Paper Menu >>
Journal Menu >>
 Chinese Medicine, 2010, 1, 5-17 doi:10.4236/cm.2010.11002 Published Online June 2010 (http://www.SciRP.org/journal/cm) Copyright © 2010 SciRes. CM Effects of Indole-3-Carbinol and Flavonoids Administered Separately and in Combination on Nitric Oxide Production and iNOS Expression in Rats Evita Rostoka1, Larisa Baumane1, Sergejs Isajevs2, Aija Line3, Karina Silina3, Maija Dzintare1, Darja Svirina2, Jelena Sharipova1, Ivars Kalvinsh1, Nikolajs Sjakste1,2 1Latvian Institute o f Organic Synthesis, Riga, Latvia 2Faculty of Medicine, University of Latvia, Riga, Latvia 3Latvian Biomedical Research and Study Centre, Riga, Latvia E-mail: Nikolajs.Sjakste@lu.lv Received January 20, 2010; revised April 29, 2010; accept ed May 10, 2010 Abstract Beneficial effects of natural compounds are often attributed to modulation of NO production; however ef- fects produced by plant extracts do not correlate with effects of purified components. The goal of our work was to study ability of flavonoids and indole-3-carbinol, as well as their combinations to modify NO produc- tion, iNOS gene and protein expression in rat tissues. Baicalein and luteolin decreased NO concentration in both intact and LPS-treated animals. Baicalein decreased iNOS gene expression. Luteolin decreased NO production in the liver and heart and number of iNOS-positive cells in the liver of LPS-treated animals. Combination of the two substances did not decrease the NO synthesis triggered by LPS, although it de- creased iNOS gene expression. Quercetin decreased NO production in the heart, kidneys and blood of intact rats, but enhanced the LPS effect in testes, spleen and blood on NO production and iNOS protein expression in the liver. Indole-3-carbinol decreased NO concentration in the cerebellum, blood, lungs and skeletal mus- cles. The drug enhanced the LPS-triggered increase of NO production in all rat organs. It increased iNOS protein expression in intact liver; however it decreased the LPS-triggered outburst of the enzyme biosynthe- sis. Combination of indole3-carbinol with quercetin decreased NO production in LPS-treated animals how- ever it slightly increased iNOS gene expression. Taken together our results suggest that modifications of NO level in tissues by a natural compound cannot be predicted from data about its effects on NOS expression or activity. Combination of substances can produce an effect differing from that of individual substances. This stresses importance of direct measurements of NO in the tissues. Keywords: Nitric Oxide, Baicalein, Luteolin, Indole-3-Carbinol, Quercetin, Inducible Nitric Oxide Synthase 1. Introduction Natural biologically active compounds of plant origin including flavonoids are main active substances of tradi- tional Chinese medicines: herbal extracts and similar preparations. Nowadays some of these substances are used in purified form as drugs. Anti-inflammatory activ- ity, antioxidant activities, anticancer activity of phyto- genic antineoplastic agents, and neuroprotective effects of Chinese herbal drugs are in focus of interest of many researchers worldwide [1]. Chinese traditional medicines are known to influence also nitric oxide enzymatic pro- duction and NO synthase activity [2]. It is supposed that flavonoid intake influences mortality from nitric ox- ide-dependent processes: ischemic heart disease, stroke, diabetes mellitus, and cancer [3], NO production is also modified by chemicals of plant origin [4]. This implies significance of flavonoid and other natural compound uptake for functions of cardiovascular, immune and nervous systems. However biological activity of poly- phenol-rich food product does not correlate with effects that could be deduced from effects of individual com- pounds on NO synthase activity. For example, red wine is known as vasodilator [5], however purified quercetin, that is abundant in red wine inhibits iNOS gene expres- sion [6,7]. It also destabilizes eNOS mRNA [8] and is  E. ROSTOKA ET AL. 6 even considered to be inhibitor of the NOS enzymatic activity [9]. Nevertheless the compound produces vaso- relaxing effects [10] despite the fact that nitric oxide re- lease in rat aorta is not detected after quercetin admini- stration [11]. In this work we have studied the ability of several natural compounds of plant origin administered separately and in combinations to modify NO production in rat tissues monitored by ESR spectroscopy of Fe (DETC)2-NO complexes conducted in parallel to evalua- tion of iNOS gene expression assayed by real-time RT- PCR technique. Combination of direct NO detection in tissues with other approaches characterizing NO produc- tion at different levels enabled us to reveal unforeseen effects of presumable NO-donors, anaesthetics and an anti-ischemic drug [12-14]. The same approach was ap- plied this time to natural compounds. Flavonoids luteolin, baicalein and quercetin as well as simple phenolic com- pound indole-3-carbinol were chosen among many other compounds after a piloting study. Luteolin, 3’, 4’, 5, 7-tetrahydroxyflavone is abundant in vegetables: roots of celery, rutabaga, red pepper, spin- ach and flowering plants: Ajuga decumbens, Taraxacum officinale (dandelion), Medicago sativa (alfalfa). Luteo- lin is known as dietary compound with antioxidant activ- ity [15]. Baicalein (5, 6, 7-trihydroxyflavone) is found in Scutellaria baicalensis Georgi roots. Quercetin (penta- hydroxyflavonol) is found in numerous higher plants. This flavonol is abundant in onions, apples, leaf vegeta- bles, beans, tea, red wine, clover, pollen. Indole-3-carbinol (3-indolmetanol) is found in Mustard family plants: (Brassica sp.): cabbage, broccoli, Brussels sprouts. The compound is widely studied as chemotherapeutic agent for cancer treatment [16]. Chemical structures of the compounds are given in Figure 1. Literature data indi- cated possible impact of all the three substances on NOS expression and/or NO production [15-22]. The chosen compounds are active substances in several drugs used in Chinese medicine. Quercetin is in important component of Shaofu Zhuyu decoction active extract [23], Saururus chinensis, a herb used traditionally in Chinese medicine for treatment of urological diseases [24], together with luteolin it is found in tree peony yellow flowers also widely used in Chinese medicine [25]. Baicalein is an active component of numerous Chinese medicines in- cluding Niu Huang Jie Du Pill [26]. The aims of the present work were: 1) To study effects NO production in several organs of intact rats and in LPS model of sepsis; 2) To reveal modifications of NO pro- duction by luteolin, baicalein, quercetin and indole-3- carbinol given separately and in combinations in both intact and LPS-treated animals; 3) To study contribution of changes in iNOS gene and protein expression in modifications of NO production by natural compounds and their modifications. Figure 1. Chemical structures of luteolin, baicalein, quer- cetin and indole-3-carbinole. 