National Agency for Food and Drug Ad- ministration Control (NAFDAC) to employ with the checking water qualities guideline values as recom- mended by World Health Organization (W.H.O) be- comes urgent. The water sachets are the products of middle class en- trepreneurs and some small scale business ventures. The objective of the present study was to find out the quality of sachet water. We have collected seven different brands of water sachet containing CM/L number and the samples were subjected to physical and bacteriological analysis. 2. MATERIALS AND METHODS 2.1. Media Used LB (Luria–Bertani) Agar, King’s B medium, Nutrient Agar and EMB (Eosine Methylene Blue) Agar were used to screen the sachet water sample for bacterial contami- nation. 2.2. Collection of Samples Seven sachet water samples supplied in and around Pal- lavaram were collected. All of them contained CM/L number along with ISI–14543, Ozonized and UV treated. Some of them were Reverse Osmosis processed. 3. PHYSICAL PARAMETERS pH: pH was checked for all the water samples immedi- ately after opened. 4. BACTERIOLOGICAL ANALYSIS ISOLATION AND IDENTIFICATION OF BACTERIA 4.1. Isolation of Bacteria Seven different sachet water samples were taken up for the present study. The samples include Freeze, VSP, VPZ, Aqua fresh, Jai, Hi-tech and Sakthi. The water samples were serially diluted and spread on EMB me- dium, Nutrient agar, King’s B medium and kept for 24 hours incubation at 37˚C. The isolated bacterial colonies were purified to homogeneity by quadrant streaking, store in LBA, NA and KBA slants periodically subcul- tured. 4.2. Identification of Organism The bacteria isolated were identified based on the bio- chemical tests outlined in the Bergey’s Manual of deter- minative bacteriology [8]. 4.3. Antibiotic Resistance/Susceptibility Screening The sensitivity/resistance of the isolates to various anti- biotics such as Chloramphenicol, Ceffriaxone, Amoxi- clav, methicillin, Nalidixic acid and Streptomycin was studied by inoculating a loopful of the overnight grown cultures on Nutrient Agar plates amended with 30μg/ml concentrations of the appropriate antibiotics and incu- bated at 37˚C. After 24 hours of incubation, the plates were observed for growth. Nutrient agar plates without antibiotics served as control. The minimum concentra- tion at which no growth was taken as the MIC (Mini- mum Inhibitory Concentration). 