Paper Menu >>
Journal Menu >>
 Chinese Medicine, 2010, 1, 28-29 doi:10.4236/cm.2010.11005 Published Online June 2010 (http://www.SciRP.org/journal/cm) Copyright © 2010 SciRes. CM Non-Exclusion Effects in Aqueous Size-Exclusion Chromatography of Polysaccharides Azamat Solievich Boymirzaev1, Abbaskhan Sabirkhanovich Turaev2 1Department of Mechanical-Technology, Namangan Engineering-Economic Institute, Namangan, Uzbekistan 2O.Sadikov Institute of Bioorganic Chemistry Uzbek Academy of Sciences, Tashkent, Uzbekistan E-mail: azamat58@mail.ru Received January 10, 2010; revised April 29, 2010; accepted May 10, 2010 Abstract This paper concerned to investigation of aggregate formation processes in aqueous Size-exclusion chroma- tography (SEC) of Na-carboxymethylcellulose (Na-CMC). Keywords: SEC, Na Carboxymethylcellulose, Aggregate Formation 1. Introduction Size-exclusion chromatography is one of the powerful methods for determination and investigation of molecu- lar weight distribution of polymers [1]. In aqueous SEC of polymers [2], the understanding of the separation mechanism demands much more attention due to the enthalpy interactions distorting a pure size-exclusion separation mechanism [1]. Because of the presence of polar, and often anionic, groups in the stationary phases used in SEC, the mobile phase must be carefully chosen to repress polymer-gel and intermolecular interactions. This is particularly important in SEC of polyelectrolytes and polar molecules such as carbohydrates [3]. Suppres- sion of interactions, such as polyelectrolyte expansion, ion-exclusion, molecular adsorption and aggregate for- mation depends on nature of electrolyte, optimal value of pH and ionic strength of eluent. The aim of this paper is to investigate of aggregate formation process in SEC of Na-CMC in order to deter- mine of the suitable aqueous eluent for true size-exclu- sion separation mechanism of macromolecules. 2. Materials and Methods SEC was performed on the liquid chromatograph, con- sisting from syringe pump Merk-Hitachi L-6000A model, Shodex RI-101 refractive index detector, multiangle laser light scattering detector DAWN NSP (Watt technology), manual sample injector Rheodine 2104, degasser of elu- ent and two chromatographic columns PL Aquagel-OH Mixed termostated at 25˚C and connected in series. Syn- thesis of Na-CMC was described in [4]. SEC analysis were performed using two types of eluent: NaCl and NaNO3 in the water with concentration 0.1 mol/L. 3. Results and Discussion Many of hydrophilic polymers are polyelectrolytes and, therefore, their elution properties in SEC is complicated by various non-exclusion effects, such as ion exclusion, polyelectrolyte expansion, molecular adsorption, and aggregate formation, which distort the normal SEC separation mechanism. These effects can be eliminated by increasing the ionic strength and changing the pH of the eluent so as to decrease the degree of dissociation of ionic groups both in the macromolecular chain and on the sorbent surface [5]. Physicochemical properties such as structure, molecular weight and shape or conformation are primary factors controlling their functional properties. A typical molar mass sensitive detector is a multi angle laser light scattering (MALLS). This detector has the advantage of providing structural information in addition to the molar masses. Analysis of CMC by SEC in 