2. Material and Methods 2.1. Chemicals Indole-3-carbinol, quercetin, baicalein and luteolin were purchased from Dayang Chemical Co., LTD (Hangzhou, China). Lipopolysaccharide, diethylthiocarbamate, fer- rous sulfate, sodium citrate, TRI reagent and all other chemicals were from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany). 2.2. Experiment Design and Drug Administration Animals were obtained from the Laboratory of Experi- mental Animals, Riga Stradins University, Riga, Latvia. All experimental procedures were carried out in accor- dance with guidelines of the Directive 86/609/EEC “European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes” (1986) and were approved by the Animal Eth- ics Committee of the Food and Veterinary Service (Riga, Latvia). Wistar male rats, each weighing 215.00 ± 5.63 g at the beginning of the experiments, were used in all the work. The environment was maintained at a temperature of 22 ± 0.5˚C with a 12-h light/dark cycle. The animals were fed a standard laboratory diet. Description of the experi- mental groups is given in Table 1. In NO production experiments substances were administered per os in con- centrations indicated in the Table 1. 3.5 hours after sub- stance administration spin trap was injected, after 30 min Copyright © 2010 SciRes. CM 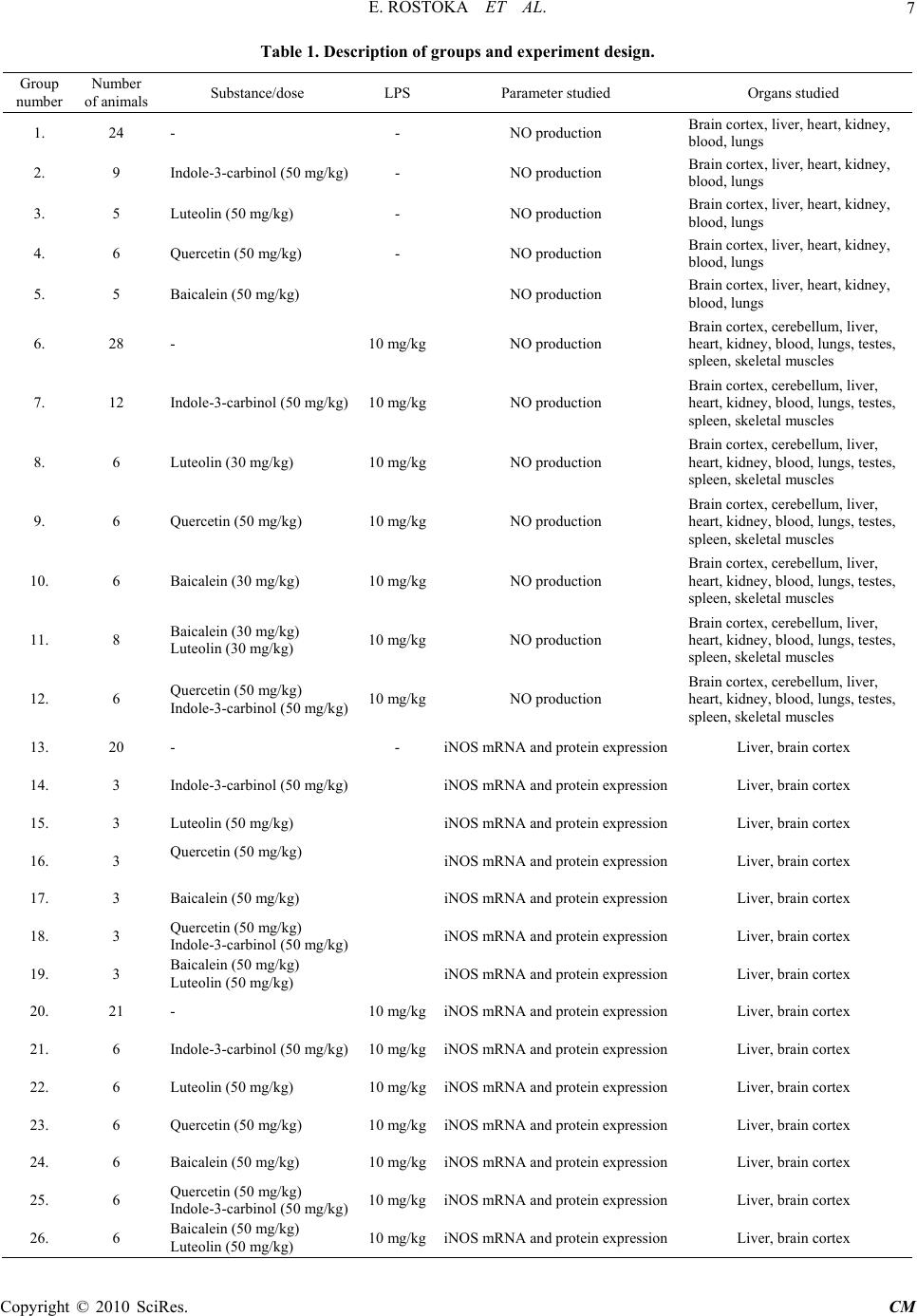 E. ROSTOKA ET AL. Copyright © 2010 SciRes. CM 7 Table 1. Description of groups and exper i ment design. Group number Number of animals Substance/dose LPS Parameter studied Organs studied 1. 24 - - NO production Brain cortex, liver, heart, kidney, blood, lungs 2. 9 Indole-3-carbinol (50 mg/kg) - NO production Brain cortex, liver, heart, kidney, blood, lungs 3. 5 Luteolin (50 mg/kg) - NO production Brain cortex, liver, heart, kidney, blood, lungs 4. 6 Quercetin (50 mg/kg) - NO production Brain cortex, liver, heart, kidney, blood, lungs 5. 5 Baicalein (50 mg/kg) NO production Brain cortex, liver, heart, kidney, blood, lungs 6. 28 - 10 mg/kgNO production Brain cortex, cerebellum, liver, heart, kidney, blood, lungs, testes, spleen, skeletal muscles 7. 12 Indole-3-carbinol (50 mg/kg) 10 mg/kgNO production Brain cortex, cerebellum, liver, heart, kidney, blood, lungs, testes, spleen, skeletal muscles 8. 6 Luteolin (30 mg/kg) 10 mg/kgNO production Brain cortex, cerebellum, liver, heart, kidney, blood, lungs, testes, spleen, skeletal muscles 9. 6 Quercetin (50 mg/kg) 10 mg/kgNO production Brain cortex, cerebellum, liver, heart, kidney, blood, lungs, testes, spleen, skeletal muscles 10. 6 Baicalein (30 mg/kg) 10 mg/kgNO production Brain cortex, cerebellum, liver, heart, kidney, blood, lungs, testes, spleen, skeletal muscles 11. 8 Baicalein (30 mg/kg) Luteolin (30 mg/kg) 10 mg/kgNO production Brain cortex, cerebellum, liver, heart, kidney, blood, lungs, testes, spleen, skeletal muscles 12. 6 Quercetin (50 mg/kg) Indole-3-carbinol (50 mg/kg) 10 mg/kgNO production Brain cortex, cerebellum, liver, heart, kidney, blood, lungs, testes, spleen, skeletal muscles 13. 20 - - iNOS mRNA and protein expressionLiver, brain cortex 14. 3 Indole-3-carbinol (50 mg/kg) iNOS mRNA and protein expressionLiver, brain cortex 15. 3 Luteolin (50 mg/kg) iNOS mRNA and protein expressionLiver, brain cortex 16. 3 Quercetin (50 mg/kg) iNOS mRNA and protein expressionLiver, brain cortex 17. 3 Baicalein (50 mg/kg) iNOS mRNA and protein expressionLiver, brain cortex 18. 3 Quercetin (50 mg/kg) Indole-3-carbinol (50 mg/kg) iNOS mRNA and protein expressionLiver, brain cortex 19. 3 Baicalein (50 mg/kg) Luteolin (50 mg/kg) iNOS mRNA and protein expressionLiver, brain cortex 20. 21 - 10 mg/kgiNOS mRNA and protein expressionLiver, brain cortex 21. 6 Indole-3-carbinol (50 mg/kg) 10 mg/kgiNOS mRNA and protein expressionLiver, brain cortex 22. 6 Luteolin (50 mg/kg) 10 mg/kgiNOS mRNA and protein expressionLiver, brain cortex 23. 6 Quercetin (50 mg/kg) 10 mg/kgiNOS mRNA and protein expressionLiver, brain cortex 24. 6 Baicalein (50 mg/kg) 10 mg/kgiNOS mRNA and protein expressionLiver, brain cortex 25. 6 Quercetin (50 mg/kg) Indole-3-carbinol (50 mg/kg) 10 mg/kgiNOS mRNA and protein expressionLiver, brain cortex 26. 6 Baicalein (50 mg/kg) Luteolin (50 mg/kg) 10 mg/kgiNOS mRNA and protein expressionLiver, brain cortex  E. ROSTOKA ET AL. Copyright © 2010 SciRes. CM 8 rats were decapitated under slight ether narcosis. In sev- eral groups lipopolysaccharide (10 mg/kg) was intrap- eritoneally injected to rats, substances or their combina- tions were administered per os in the same time, spin traps were administered 3.5 hours later, 30 minutes after spin trap injection rats were decapitated under slight ether narcosis. In additional piloting series of experi- ments (not shown) iNOS inhibitor AMT (2 mg/kg), was administered intraperitoneally shortly before spin-trap administration, 30 minutes later rats were decapitated. For real-time PCR and immunochemistry rats were de- capitated under slight ether narcosis; liver tissue was taken for RNA extraction and immunohistochemical examination. Brain cortex tissue was also taken from some animals for immunohistochemistry. Natural com- pounds and LPS were administered following the above time schedule. 2.3. Administration of Spin Trap Agents To determine production level of nitric oxide in the tis- sues we used ESR spectroscopy of paramagnetic Fe- diethylthiocarbamate–nitric oxide complex (Fe (DETC)2- NO) [27]. Spin traps were administered 30 minutes be- fore the sacrifice. Rats were administered 400 mg/kg of the nitric oxide scavenger diethylthiocarbamate via in- traperitoneal injection and ferrous citrate subcutaneously (40 mg/kg ferrous sulphate + 200 mg/kg sodium citrate). Diethylthiocarbamate binds ferrous ion, the resulting complex traps nitric oxide converting to the paramag- netic Fe (DETC)2-NO complex that is detected by ESR spectroscopy. 