4.4. Heavy Metal Tolerance Spectrum The tolerance of the bacterial isolates to various heavy metals such as zinc (zinc sulphate), lead (lead acetate), copper (copper sulphate), chromium (potassium chro- mate) was studied by inoculating loopful of overnight grown cultures on Nutrient Agar plates amended with 1, 3 and 5mM concentrations of heavy metals and incu- bated at 37˚C. After 24 hours of incubation, the plates were observed for growth. Nutrient agar plates without heavy metal served as control. The minimum concentra- tion at which there was no growth was taken as the MIC  N. Banu et al. / HEALTH 2 (2010) 582-588 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 584 value. 4.5. Screening for Extra Cellular Enzyme Production Four industrially important extra cellular enzymes were selected and screened for primary extra cellular enzyme production. 4.6. Protease Nutrient agar along with 1% Gelatin (substrate) and 1% casein (substrate) was taken in separate conical flasks and autoclaved. They were poured into respective petr- iplates (triplicates). After solidifying, isolates were stre- aked on them and kept for incubation at 37˚C. After 24 hours of incubation, the plates were stained with 15% HgCl2 (indicator) and observed for zone of inhibition. 4.7. Amylase Nutrient agar along with 1% soluble starch (substrate) was taken in a conical flask and then autoclaved. They were poured into respective petriplates (triplicates). Af- ter solidifying, isolates were streaked on them and kept for incubation at 37˚C. After 24 hours of incubation, the plates were stained with Gram’s Iodine (indicator) and observed for zone of inhibition. 4.8. Cellulase Nutrient agar along with 1% cellulose (substrate) was taken in a conical flask and then autoclaved. They were poured into respective petriplates (triplicates). After so- lidifying, isolates were streaked on them and kept for incubation at 37˚C. After 24 hours of incubation, the plates were stained with 0.3% of Congo red (indicator); plates were kept in orbital shaker (mild shaking) for 15 minutes. Congo red was discarded and then 1 N NaCl was added to the plate and kept in shaker for 10 minutes and observed for zone of inhibition. 4.9. Antibacterial Activity of Pure Bacterial Isolates Four bacterial isolates (Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas vesicularis and Pseudomo- nas aeruginosae) from sachet water were selected for antibacterial activity against the four human pathogenic bacteria (Micrococcus luteus, Salmonella paratyphium, Proteus mirabilis and Klebsiella pneumoniae) obtained from CAS Botany, University of Madras. Isolated bacte- ria were grown in King’s B Broth and kept for incuba- tion at 37˚C