0.1 М NaNO3 solutions were complicated by presence of the low amount associates forming due to intermolecular interac- tions [6,7]. To avoid of the aggregates of macromolecules Hoogendam C.W. [7] demonstrated that the solutes Na-CMC in first step were prepared in pure water, after 0.1 M NaNO3 were added to sample solution. We have received bimodal chromatograms of CMC from MALLS detector in SEC analysis when used of water consisting NaNO3 with concentration 0.1 mol/L (Figure 1(a)). Same result was occurring, when we used 0.1 M NaNO3 in water as eluent. But when 0.1 M NaCl was used first peak in the chromatogram is disappeared indicating that 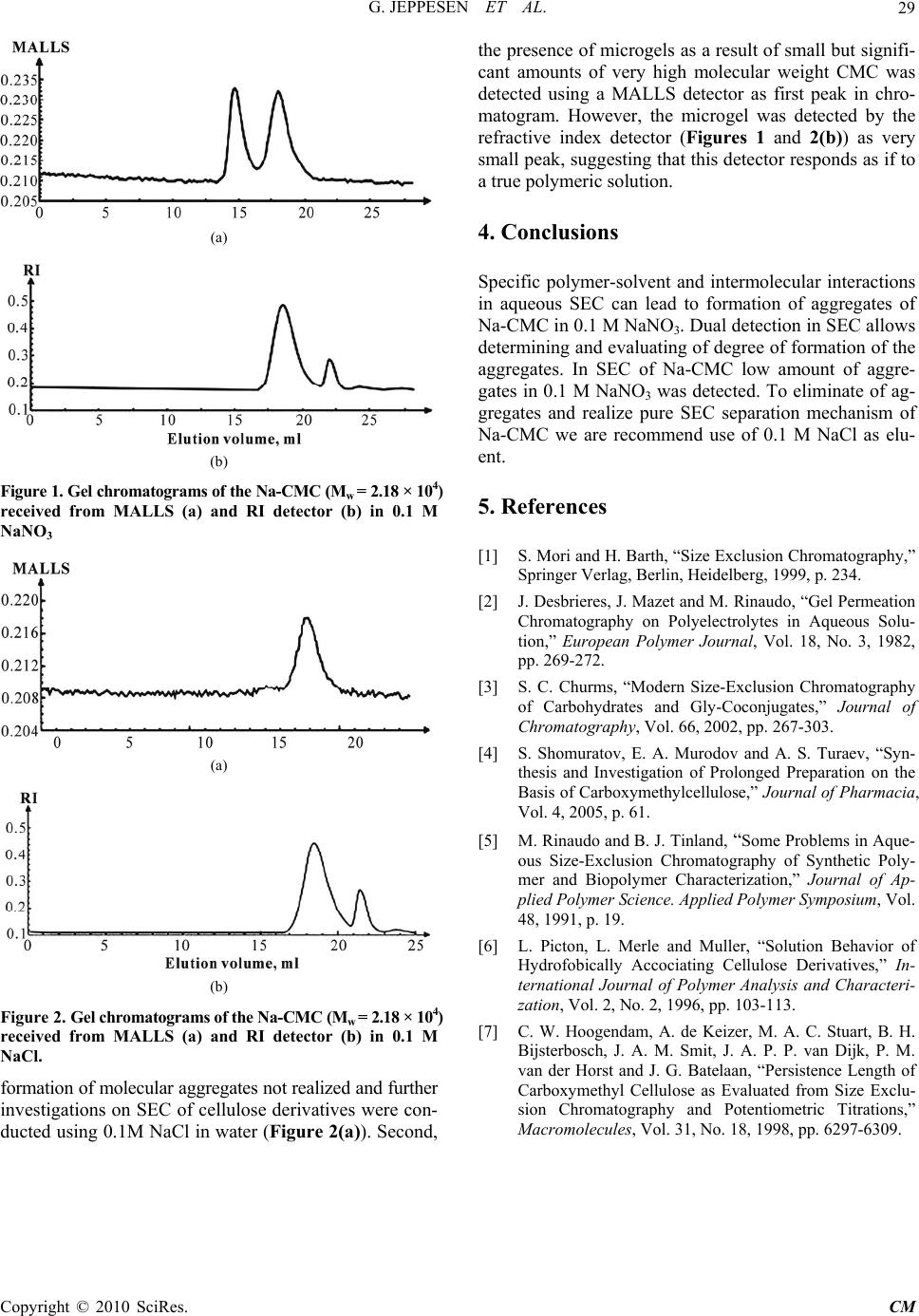 G. JEPPESEN ET AL.29 (a) (b) Figure 1. Gel chromatograms of the Na-CMC (Mw = 2.18 × 104) received from MALLS (a) and RI detector (b) in 0.1 M NaNO3 (a) (b) Figure 2. Gel chromatograms of the Na-CMC (Mw = 2.18 × 104) received from MALLS (a) and RI detector (b) in 0.1 M NaCl. formation of molecular aggregates not realized and further investigations on SEC of cellulose derivatives were con- ducted using 0.1M NaCl in water (Figure 2(a)). Second, the presence of microgels as a result of small but signifi- cant amounts of very high molecular weight CMC was detected using a MALLS detector as first peak in chro- matogram. However, the microgel was detected by the refractive index detector (Figures 1 and 2(b)) as very small peak, suggesting that this detector responds as if to a true polymeric solution. 4. Conclusions Specific polymer-solvent and intermolecular interactions in aqueous SEC can lead to formation of aggregates of Na-CMC in 0.1 M NaNO3. Dual detection in SEC allows determining and evaluating of degree of formation of the aggregates. In SEC of Na-CMC low amount of aggre- gates in 0.1 M NaNO3 was detected. To eliminate of ag- gregates and realize pure SEC separation mechanism of Na-CMC we are recommend use of 0.1 M NaCl as elu- ent. 5. References [1] S. Mori and H. Barth, “Size Exclusion Chromatography,” Springer Verlag, Berlin, Heidelberg, 1999, p. 234. [2] J. Desbrieres, J. Mazet and M. Rinaudo, “Gel Permeation Chromatography on Polyelectrolytes in Aqueous Solu- tion,” European Polymer Journal, Vol. 18, No. 3, 1982, pp. 269-272. [3] S. C. Churms, “Modern Size-Exclusion Chromatography of Carbohydrates and Gly-Coconjugates,” Journal of Chromatography, Vol. 66, 2002, pp. 267-303. [4] S. Shomuratov, E. A. Murodov and A. S. Turaev, “Syn- thesis and Investigation of Prolonged Preparation on the Basis of Carboxymethylcellulose,” Journal of Pharmacia, Vol. 4, 2005, p. 61. [5] M. Rinaudo and B. J. Tinland, “Some Problems in Aque- ous Size-Exclusion Chromatography of Synthetic Poly- mer and Biopolymer Characterization,” Journal of Ap- plied Polymer Science. Applied Polymer Symposium, Vol. 48, 1991, p. 19. [6] L. Picton, L. Merle and Muller, “Solution Behavior of Hydrofobically Accociating Cellulose Derivatives,” In- ternational Journal of Polymer Analysis and Characteri- zation, Vol. 2, No. 2, 1996, pp. 103-113. [7] C. W. Hoogendam, A. de Keizer, M. A. C. Stuart, B. H. Bijsterbosch, J. A. M. Smit, J. A. P. P. van Dijk, P. M. van der Horst and J. G. Batelaan, “Persistence Length of Carboxymethyl Cellulose as Evaluated from Size Exclu- sion Chromatography and Potentiometric Titrations,” Macromolecules, Vol. 31, No. 18, 1998, pp. 6297-6309. Copyright © 2010 SciRes. CM |