2.4. Sacrifice, Organ Dissection and Sample Preparation for Electron Paramagnetic Resonance Spectroscopy Following the drug and spin trap administration the rats were decapitated under slight ether anesthesia, samples of brain cortex, cerebellum, myocardium tissue, liver, kidney, testes, skeletal muscles, lungs and blood were compacted in a glass tube 30 mm in length with inner diameter 4 mm and immediately frozen in liquid nitrogen. Before recording the ESR spectra, the specimen was placed in a quartz finger Dewar flask ER 167 FDS-Q (Bruker, Karlsruhe, Germany) filled with liquid nitrogen. 2.5. ESR Measurements ESR spectra were recorded in liquid nitrogen using an ESR spectrometer “Radiopan” SE/X2544 (Radiopan, Poznan, Poland). The conditions of the electron paramag- netic resonance measurements were: operation at X-band, 25 mW microwave power, 100 kHz modulation frequency, 5 G modulation amplitude, receiver gain 0.5 × 104, and time constant 1 s. Spectra were recorded for 4 minutes. The nitric oxide content in the samples was evaluated from the height of the third component of the NO signal at g = 2.031. The NO concentration (ng/g of tissue) was calculated on the basis of calibration curves as described previously. Briefly, different quantities of NaNO2 (final concentra- tions 10, 20, 30, 40, 60, 100 M) were mixed with DETC (33 mg/mL) and FeSO4·7H2O (3.3 mmol/L), an excess of Na2S2O4 (2 mol/L) was added to the mixture. The EPR spectra were taken as described above. Further details are given in our previous publications [12-14,28-30]. 2.6. RNA Extraction and cDNA Preparation Total RNA was isolated from liver and brain cortex us- ing TRI reagent (Sigma Aldrich, Taufkirchen, Germany). DNA contaminations were removed with RNA-free kit (Ambion, Austin, TX, USA). The resulting RNA quan- tity and purity were determined by spectrophotometry, integrity of RNA molecules was monitored by gel elec- trophoresis, and only specimens with well-pronounced rRNA bands were taken for reactions. RNA (2 μg) was reverse-transcribed using a random hexamer primer (RevertAid™ First Strand cDNA Synthesis Kit, Fermen- tas, Vilnius, Lithuania) to obtain cDNA. 2.7. Real Time RT-PCR The mRNA expression rates of the brain cortex, liver iNOS and reference gene were determined using the SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) according to the instructions pro- vided by the manufacturer. Amplification and detection of specific products were performed on a StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using the following temperature-time profile: one cycle of 95˚C for 10.00 min; and 40 cycles of 95˚C for 0.15 min, 60˚C for 1.00 min. To check speci- ficity of amplification products, the dissociation curve mode was used (one cycle at 95˚C for 0.15 min, 60˚C for 1 min and 95˚C for 0.15 min). To evaluate the suitability of candidates as reference genes, we applied the GeNorm program [31]. Primers were designed using Primer3 software. The primers were supplied by Metabion inter- national AG, Germany. The 2–∆∆CT method was applied for analysis of the results. Primer sequences for iNOS gene were 5’-GCTACACTTCCAACGCAACA-3’ for forward and 5’-CATGGTGAACACGTTCTTGG for rev- erse primer, the expected size of the product was 116 bp. RNA-polymerase II [32] was used as reference gene (5’- GCCAGAGTCTCCCATGTGTT-3’and 5’-GTCGGTGG GACTCTGTTTGT-3’, 135 bp).  E. ROSTOKA ET AL.9 2.8. Histological and Immunohistochemical Examination Paraffin-embedded tissue was cut in 4-micron-thick sec- tions and stained with haematoxylin and eosin for mor- phological examination. Infiltration of inflammatory cells in brain tissue was assessed in subcortical perivascular, subcortical parenchymal, and intracortical peri-vascular regions (magnification × 400). Perivascular infiltrates were defined as inflammatory cells, which are located not fur- ther than three cell layers from blood vessels. Inflamma- tory cells further than three layers from a blood vessel wall were defined as parenchymal infiltrates. Infiltration of inflammatory cells was assessed according to four score scale: 0-no infiltration; 1-light infiltration; 2- medium infiltration; 3-marked infiltration; 4-very marked infiltration (more than 25% of the total field of vision). The morphology of liver tissue was evaluated by evaluating the histological activity index (HAI), as de- scribed [33]: infiltration of inflammatory cells (0-4 scores); necrosis of hepatocytes around a central vein (0-6 scores); necrosis of hepatocytes and apoptosis in periphery lobules (0-4 scores); inflammatory changes of portal tracts (0-4 scores). Tissue sections were stained for visualization of iNOS positive cells by an immunochemical approach as previ- ously described [34]. Briefly, antigen retrieval was achieved by treatment in a microwave oven for 20 min at 300 W in citrate buffer, pH = 6.0. Endogenous peroxi- dase activity was blocked by 0.5 % H2O2 for 10 min. Nonspecific primary antibody binding was blocked by serum-free protein block for 10 min. Rabbit polyclonal active iNOS antibody Abcam Inc. (Cambridge, MA, USA) was applied in 1:200 dilution and incubated for 1h at room temperature in a humidified chamber. Detection of primary antibody binding was performed using spe- cific peroxidase conjugated polyclonal goat anti-rabbit IgG (1:100 for 30 min) and subsequently peroxidase conjugated polyclonal rabbit anti-goat IgG (1:100 for 30 min). The immunoperoxidase color reaction was devel- oped by incubation with diaminobenzidine (7 min). A negative control without primary antibody was included in each staining run. iNOS positive cells were counted in twenty high-powered fields at magnification × 400. All cell counts were expressed as cells per square millimeter. For morphological examination, at least 3 replicate measurements of iNOS positive cells were performed by the same observer in 10 randomly selected slides, and the intraobserver reproducibility was assessed with the coef- ficient of variation and with the interclass correlation coefficient. The intraobserver coefficient of variation was 4%, and the intraobserver correlation coefficient was 0.94. 2.9. Statistical Analysis Results were expressed as mean ± SD. The significance of differences in NO concentration and iNOS expression between groups was evaluated according to Student's unpaired t-test, the Mann-Whitney U test was used for quantification of immunohistochemical experiments. Re- sults were considered to be significant when P was less than 0.05. 