in orbital shaking incubator for 48 hours. After 48 hours incubation, samples were transferred to sterile centrifuge tubes and centrifuged at 8000 rpm for 15 minutes at 4˚C. Then supernatant was collected and it is filtered using 0.45 μ membrane filter. Filtered super- natant was stored in refrigerator for further study. Human pathogens as mentioned above were grown in Nutrient Broth and incubated for 24 hours in orbital shaking incubator at 37˚C. After 24 hours pathogens were stored in refrigerator for further study. Nutrient agar plates were prepared and pathogens were swabbed on it and well were made on the plates with the help of borer. 100 μl of filtered supernatant of bacterial isolates was added into the wells and kept for incubation for 48 hours. After 48 hours, plates were ob- served for antibacterial activity. 4.10. Antibacterial Activity of Isolates with Organic Solvent The supernatant of the bacterial isolates as mentioned above was taken. To that equal amount of Ethyl Acetate (EA), an universally proved polar organic solvent that could dissolve many compounds was added and kept in orbital shaker for one hour or more preferably overnight. Then transfer the organic layer and distribute it equally in to different conical flasks and cover the flask with cheese cloth to prevent contamination and after complete drying, add EA. To the residue presenting the flask, the supernatant was used for further study. Human pathogens as mentioned above were grown in Nutrient Broth and incubated for 24 hours in orbital shaking incubator at 37˚C. After 24 hours, pathogens were stored in refrigerator for further study. Nutrient agar plates were prepared and pathogens were swabbed on it and well were made on the plates with the help of borer. 100 μl of filtered EA supernatant was added into the wells and keep it for incubation for 48hours. After 48 hours, the plates were observed for antibacterial activity. 5. RESULTS AND DISCUSSION The objective of the present study was to find out the quality of sachet water. For this, we randomly selected seven different brands of sachet water and they were collected from in and around pallavaram, Chennai. The samples were subjected to physical and bacteriological analysis. The water samples were assessed for coliform and other bacteria using Nutrient agar, KB agar and EMB agar. The results for this study support an earlier observation that the sachet water being produced is of questionable quality [9,10]. As useful as sachet water is to the society, the result of the analysis raised