3. Results 3.1. Effects of Natural Compounds and their Combinations on NO Production in Intact and LPS-Treated Rats In order to test the ability of the natural compounds to modify NO production in animals the radical concentra- tion was monitored in several rat organs and tissues. Data are summarized in Figure 2. ESR spectra of the different organs had a typical Cu-DETC spectrum shape with a superposed Fe(DETC)2-NO peak, spectra were published previously [14]. The NO production reached the highest levels in the brain cortex, liver, lungs, and blood Figure 2. The NO production in heart and kidneys was an order of magnitude lower. When control group of animals was compared to ani- mals treated with natural substances it turned out that baicalein decreased NO concentration in heart, kidney, liver and lungs (Figure 2). Luteolin decreased NO pro- duction in the liver and heart. Quercetin induced signifi- cant decrease of NO production the heart, kidneys and blood. Indole -3-carbinol caused a significant decrease of NO production in the cerebellum, spleen, blood, lungs and skeletal muscles. In the following set of experiments the eventual activ- ity of the compounds as modifiers of NO production was tested against the background of the iNOS induction. Intraperitoneal injection of LPS to the animals caused a drastic increase of NO production levels in all tissues studied (Figure 2). The highest production of nitric ox- ide was detected in liver, whereas very strong increases in nitric oxide accumulation (50-100 fold compared to control) were observed in heart, blood and kidney. However, the effects of LPS were less pronounced in brain tissues where nitric oxide increased 4-6 times only. Nitric oxide production increase in testes was of compa- rable magnitude. Baicalein (30 mg/kg) decreased NO concentration in brain cortex, liver, heart and kidneys. Luteolin (30 mg/kg) decreased NO outburst in all organs except skeletal mus- cles. In contrast, administration of the indole-3-carbinol (50 mg/kg) enhanced the LPS-induced increase of NO production in all organs except spleen and testes, Figure 2. Quercetin (50 mg/kg) produced similar effect: NO Copyright © 2010 SciRes. CM 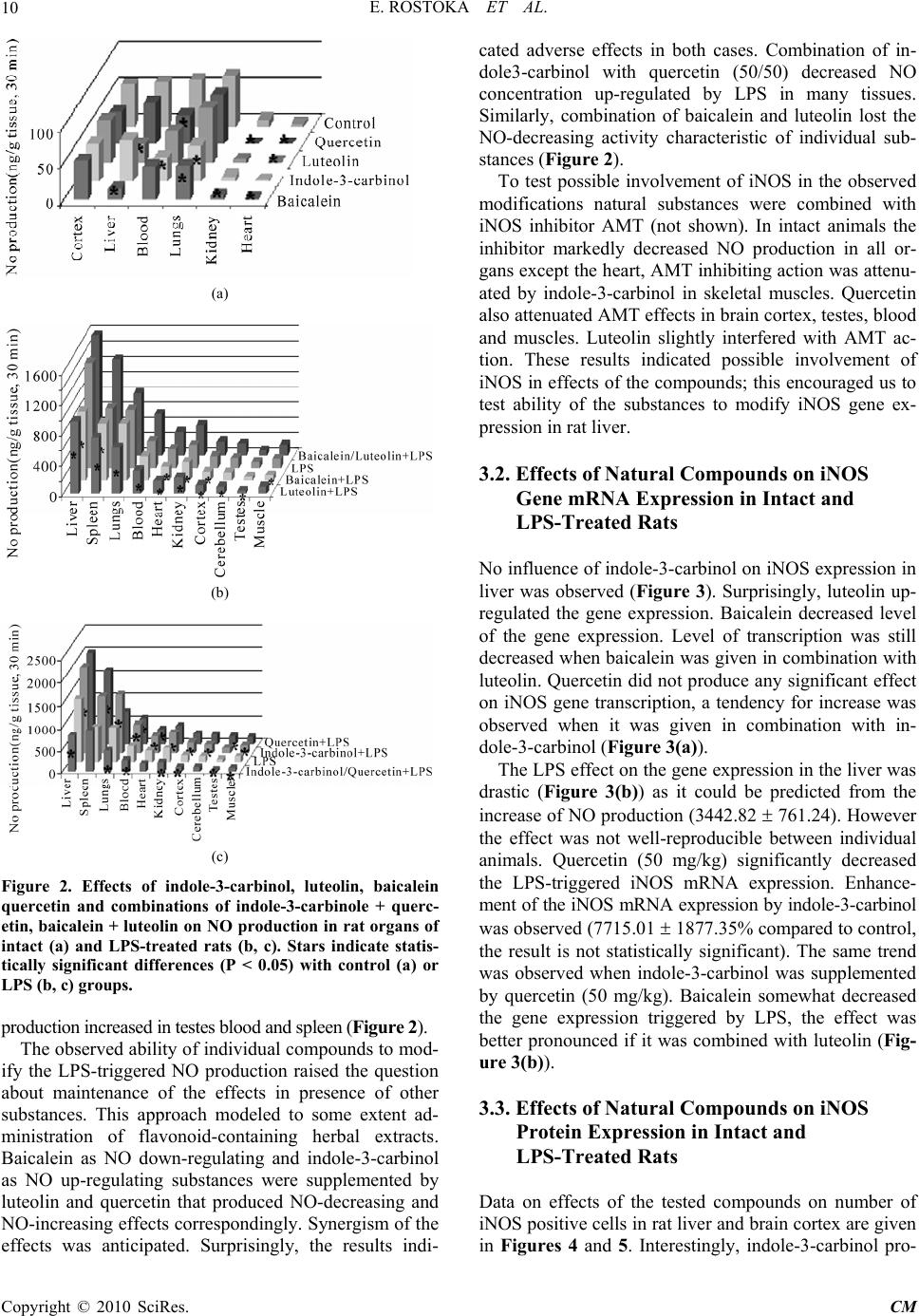 E. ROSTOKA ET AL. 10 (a) (b) (c) Figure 2. Effects of indole-3-carbinol, luteolin, baicalein quercetin and combinations of indole-3-carbinole + querc- etin, baicalein + luteolin on NO production in rat organs of intact (a) and LPS-treated rats (b, c). Stars indicate statis- tically significant differences (P < 0.05) with control (a) or LPS (b, c) groups. production increased in testes blood and spleen (Figure 2). The observed ability of individual compounds to mod- ify the LPS-triggered NO production raised the question about maintenance of the effects in presence of other substances. This approach modeled to some extent ad- ministration of flavonoid-containing herbal extracts. Baicalein as NO down-regulating and indole-3-carbinol as NO up-regulating substances were supplemented by luteolin and quercetin that produced NO-decreasing and NO-increasing effects correspondingly. Synergism of the effects was anticipated. Surprisingly, the results indi- cated adverse effects in both cases. Combination of in- dole3-carbinol with quercetin (50/50) decreased NO concentration up-regulated by LPS in many tissues. Similarly, combination of baicalein and luteolin lost the NO-decreasing activity characteristic of individual sub- stances (Figure 2). To test possible involvement of iNOS in the observed modifications natural substances were combined with iNOS inhibitor AMT (not shown). In intact animals the inhibitor markedly decreased NO production in all or- gans except the heart, AMT inhibiting action was attenu- ated by indole-3-carbinol in skeletal muscles. Quercetin also attenuated AMT effects in brain cortex, testes, blood and muscles. Luteolin slightly interfered with AMT ac- tion. These results indicated possible involvement of iNOS in effects of the compounds; this encouraged us to test ability of the substances to modify iNOS gene ex- pression in rat liver. 3.2. Effects of Natural Compounds on iNOS Gene mRNA Expression in Intact and LPS-Treated Rats No influence of indole-3-carbinol on iNOS expression in liver was observed (Figure 3). Surprisingly, luteolin up- regulated the gene expression. Baicalein decreased level of the gene expression. Level of transcription was still decreased when baicalein was given in combination with luteolin. Quercetin did not produce any significant effect on iNOS gene transcription, a tendency for increase was observed when it was given in combination with in- dole-3-carbinol (Figure 3(a)). The LPS effect on the gene expression in the liver was drastic (Figure 3(b)) as it could be predicted from the increase of NO production (3442.82 761.24). However the effect was not well-reproducible between individual animals. Quercetin (50 mg/kg) significantly decreased the LPS-triggered iNOS mRNA expression. Enhance- ment of the iNOS mRNA expression by indole-3-carbinol was observed (7715.01 1877.35% compared to control, the result is not statistically significant). The same trend was observed when indole-3-carbinol was supplemented by quercetin (50 mg/kg). Baicalein somewhat decreased the gene expression triggered by LPS, the effect was better pronounced if it was combined with luteolin (Fig- ure 3(b)). 3.3. Effects of Natural Compounds on iNOS Protein Expression in Intact and LPS-Treated Rats Data on effects of the tested compounds on number of iNOS positive cells in rat liver and brain cortex are given in Figures 4 and 5. Interestingly, indole-3-carbinol pro- Copyright © 2010 SciRes. CM 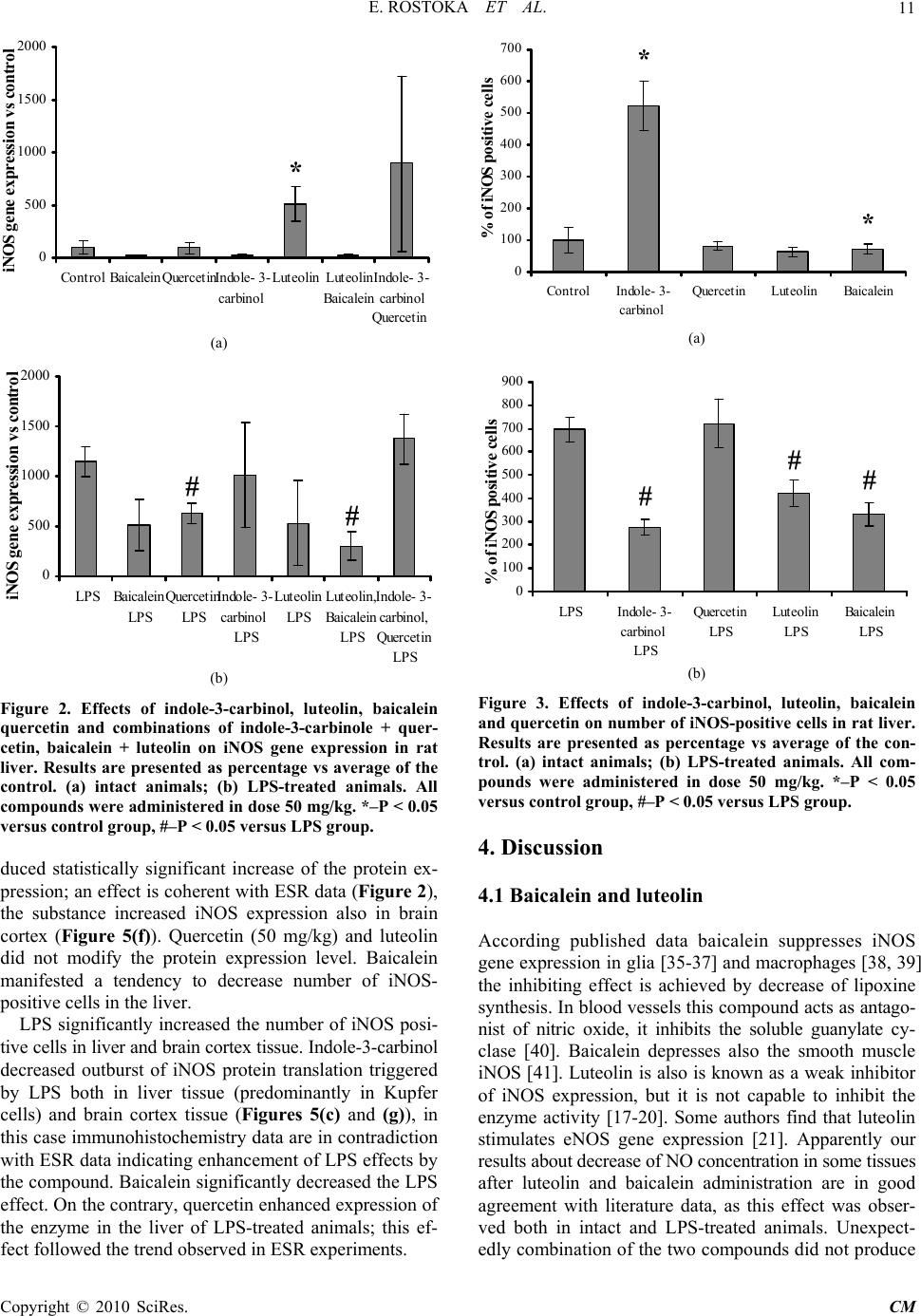 E. ROSTOKA ET AL.11 * 0 500 1000 1500 2000 ControlBaicaleinQuercetinIndole- 3- carbinol LuteolinLuteolin Baicalein Indole- 3- carbin ol Quercetin iN O S gene exp ression vs cont rol A (a) ## 0 500 1000 1500 2000 LPS Baicalein LPS Quercetin LPS Indole- 3- carbinol LPS Luteolin LPS Luteolin , Ba icalein LPS Indole- 3- carbinol, Querce tin LP S iN OS gene exp ression vs cont rol B (b) Figure 2. Effects of indole-3-carbinol, luteolin, baicalein quercetin and combinations of indole-3-carbinole + quer- cetin, baicalein + luteolin on iNOS gene expression in rat liver. Results are presented as percentage vs average of the control. (a) intact animals; (b) LPS-treated animals. All compounds were administered in dose 50 mg/kg. *–P < 0.05 versus control gr oup, #–P < 0.05 versus LPS group. duced statistically significant increase of the protein ex- pression; an effect is coherent with ESR data (Figure 2), the substance increased iNOS expression also in brain cortex (Figure 5(f)). Quercetin (50 mg/kg) and luteolin did not modify the protein expression level. Baicalein manifested a tendency to decrease number of iNOS- positive cells in the liver. LPS significantly increased the number of iNOS posi- tive cells in liver and brain cortex tissue. Indole-3-carbinol decreased outburst of iNOS protein translation triggered by LPS both in liver tissue (predominantly in Kupfer cells) and brain cortex tissue (Figures 5(c) and (g)), in this case immunohistochemistry data are in contradiction with ESR data indicating enhancement of LPS effects by the compound. Baicalein significantly decreased the LPS effect. On the contrary, quercetin enhanced expression of the enzyme in the liver of LPS-treated animals; this ef- fect followed the trend observed in ESR experiments. * * 0 100 200 300 400 500 600 700 ControlIndole- 3- carbinol Quercetin LuteolinBaicalein % of iNOS positive cells A (a) # # # 0 100 200 300 400 500 600 700 800 900 LPSIndole- 3- carbinol LP S Quercetin LPS Lute o lin LPS Baic al ein LPS % of iNO S pos it ive c ells B (b) Figure 3. Effects of indole-3-carbinol, luteolin, baicalein and quercetin on number of iNOS-positive cells in rat liver. Results are presented as percentage vs average of the con- trol. (a) intact animals; (b) LPS-treated animals. All com- pounds were administered in dose 50 mg/kg. *–P < 0.05 versus control gr oup, #–P < 0.05 versus LPS group. 4. Discussion 4.1 Baicalein and luteolin According published data baicalein suppresses iNOS gene expression in glia [35-37] and macrophages [38, 39] the inhibiting effect is achieved by decrease of lipoxine synthesis. In blood vessels this compound acts as antago- nist of nitric oxide, it inhibits the soluble guanylate cy- clase [40]. Baicalein depresses also the smooth muscle iNOS [41]. Luteolin is also is known as a weak inhibitor of iNOS expression, but it is not capable to inhibit the enzyme activity [17-20]. Some authors find that luteolin stimulates eNOS gene expression [21]. Apparently our results about decrease of NO concentration in some tissues after luteolin and baicalein administration are in good agreement with literature data, as this effect was obser- ved both in intact and LPS-treated animals. Unexpect- edly combination of the two compounds did not produce Copyright © 2010 SciRes. CM 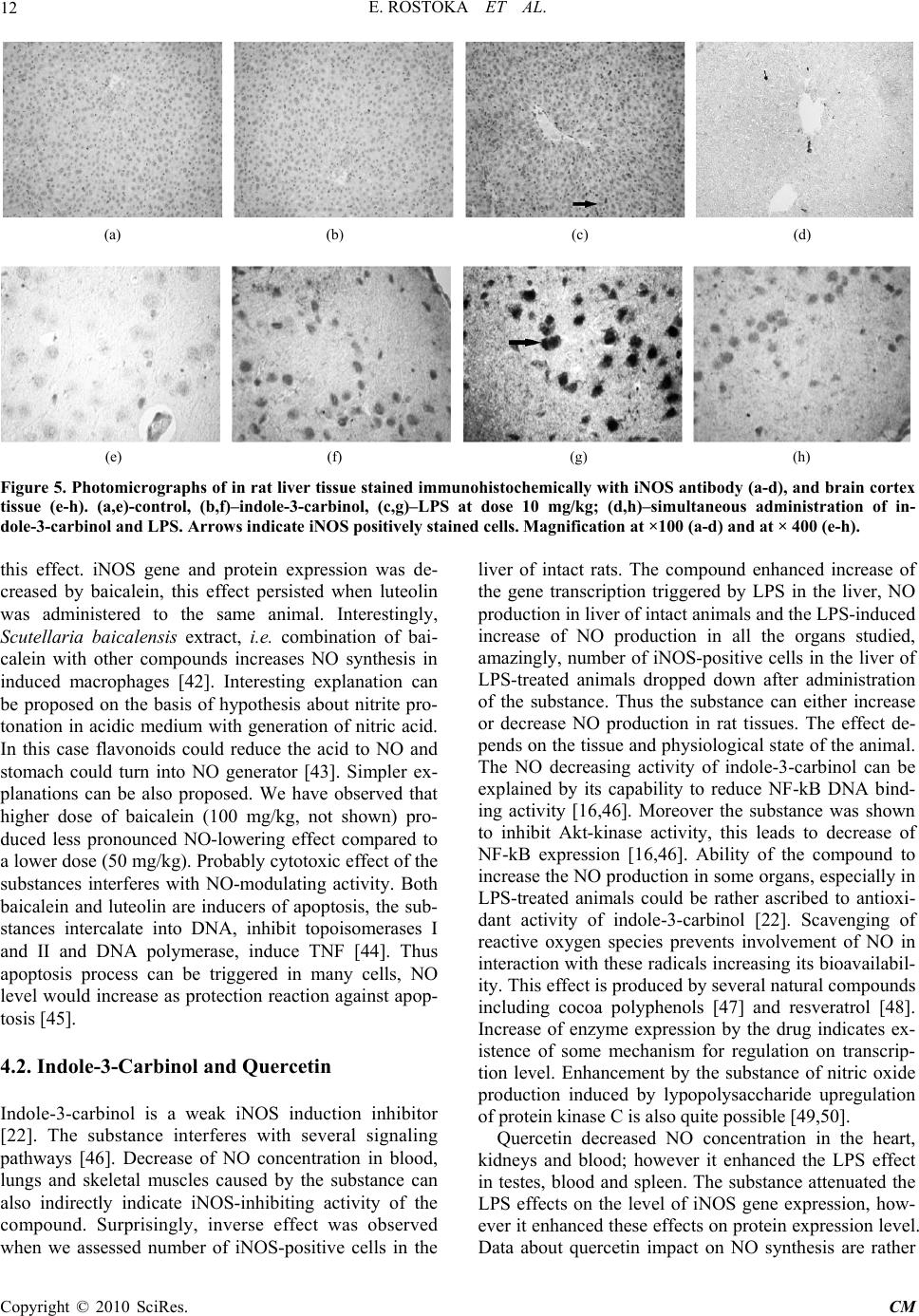 E. ROSTOKA ET AL. Copyright © 2010 SciRes. CM 12 (a) (b) (c) (d) (e) (f) (g) (h) Figure 5. Photomicrographs of in rat liver tissue stained immunohistochemically with iNOS antibody (a-d), and brain cortex tissue (e-h). (a,e)-control, (b,f)–indole-3-carbinol, (c,g)–LPS at dose 10 mg/kg; (d,h)–simultaneous administration of in- dole-3-carbinol and LPS. Arrows indicate iNOS positively stained cells. Magnification at ×100 (a-d) and at × 400 (e-h). this effect. iNOS gene and protein expression was de- creased by baicalein, this effect persisted when luteolin was administered to the same animal. Interestingly, Scutellaria baicalensis extract, i.e. combination of bai- calein with other compounds increases NO synthesis in induced macrophages [42]. Interesting explanation can be proposed on the basis of hypothesis about nitrite pro- tonation in acidic medium with generation of nitric acid. In this case flavonoids could reduce the acid to NO and stomach could turn into NO generator [43]. Simpler ex- planations can be also proposed. We have observed that higher dose of baicalein (100 mg/kg, not shown) pro- duced less pronounced NO-lowering effect compared to a lower dose (50 mg/kg). Probably cytotoxic effect of the substances interferes with NO-modulating activity. Both baicalein and luteolin are inducers of apoptosis, the sub- stances intercalate into DNA, inhibit topoisomerases I and II and DNA polymerase, induce TNF [44]. Thus apoptosis process can be triggered in many cells, NO level would increase as protection reaction against apop- tosis [45]. 4.2. Indole-3-Carbinol and Quercetin Indole-3-carbinol is a weak iNOS induction inhibitor [22]. The substance interferes with several signaling pathways [46]. Decrease of NO concentration in blood, lungs and skeletal muscles caused by the substance can also indirectly indicate iNOS-inhibiting activity of the compound. Surprisingly, inverse effect was observed when we assessed number of iNOS-positive cells in the liver of intact rats. The compound enhanced increase of the gene transcription triggered by LPS in the liver, NO production in liver of intact animals and the LPS-induced increase of NO production in all the organs studied, amazingly, number of iNOS-positive cells in the liver of LPS-treated animals dropped down after administration of the substance. Thus the substance can either increase or decrease NO production in rat tissues. The effect de- pends on the tissue and physiological state of the animal. The NO decreasing activity of indole-3-carbinol can be explained by its capability to reduce NF-kB DNA bind- ing activity [16,46]. Moreover the substance was shown to inhibit Akt-kinase activity, this leads to decrease of NF-kB expression [16,46]. Ability of the compound to increase the NO production in some organs, especially in LPS-treated animals could be rather ascribed to antioxi- dant activity of indole-3-carbinol [22]. Scavenging of reactive oxygen species prevents involvement of NO in interaction with these radicals increasing its bioavailabil- ity. This effect is produced by several natural compounds including cocoa polyphenols [47] and resveratrol [48]. Increase of enzyme expression by the drug indicates ex- istence of some mechanism for regulation on transcrip- tion level. Enhancement by the substance of nitric oxide production induced by lypopolysaccharide upregulation of protein kinase C is also quite possible [49,50]. Quercetin decreased NO concentration in the heart, kidneys and blood; however it enhanced the LPS effect in testes, blood and spleen. The substance attenuated the LPS effects on the level of iNOS gene expression, how- ever it