doubts as to its quality. The pH of wa- ter sachets has an upper range of 9.26 (Table 1), a value higher than the upper limit of pH 8.5, as recommended by W.H.O. Even though pH has no direct effect on health, its indirect action on physiological processes 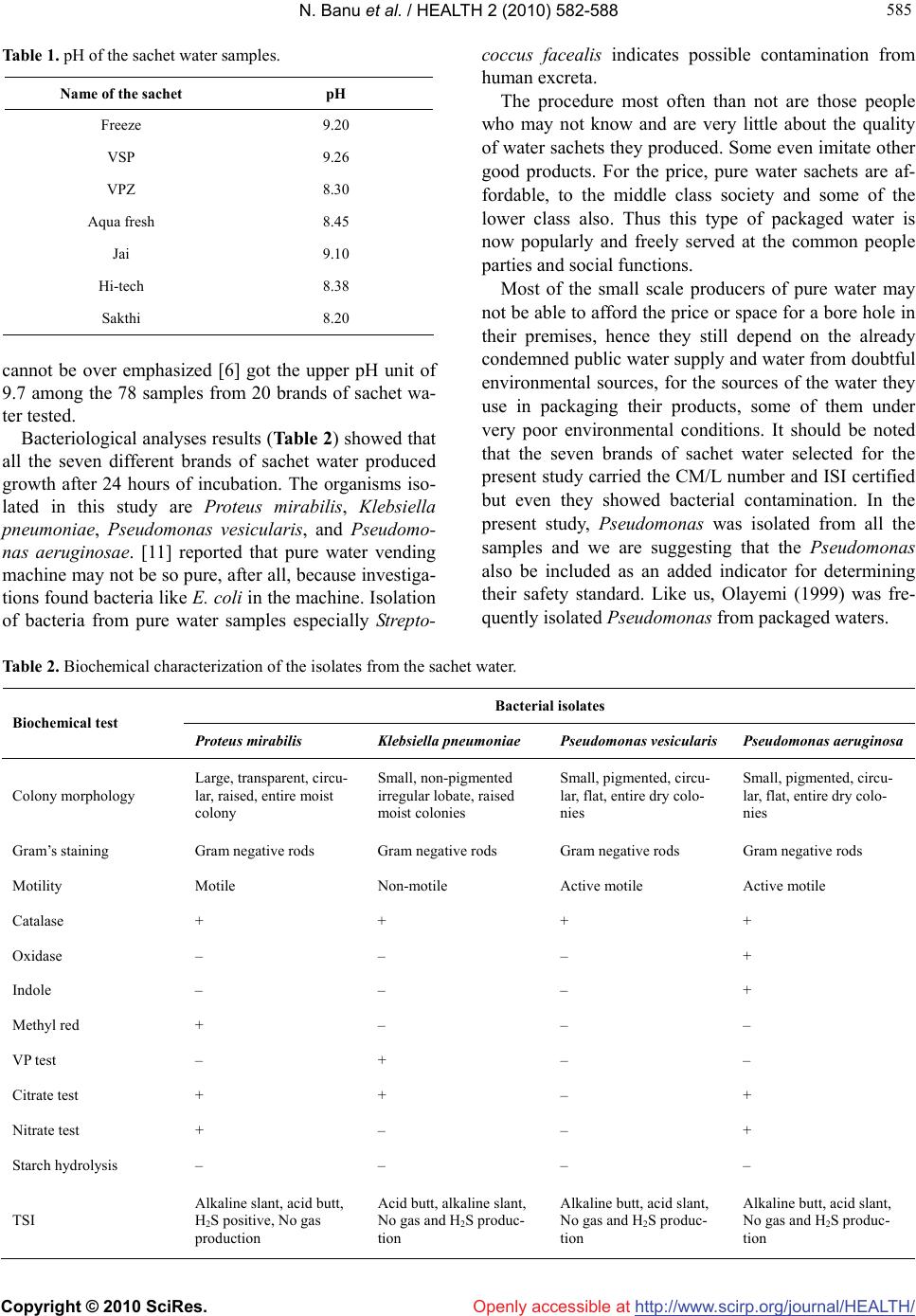 N. Banu et al. / HEALTH 2 (2010) 582-588 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 585 585 Table 1. pH of the sachet water samples. Name of the sachet pH Freeze 9.20 VSP 9.26 VPZ 8.30 Aqua fresh 8.45 Jai 9.10 Hi-tech 8.38 Sakthi 8.20 cannot be over emphasized [6] got the upper pH unit of 9.7 among the 78 samples from 20 brands of sachet wa- ter tested. Bacteriological analyses results (Table 2) showed that all the seven different brands of sachet water produced growth after 24 hours of incubation. The organisms iso- lated in this study are Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas vesicularis, and Pseudomo- nas aeruginosae. [11] reported that pure water vending machine may not be so pure, after all, because investiga- tions found bacteria like E. coli in the machine. Isolation of bacteria from pure water samples especially Strepto- coccus facealis indicates possible contamination from human excreta. The procedure most often than not are those people who may not know and are very little about the quality of water sachets they produced. Some even imitate other good products. For the price, pure water sachets are af- fordable, to the middle class society and some of the lower class also. Thus this type of packaged water is now popularly and freely served at the common people parties and social functions. Most of the small scale producers of pure water may not be able to afford the price or space for a bore hole in their premises, hence they still depend on the already condemned public water supply and water from doubtful environmental sources, for the sources of the water they use in packaging their products, some of them under very poor environmental conditions. It should be noted that the seven brands of sachet water selected for the present study carried the CM/L number and ISI certified but even they showed bacterial contamination. In the present study, Pseudomonas was isolated from all the samples and we are suggesting that the Pseudomonas also be included as an added indicator for determining their safety standard. Like us, Olayemi (1999) was fre- quently isolated Pseudomonas from packaged waters. Table 2. Biochemical characterization of the isolates from the sachet water. Bacterial isolates Biochemical test Proteus mirabilis Klebsiella pneumoniae Pseudomonas vesicularis Pseudomonas aeruginosa Colony morphology Large, transparent, circu- lar, raised, entire moist colony Small, non-pigmented irregular lobate, raised moist colonies Small, pigmented, circu- lar, flat, entire dry colo- nies Small, pigmented, circu- lar, flat, entire dry colo- nies Gram’s staining Gram negative rods Gram negative rods Gram negative rods Gram negative rods Motility Motile Non-motile Active motile Active motile Catalase + + + + Oxidase – – – + Indole – – – + Methyl red + – – – VP test – + – – Citrate test + + – + Nitrate test + – – + Starch hydrolysis – – – – TSI Alkaline slant, acid butt, H2S positive, No gas production Acid butt, alkaline slant, No gas and H2S produc- tion Alkaline butt, acid slant, No gas and H2S produc- tion Alkaline butt, acid slant, No gas and H2S produc- tion 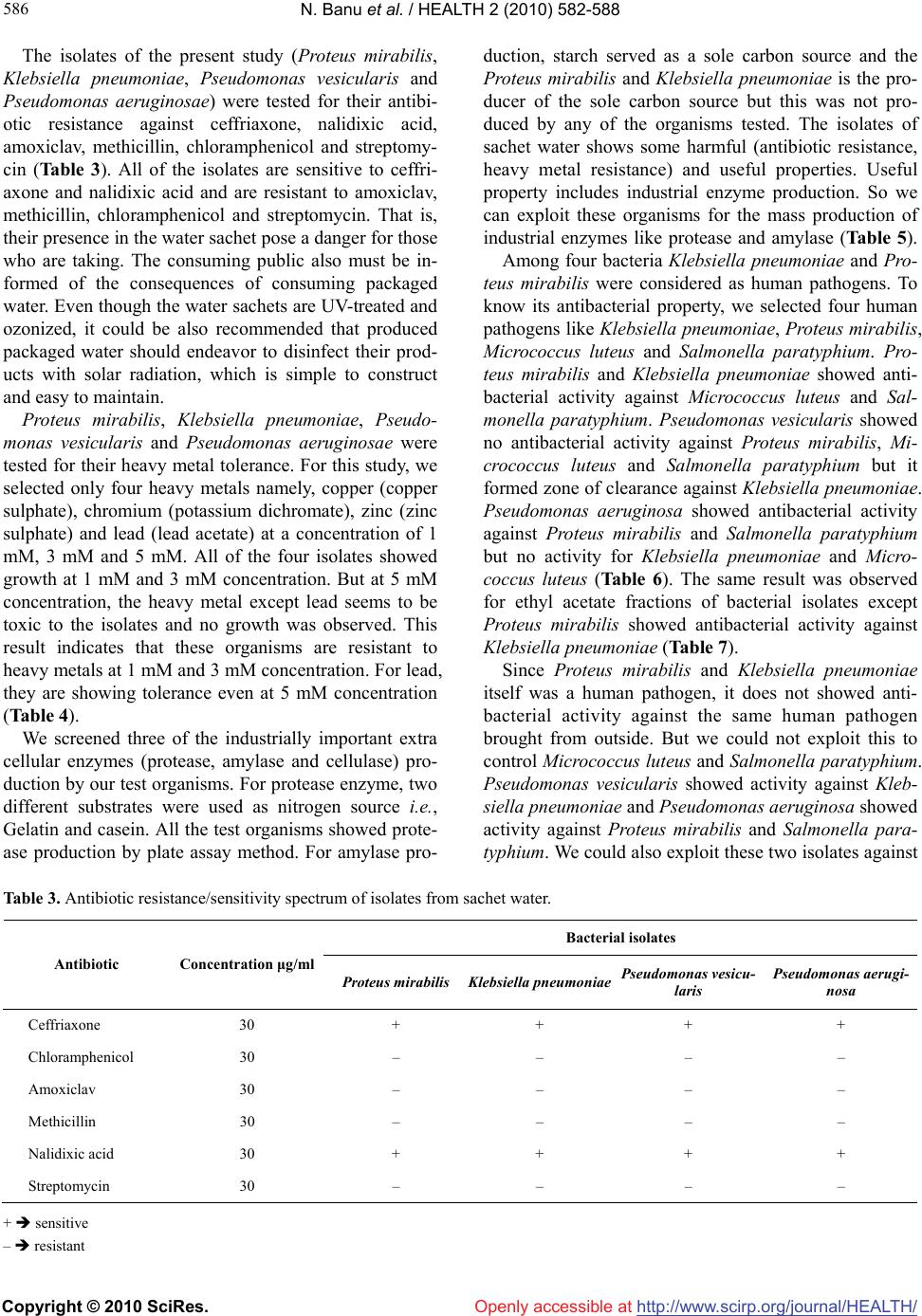 N. Banu et al. / HEALTH 2 (2010) 582-588 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 586 The isolates of the present study (Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas vesicularis and Pseudomonas aeruginosae) were tested for their antibi- otic resistance against ceffriaxone, nalidixic acid, amoxiclav, methicillin, chloramphenicol and streptomy- cin (Table 3). All of the isolates are sensitive to ceffri- axone and nalidixic acid and are resistant to amoxiclav, methicillin, chloramphenicol and streptomycin. That is, their presence in the water sachet pose a danger for those who are taking. The consuming public also must be in- formed of the consequences of consuming packaged water. Even though the water sachets are UV-treated and ozonized, it could be also recommended that produced packaged water should endeavor to disinfect their prod- ucts with solar radiation, which is simple to construct and easy to maintain. Proteus mirabilis, Klebsiella pneumoniae, Pseudo- monas vesicularis and Pseudomonas aeruginosae were tested for their heavy metal tolerance. For this study, we selected only four heavy metals namely, copper (copper sulphate), chromium (potassium dichromate), zinc (zinc sulphate) and lead (lead acetate) at a concentration of 1 mM, 3 mM and 5 mM. All of the four isolates showed growth at 1 mM and 3 mM concentration. But at 5 mM concentration, the heavy metal except lead seems to be toxic to the isolates and no growth was observed. This result indicates that these organisms are resistant to heavy metals at 1 mM and 3 mM concentration. For lead, they are showing tolerance even at 5 mM