enhanced these effects on protein expression level. Data about quercetin impact on NO synthesis are rather  E. ROSTOKA ET AL.13 contradictory. The substance inhibits iNOS induction, the effect is better detectable in in vitro cultured cells [6,7,51]. In vivo quercetin did not decrease NFkB activa- tion in kidney cortex [52]. In systems, where iNOS ex- pression decrease by quercetin was observed, the flavon- oid did not down-regulate NFkB activation [7,53]. Proba- bly, quercetin interferes with tyrosin kinase-mediated pathways [54], and decreases TNFα expression [55]. However other authors observed the quercetin-induced inhibition of IkBα (inhibitor of kappa B alpha) degrada- tion via depression of IkB kinase activity, this leads to inhibition of NFkB [56]. Other reports inform about prevention and/or inhibition of IkB phosphorylation [57, 58], depression of NFkB activation by interleukin [59] and hydrogen peroxide [60] produced by the flavonoid. Inhibition of iNOS expression by quercetin is often as- cribed to inhibition of the NFkB pathway [61,62]. Inter- estingly, some authors [63] find out that quercetin does not inhibit iNOS gene expression; however it inhibits iNOS enzyme expression. We came up to quite an oppo- site conclusion; however both the cited and our data in- dicate separate mechanisms for regulation of the iNOS gene and protein expression by this substance. Quercetin also destabilizes eNOS mRNA [8]. There are even data about ability of the substance to inhibit NOS enzymatic activity [9]. In the same time quercetin protects epithe- lium against lesions produced by NOS inhibitors [64] and stimulates NO synthesis in leukemia cells as protec- tion reaction against induction of apoptosis [45]. Quercetin is considered to be the main active compound of red wine; some authors have observed NO-dependent vaso- relaxation induced by quercetin [10]. Supplementation of diet with quercetin favors NO production in endothelium [5]. However the nitric oxide release from endothelium was not detected in special studies [11]. Quercetin is also one of the active substances of the Gingko biloba ex- tracts, both purified quercetin and Gingko biloba extracts (i.e. mixture of quercetin with other natural compounds) decrease LPS-induced iNOS expression, however the extract acts via NF-kB inhibition, but quercetin inhibits TNFa pathway [65,66]. In our studies quercetin in- creased the LPS-triggered NO outburst in lungs, testes and lungs. Apparently, the NOS-inhibiting effect was not detectable on organism level. Our data contradict for- merly published reports about decrease of nitrite produc- tion in brain [67] and blood plasma of LPS-treated ani- mals [68] or streptozotocin-treated animals [69] by quercetin. However nitrite and NO production levels do not always correlate. When NO production was assessed by an approach similar to ours the quercetin-induced increase of NO production in rat brain was also observed [70]. Moreover the substance did not modify much iNOS gene expression in both healthy and LPS-treated animals. This effect can be associated with apoptosis-promoting activity of quercetin [71]. Probably simultaneous ad- ministration of indole-3-carbinol and quercetin favored manifestation of NOS-inhibiting activity of quercetin and abolished increase of NO bioavailability produced by indole-3-carbinol, as no decrease of iNOS expression was observed in this case. NO scavenging activity of quercetin [69-73] also could manifest itself in this case. 5. Conclusions Taken together our results suggest that modifications of NO level in tissues by the studied natural compounds cannot be predicted from data about its effects on NOS expression or activity. Effects of individual compounds are not additive when these are administered in combina- tion. This stresses importance of direct measurements of NO in the tissues using ESR method. 6. Acknowledgements This study was supported in part by the National Re- serach Program 2010.10.-4/VPP4 “Creation of novel means and methods for prophylaxis, treatment and diag- nostics, elaboration of biomedical technologies for im- provement of social health” and the grant 04.1317 “Pathological production of nitric oxide, possibilities of its pharmacological correction” awarded to N. Sjakste by the Latvian Council of Science. We thank L. Lauberte for technical assistance. Participation of D. Meirena in experiment design and discussion is greatly appreciated. 7. References [1] H. Y. Li, L. Cui and M. Cui, “Hot Topics in Chinese Herbal Drugs Research Documented in PubMed/Medline by Authors inside China and Outside of China in the Past 10 Years: Based on Co-Word Cluster Analysis,” Journal of Alternative and Complementary Medicine, Vol. 15, No. 7, 2009, pp. 779-785. [2] H. Zeng, X. Liu, S. Dou, W. Xu, N. Li, X. Liu, W. Zhang, Z. Hu and R. Liu, “Huang-Lian-Jie-Du-Tang Exerts Anti- Inflammatory Effects in Rats through Inhibition of Nitric Oxide Production and Eicosanoid Biosynthesis via the Lipoxygenase Pathway” Journal of Pharmacy and Pharmacology, Vol. 61, No. 2, 2009, pp. 1699-1707. [3] V. Bayard, F. Chamorro, J. Motta and N. K. Hollenberg, “Does Flavanol Intake Influence Mortality from Nitric Oxide-Dependent Processes? Ischemic Heart Disease, Stroke, Diabetes Mellitus, and Cancer in Panama,” In- ternational Journal of Medical Microbiology, Vol. 4, No. 1, 2007, pp. 53-58. [4] C. A. Schmitt and V. M. Dirsch, “Modulation of Endothe Lial Nitric Oxide by Plant-Derived Products,” Nitric Ox- ide, Vol. 21, No. 2, 2009, pp. 77-91. [5] S. Benito, D. Lopez, M. P. Saiz, S. Buxaderas, J. Sanchez, P. Puig-Parellada and M. T. Mitjavila, “A Flavonoid-Rich Diet Increases Nitric Oxide Production in Rat Aorta,” British Journal of Pharmacology, Vol. 135, No. 4, 2002, Copyright © 2010 SciRes. CM  E. ROSTOKA ET AL. 14 pp. 910-916. [6] R. Olszanecki, A. Gebska, V. I. Kozlovski and R. J. Gry- glewski, “Flavonoids and Nitric Oxide Synthase,” Jour- nal of Physiology and Pharmacology, Vol. 53, No. 4, 2002, pp. 571-584. [7] B. H. Kim, S. M. Cho, A. M. Reddy, Y. S. Kim, K. R. Min and Y. Kim, “Down-regulatory Effect of Quercitin Gallate on Nuclear Factor-Kappa B-Dependent Inducible Nitric Oxide Synthase Expression in Lipopolysaccharide- Stimulated Macrophages RAW 264.7,” Biochemical Pharmacology, Vol. 69, No. 11, 2005, pp. 1577-1183. [8] T. Wallerath, H. Li, U. Godtel-Ambrust, P. M. Schwarz and U. Forstermann, “A Blend of