concentration (Table 4). We screened three of the industrially important extra cellular enzymes (protease, amylase and cellulase) pro- duction by our test organisms. For protease enzyme, two different substrates were used as nitrogen source i.e., Gelatin and casein. All the test organisms showed prote- ase production by plate assay method. For amylase pro- duction, starch served as a sole carbon source and the Proteus mirabilis and Klebsiella pneumoniae is the pro- ducer of the sole carbon source but this was not pro- duced by any of the organisms tested. The isolates of sachet water shows some harmful (antibiotic resistance, heavy metal resistance) and useful properties. Useful property includes industrial enzyme production. So we can exploit these organisms for the mass production of industrial enzymes like protease and amylase (Table 5). Among four bacteria Klebsiella pneumoniae and Pro- teus mirabilis were considered as human pathogens. To know its antibacterial property, we selected four human pathogens like Klebsiella pneumoniae, Proteus mirabilis, Micrococcus luteus and Salmonella paratyphium. Pro- teus mirabilis and Klebsiella pneumoniae showed anti- bacterial activity against Micrococcus luteus and Sal- monella paratyphium. Pseudomonas vesicularis showed no antibacterial activity against Proteus mirabilis, Mi- crococcus luteus and Salmonella paratyphium but it formed zone of clearance against Klebsiella pneumoniae. Pseudomonas aeruginosa showed antibacterial activity against Proteus mirabilis and Salmonella paratyphium but no activity for Klebsiella pneumoniae and Micro- coccus luteus (Table 6). The same result was observed for ethyl acetate fractions of bacterial isolates except Proteus mirabilis showed antibacterial activity against Klebsiella pneumoniae (Table 7). Since Proteus mirabilis and Klebsiella pneumoniae itself was a human pathogen, it does not showed anti- bacterial activity against the same human pathogen brought from outside. But we could not exploit this to control Micrococcus luteus and Salmonella paratyphium. Pseudomonas vesicularis showed activity against Kleb- siella pneumoniae and Pseudomonas aeruginosa showed activity against Proteus mirabilis and Salmonella para- typhium. We could also exploit these two isolates against Table 3. Antibiotic resistance/sensitivity spectrum of isolates from sachet water. Bacterial isolates Antibiotic Concentration μg/ml Proteus mirabilis Klebsiella pneumoniaePseudomonas vesicu- laris Pseudomonas aerugi- nosa Ceffriaxone 30 + + + + Chloramphenicol 30 – – – – Amoxiclav 30 – – – – Methicillin 30 – – – – Nalidixic acid 30 + + + + Streptomycin 30 – – – – + sensitive – resistant 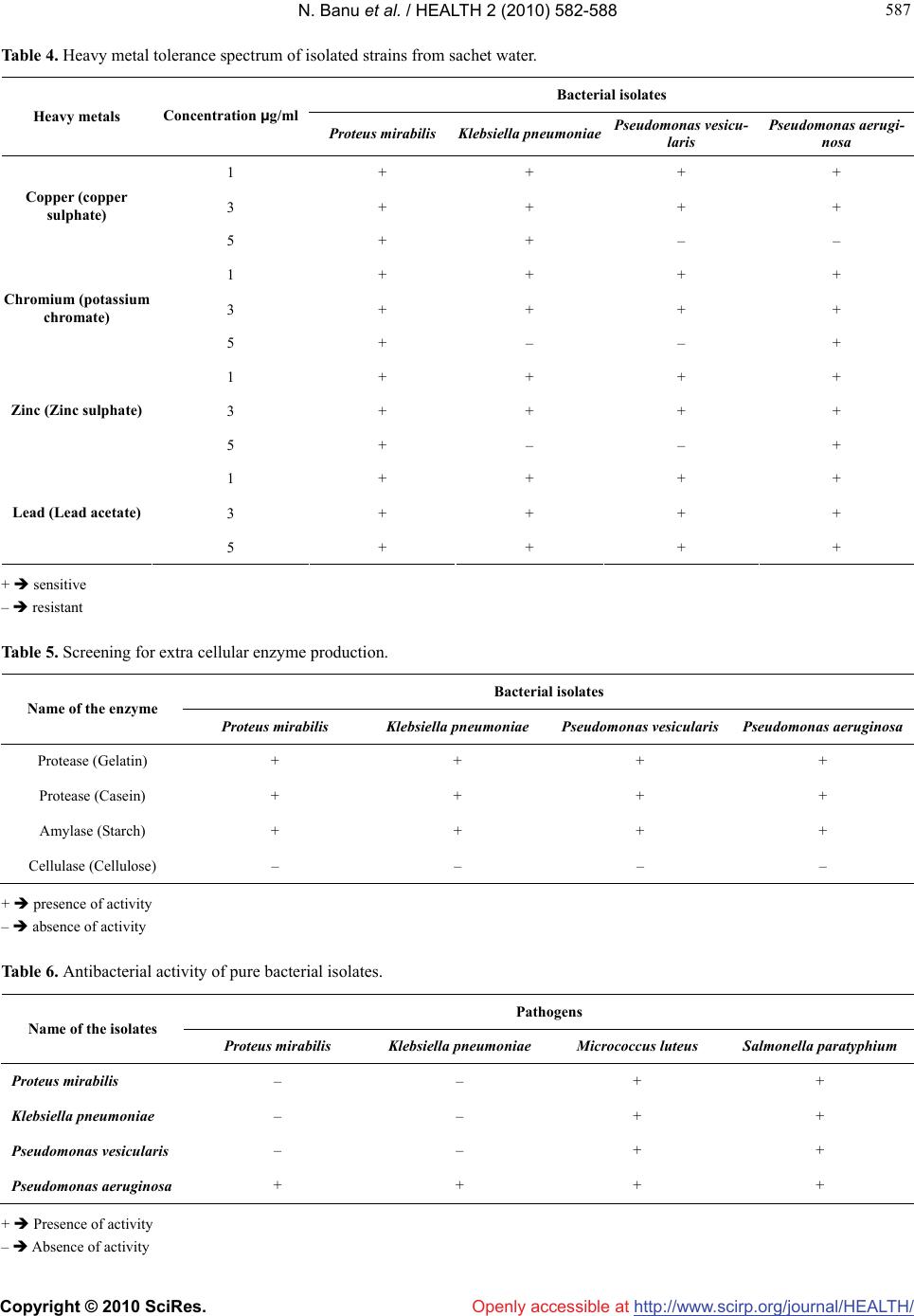 N. Banu et al. / HEALTH 2 (2010) 582-588 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 587 587 Table 4. Heavy metal tolerance spectrum of isolated strains from sachet water. Bacterial isolates Heavy metals Concentration μg/ml Proteus mirabilis Klebsiella pneumoniaePseudomonas vesicu- laris Pseudomonas aerugi- nosa 1 + + + + 3 + + + + Copper (copper sulphate) 5 + + – – 1 + + + + 3 + + + + Chromium (potassium chromate) 5 + – – + 1 + + + + 3 + + + + Zinc (Zinc sulphate) 5 + – – + 1 + + + + 3 + + + + Lead (Lead acetate) 5 + + + + + sensitive – resistant Table 5. Screening for extra cellular enzyme production. Bacterial isolates Name of the enzyme Proteus mirabilis Klebsiella pneumoniae Pseudomonas vesicularis Pseudomonas aeruginosa Protease (Gelatin) + + + + Protease (Casein) + + + + Amylase (Starch) + + + + Cellulase (Cellulose) – – – – + presence of activity – absence of activity Table 6. Antibacterial activity of pure bacterial isolates. Pathogens Name of the isolates Proteus mirabilis Klebsiella pneumoniae Micrococcus luteus Salmonella paratyphium Proteus mirabilis – – + + Klebsiella pneumoniae – – + + Pseudomonas vesicularis – – + + Pseudomonas aeruginosa + + + + + Presence of activity – Absence of activity 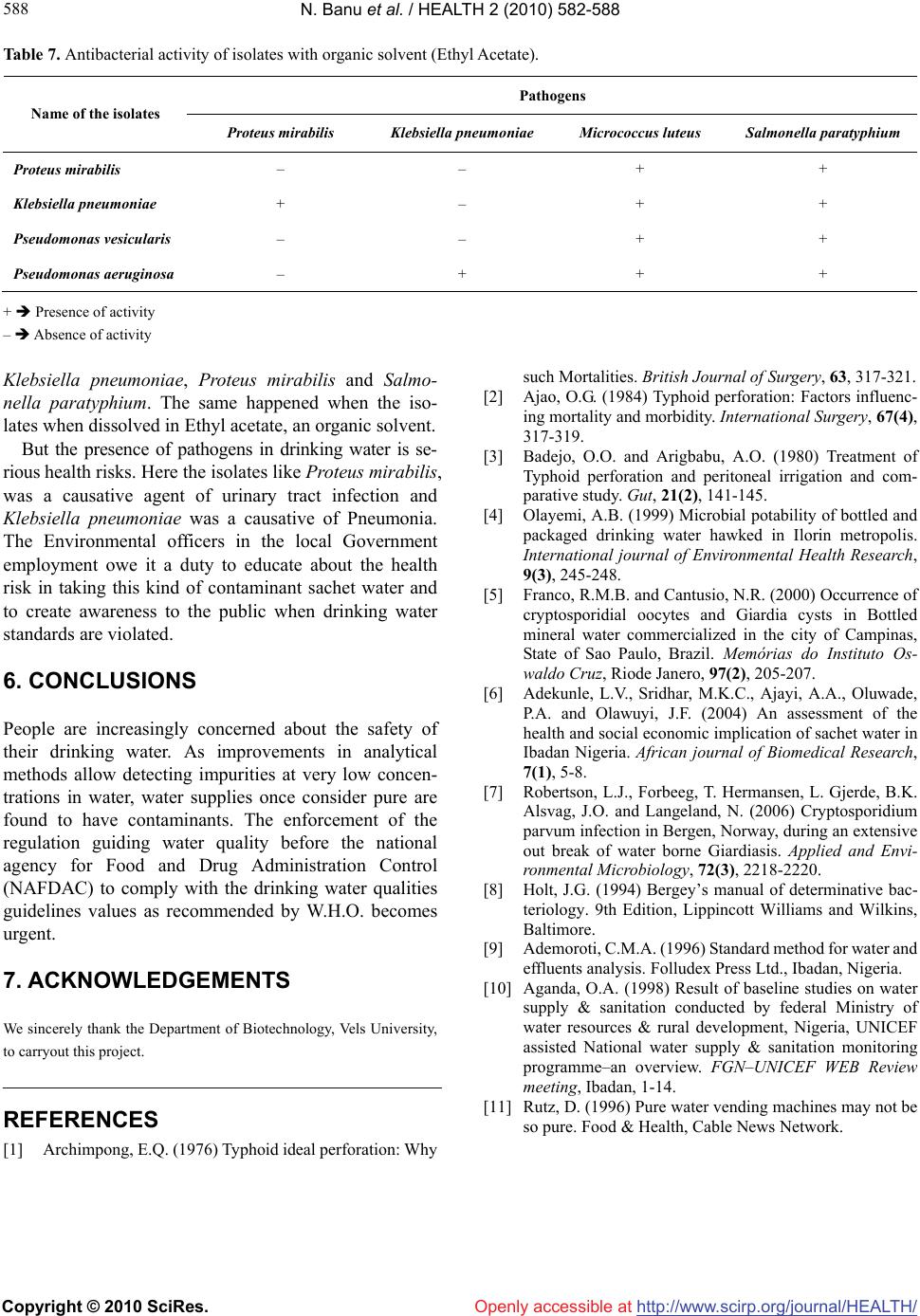 N. Banu et al. / HEALTH 2 (2010) 582-588 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 588 Table 7. Antibacterial activity of isolates with organic solvent (Ethyl Acetate). Pathogens Name of the isolates Proteus mirabilis Klebsiella pneumoniae Micrococcus luteus Salmonella paratyphium Proteus mirabilis – – + + Klebsiella pneumoniae + – + + Pseudomonas vesicularis – – + + Pseudomonas aeruginosa – + + + + Presence of activity – Absence of activity Klebsiella pneumoniae, Proteus mirabilis and Salmo- nella paratyphium. The same happened when the iso- lates when dissolved in Ethyl acetate, an organic solvent. But the presence of pathogens in drinking water is se- rious health risks. Here the isolates like Proteus mirabilis, was a causative agent of urinary tract infection and Klebsiella pneumoniae was a causative of Pneumonia. The Environmental officers in the local Government employment owe it a duty to educate about the health risk in taking this kind of contaminant sachet water and to create awareness to the public when drinking water standards are violated. 6. CONCLUSIONS People are increasingly concerned about the safety of their drinking water. As improvements in analytical methods allow detecting impurities at very low concen- trations in water, water supplies once consider pure are found to have contaminants. The enforcement of the regulation guiding water quality before the national agency for Food and Drug Administration Control (NAFDAC) to comply with the drinking water qualities guidelines values as recommended by W.H.O. becomes urgent. 7. ACKNOWLEDGEMENTS We sincerely thank the Department of Biotechnology, Vels University, to carryout this project. REFERENCES [1] Archimpong, E.Q. (1976) Typhoid ideal perforation: Why such Mortalities. British Journal of Surgery, 63, 317-321. [2] Ajao, O.G. (1984) Typhoid perforation: Factors influenc- ing mortality and morbidity. International Surgery, 67(4), 317-319. [3] Badejo, O.O. and Arigbabu, A.O. (1980) Treatment of Typhoid perforation and peritoneal irrigation and com- parative study. Gut, 21(2), 141-145. [4] Olayemi, A.B. (1999) Microbial potability of bottled and packaged drinking water hawked in Ilorin metropolis. International journal of Environmental Health Research, 9(3), 245-248. [5] Franco, R.M.B. and Cantusio, N.R. (2000) Occurrence of cryptosporidial oocytes and Giardia cysts in Bottled mineral water commercialized in the city of Campinas, State of Sao Paulo, Brazil. Memórias do Instituto Os- waldo Cruz, Riode Janero, 97(2), 205-207. [6] Adekunle, L.V., Sridhar, M.K.C., Ajayi, A.A., Oluwade, P.A. and Olawuyi, J.F. (2004) An assessment of the health and social economic implication of sachet water in Ibadan Nigeria. African journal of Biomedical Research, 7(1), 5-8. [7] Robertson, L.J., Forbeeg, T. Hermansen, L. Gjerde, B.K. Alsvag, J.O. and Langeland, N. (2006) Cryptosporidium parvum infection in Bergen, Norway, during an extensive out break of water borne Giardiasis. Applied and Envi- ronmental Microbiology, 72(3), 2218-2220. [8] Holt, J.G. (1994) Bergey’s manual of determinative bac- teriology. 9th Edition, Lippincott Williams and Wilkins, Baltimore. [9] Ademoroti, C.M.A. (1996) Standard method for water and effluents analysis. Folludex Press Ltd., Ibadan, Nigeria. [10] Aganda, O.A. (1998) Result of baseline studies on water supply & sanitation conducted by federal Ministry of water resources & rural development, Nigeria, UNICEF assisted National water supply & sanitation monitoring programme–an overview. FGN–UNICEF WEB Review meeting, Ibadan, 1-14. [11] Rutz, D. (1996) Pure water vending machines may not be so pure. Food & Health, Cable News Network. |