Polyphenolic Com- pounds Explains the Stimulatory Effect of Red Wine on Human Endothelial NO Synthase,” Nitric Oxide, Vol. 12, No. 2, 2005, pp. 97-104. [9] L. Luo, Q. Sun, Y. Y. Mao, Y. H. Lu and R. X. Tan, “In- hibitory Effects of Flavonoids from Hypericum Perfora- tum on Nitric Oxide Synthase,” Journal of Ethnophar- macology, Vol. 93, No. 2-3, 2004, pp. 221-225. [10] C. K. Chen and C. R. Pace-Asciak, “Vasorelaxing Activ- ity of Resveratrol and Quercetin in Isolated Rat Aorta,” General Pharmacology, Vol. 27, No. 2, 1996, pp. 363- 366. [11] J. C. Stoclet, A. Kleschyov, E. Andriambeloson, M. Die- bolt and R. Andriantsitohaina, “Endothelial NO Release Caused by Red Wine Polyphenols,” Journal of Physiol- ogy and Pharmacology, Vol. 50, No. 4, 1999, pp. 535- 540. [12] N. Sjakste, A. L. Kleschyov, J. L. Boucher, L. Baumane, M. Dzintare, D. Meirena, J. Sjakste, K. Sydow, T. Mün- zel and I. Kalvinsh, “Endothelium- and Nitric Oxide- Dependent Vasorelaxing Activities of Gamma-Butyrobetaine Esters: Possible Link to the Antiischemic Activities of Mildronate,” European Journal of Pharmacology, Vol. 495, No. 1, 2004, pp. 67-73. [13] N. Sjakste, J. Sjakste, J. L. Boucher, L. Baumane, T. Sjakste, M. Dzintare, D. Meirena, J. Sharipova and I. Kalvinsh, “Putative Role of Nitric Oxide Synthase Iso- forms in the Changes of Nitric Oxide Concentration in Rat Brain Cortex and Cerebellum Following Sevoflurane and Isoflurane Anaesthesia,” European Journal of Phar- macology, Vol. 513, No. 3, 2005, pp. 193-205. [14] N. Sjakste, V. G. Andrianov, J. L. Boucher, I. Shestakova, L. Baumane, M. Dzintare, D. Meirena and I. Kalvins, “Paradoxical Effects of Two Oximes on Nitric Oxide Production by Purified NO Synthases, in Cell Culture and in Animals,” Nitric Oxide, Vol. 17, No. 3-4, 2007, pp. 107-114. [15] M. E. van Meeteren, J. J. Hendriks, C. D. Dijkstra and E. A. van Tol, “Dietary Compounds Prevent Oxidative Damage and Nitric Oxide Production by Cells Involved in Demyelinating Disease,” Biochemical Pharmacology, Vol. 67, No. 5, 2004, pp. 967-975. [16] K. M. Rahman, S. Ali, A. Aboukameel, S. H. Sarkar, Z. Wang, P. A. Philip, W. A. Sakr and A. Raz, “Inactivation of NF-kappaB by 3,3'-diindolylmethane Contributes to Increased Apoptosis Induced by Chemotherapeutic Agent in Breast Cancer Cells,” Molecular Cancer Therapeutics, Vol. 6, 2007, pp. 2757-2765. [17] S. J. Kim, H. Park and H. P. Kim, “Inhibition of Nitric Oxide Production from Lipopolysaccharide-Treated RAW 264.7 Cells by Synthetic Flavones: Structure- Activity Relationship and Action Mechanism,” Archives of Pharmacal Research, Vol. 27, No. 9, 2004, pp. 937- 943. [18] J. S. Kim, H. J. Lee, M. H. Lee, J. Kim, C. Jin and J. H. Ryu “Luteolin Inhibits LPS-Stimulated Inducible Nitric Oxide Synthase Expression in BV-2 Microglial Cells,” Planta Medica, Vol. 72, No. 1, 2006, pp. 65-68. [19] C. Hu and D. D. Kitts, “Luteolin and Luteolin-7-O-gluco- side from Dandelion Flower Suppress iNOS and COX-2 in RAW264.7 Cells,” Molecular and Cellular Biochem- istry, Vol. 265, 2004, pp. 107-113. [20] L. S. Scuro, P. U. Simioni, D. L. Grabriel, E. E. Saviani, L. V. Modolo, W. M. Tamashiro and I. Salgado, “Sup- pression of Nitric Oxide Production in Mouse Macro- phages by Soybean Flavonoids Accumulated in Response to Nitroprusside and Fungal Elicitation,” BMC Medicine, Vol. 5, 2004, p. 5. [21] H. Li, N. Xia, I. Brausch, Y. Yao and U. Forstermann, “Flavonoids from Artichoke (Cynara scolymus L.) up- Regulate Endothelial-Type Nitric-Oxide Synthase Gene Expression in Human Endothelial Cells,” Journal of Pharmacology and Experimental Therapeutics, Vol. 310, No. 3, 2004, pp. 926-932. [22] C. Gerhäuser, K. Klimo, E. Heiss, I. Neumann, A. Ga- mal-Eldeen, J. Knauft, G. Y. Liu, S. Sitthimonchai and N. Frank, “Mechanism-based in Vitro Screening of Potential Cancer Chemopreventive Agents,” Mutation Research, Vol. 523-524, 2003, pp. 163-172. [23] S. Su, J. Guo, J. Duan, T. Wang, D. Qian, E. Shang and Y. Tang, “Ultra-performance Liquid Chromatography- Tandem Mass Spectrometry Analysis of the Bioactive Components and their Metabolites of Shaofu Zhuyu De- coction Active Extract in Rat Plasma,” Journal of Chro- matography B Analytical Technologies in the Biomedical and Life Sciences, 2009. (in press) [24] H. J. Chen, X. Li, J. W. Chen, S. Guo and B. C. Cai, “Simultaneous Determination of Eleven Bioactive Com- pounds in Saururus Chinensis from Different Harvesting Seasons by HPLC-DAD,” Journal of Pharmaceutical and Biomedical Analysis, 2010. (in press) [25] C. Li, H. Du, L. Wang, Q. Shu, Y. Zheng, Y. Xu, J. Zhang, J. Zhang, R. Yang and Y. Ge, “Flavonoid Com- position and Antioxidant Activity of Tree Peony (Paeonia section moutan) Yellow Flowers,” Journal of Agricul- tural and Food Chemistry, Vol. 57, No. 18, 2009, pp. 8496-8503. [26] X. Liang, L. Zhang, X. Zhang, W. Dai, H. Li, L. Hu, H. Liu, J. Su and W. Zhang, “Qualitative and Quantitative Analysis of Traditional Chinese Medicine Niu Huang Jie Du Pill Using Ultra Performance Liquid Chromatography Coupled with Tunable UV Detector and Rapid Resolution Liquid Chromatography Coupled with Time-of-Flight Tandem Mass Spectrometry,” Journal of Pharmaceutical Copyright © 2010 SciRes. CM  E. ROSTOKA ET AL.15 and Biomedical Analysis, Vol. 51, No. 3, 2010, pp. 565- 571. [27] A. L. Kleschyov, P. Wenzel and T. Münzel, “Electron Paramagnetic Resonance (EPR) Spin Trapping of Bio- logical Nitric Oxide,” Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sci- ences, Vol. 851, No. 1-2, 2007, pp. 12-20. [28] N. Sjakste, L. Baumane, D. Meirena, L. Lauberte, M. Dzintare and I. Kalvinsh, “Drastic Increase in Nitric Ox- ide Content in Rat Brain under Halothane Anesthesia, Revealed by EPR Method,” Biochemical Pharmacology, Vol. 58, No. 12, 1999, pp. 1955-1959. [29] L. Baumane, M. Dzintare, L. Zvejniece, D. Meirena, L. Lauberte, V. Sile, I. Kalvinsh and N. Sjakste, “Increased Synthesis of Nitric Oxide in Rat Brain Cortex Due to Halogenated Volatile Anesthetics Confirmed by ESR Spectroscopy,” Acta Anaesthesiologica Scandinavica, Vol. 46, No. 4, 2002, pp. 378-383. [30] E. Rostoka, L. Baumane, S. Isajevs, A. Line, M. Dzintare, D. Svirina, J. Sharipova, K. Silina, I. Kalvinsh and N. Sjakste, “Effects of Kaempferol and Myricetin on Induc- ible Nitric Oxide Synthase Expression and NO Produc- tion in Rats,” Basic & Clinical Pharmacology & Toxi- cology, 2010. (in press) [31] J. Vandesompele, K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe and F. Speleman, “Accurate Nor- malization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes,” Genome Biology, Vol. 18, 2002. [32] A. Radonić, S. Thulke, I. M. Mackay, O. Landt, W. Siegert and A. Nitsche, “Guideline to Reference Gene Selection for Quantitative Real-Time PCR,” Biochemical and Biophysical Research Communications, Vol. 313, No. 4, 2004, pp. 856-862. [33] K. Ishak, A. Baptista, L. Bianchi, F. Callea, J. De Groote, J. Gudat, F. Gudat, H. Denk, V. Desmet, G. Korb, R. N. MacSween, “Histological Grading and Staging of Chronic Hepatitis,” Journal of Hepatology, Vol. 22, No. 6, 1995, pp. 696-699. [34] A. D. Stefano, G. Caramori, T. Oates, A. Capelli, M. Lusuardi, I. Gnemmi, F. Ioli, K. F. Chung, C. F. Donner, P. J. Barnes and I. M. Adcock, “Increased Expression of Nuclear Factor-kB in Bronchial Biopsies from Smokers and Patients with COPD,” European Respiratory Journal, Vol. 20, 2000, pp. 556-563. [35] J. S. Won, Y. B. Im, M. Khan, A. K. Singh and I. Singh, “Involvement of Phospholipase A2 and Lipoxygenase in Lipopolysaccharide-Induced Inducible Nitric Oxide Syn- Thase Expression in Glial Cells,” Glia, Vol. 51, 2005, pp. 13-21. [36] C. J. Chen, S. L. Raung, S. L. Liao and S. Y. Chen “Inhi- bition of Inducible Nitric Oxide Synthase Expression by Baicalein in Endotoxin/Cytokine-Stimulated Microglia,” Biochemical Pharmacology, Vol. 67, No. 5, 2004, pp. 957-965. [37] K. Suk, H. Lee, S. S. Kang, G. J. Cho and W. S. Choi, “Flavonoid Baicalein Attenuates Activation-Induced Cell Death of Brain Microglia,” Journal of Pharmacology and Experimental Therapeutics, Vol. 305, No. 2, 2003, pp. 638-645. [38] M. Vivancos and J. J. Moreno, “Role of Ca2+-Independent Phospholipase A(2) and Cyclooxygenase/Lip-Oxygenase Pathways in the Nitric Oxide Production by Murine Macrophages Stimulated by Lipopolysaccharides,” Nitric Oxide, Vol. 6, No. 3, 2002, pp. 255-262. [39] I. Wakabayashi, “Inhibitory Effects of Baicalein and Wogonin on Lipopolysaccharide-Induced Nitricoxide Production in Macrophages,” Pharmacology & Toxicol- ogy, Vol. 84, 1999, pp. 288-291. [40] Y. Huang, C. M. Wong, C. W. Lau, X. Yao, S. Y. Tsang, Y. L. Su and Z. Y. Chen, “Inhibition of Nitric Ox- ide/Cyclic GMP-Mediated Relaxation by Purified Fla- vonoids, Baicalin and Baicalein, in Rat Aortic Rings,” Biochemical Pharmacology, Vol. 67, No. 4, 2004, pp. 787-794. [41] T. Hashimoto, M. Kihara, K. Yokoyama, T. Fujita, S. Kobayashi, K. Matsushita, K. Tamura, N. Hirawa, Y. Toya and S. Umemura, “Lipooxygenase Products Regu- late Nitric Oxide and Inducible Nitric Oxide Synthase Production in Interleukin-1beta Stimulated Vascular Smooth Muscle Cells,” Hypertension Research, Vol. 26, 2003, pp. 177-184. [42] H. M. Kim, E. J. Moon, E. Li, K. M. Kim, S. Y. Nam and C. K. Chung, “The Nitric Oxide-Producing Activities of Scutellaria Baicalensis,” Toxicology, Vol. 135, No. 2-3, 1999, pp. 109-115. [43] U. Takahama, T. Oniki and S. Hirota, “Oxidation of Quercetin by Salivary Components. Quercetin-Dependent Reduction of Salivary Nitrite under Acidic Conditions Producing Nitric Oxide,” Journal of Agricultural and Food Chemistry, Vol. 50, 2002, pp. 4317-4322. [44] R. D. Snyder and P. J. Gillies, “Evaluation of the Clasto- genic, DNA Intercalative, and Topoisomerase II-Interactive Properties of Bioflavonoids in Chinese Hamster V79 Cells,” Environmental and Molecular Mutagenesis, Vol. 40, 2002, pp. 266-276. [45] C. Kellner and S. J Zunino, “Nitric Oxide Is Synthesized in Acute Leukemia Cells after Exposure to Phenolic Anti- Oxidants and Initially Protects against Mitochondrial Membrane Depolarization,” Cancer Letter, Vol. 215, No. 1, 2004, pp. 43-52. [46] F. H. Sarkar and Y. Li, “Cell Signaling Pathways Altered by Natural Chemopreventive Agents,” Mutation Re- search, Vol. 555, No. 1-2, 2004, pp. 53-64. [47] H. Sies, T. Schewe, C. Heiss and M. Kelm, “Cocoa Polyphenols and Inflammatory Mediators,” American Journal of Clinical Nutrition, Vol. 81, 2005, pp. 304S- 312S. [48] F. Orallo, E. Alvarez, M. Camiña, J. M. Leiro, E. Gómez and P. Fernández, “The Possible Implication of Trans- Resveratrol in the Cardioprotective Effects of Long-Term Moderate Wine Consumption,” Molecular Pharmacology, Vol. 61, No. 2, 2002, pp. 294-302. [49] K. Fukuzawa, K. Kogure, M. Morita, S. Hama, S. Ma- nabe and A. Tokumura, “Enhancement of Nitric Oxide and Superoxide Generations by Alpha-Tocopheryl Suc- Copyright © 2010 SciRes. CM  E. ROSTOKA ET AL. 16 cinate and its Apoptotic and Anticancer Effects,” Bio- chemistry, Vol. 69, 2004, pp. 50-57. [50] K. Kogure, M. Morita, S. Hama, S. Nakashima, A. To- kumura and K. Fukuzawa, “Enhancement by Alpha- to-Copheryl Hemisuccinate of Nitric Oxide Production Induced by Lypopolysaccharide and Interferon-Gamma through the Upregulation of Protein Kinase C in Rat Vas- cular Smooth Muscle Cells,” European Journal of Bio- chemistry, Vol. 269, 2002, pp. 2367-2372. [51] G. M. Raso, R. Meli, G. Di Carlo, M. Pacilio and R. D. Carlo, “Inhibition of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Expression by Flavonoids in Macro- phage J774A.1,” Life Sciences, Vol. 68, 2001, pp. 921-931. [52] G. K. Rangan, Y. Wang and D. C. Harris, “Dietary Quercetin Augments Activator Protein-1 and Does Not Reduce Nuclear Factor-Kappa B in the Renal Cortex of Rats with Established Chronic Glomerular Disease,” Nephron, Vol. 90, No. 3, 2002, pp. 313-319. [53] T. L. Wadsworth and D. R. Koop, “Effects of the Wine Polyphenolics Quercetin and Resveratrol on Pro- inflammatory Cytokine Expression in RAW 264.7 Macro- phages,” Biochemical Pharmacology, Vol. 57, No. 8, 1999, pp. 941-949. [63] [54] S. Wang, V. L. DeGroff and S. K. Clinton, “Tomato and Soy Polyphenols Reduce Insulin-Like Growth Factor-I- Stimulated Rat Prostate Cancer Cell Proliferation and Apoptotic Resistance in Vitro via Inhibition of Intracel- lular Signaling Pathways Involving Tyrosine Kinase,” Journal of Nutrition, Vol. 133, 2003, pp. 2367-2376. [55] J. L. Pang, D. A. Ricupero, S. Huang, N. Fatma, D. P. Sing, J. S. Romero and N. Chattopadhyay, “Differential Activity of Kaempferol and Quercetin in Attenuating Tumor Necrosis Factor Receptor Family Signaling in Bone Cells,” Biochemical Pharmacology, Vol. 71, No. 6, 2006, pp. 818-886. [56] V. García-Mediavilla, I. Crespo, P. S. Collado, A. Esteller, S. Sánchez-Campos, M. J. Tuñón and J. González-Gallego, “The Anti-Inflammatory Flavones Quercetin and Kaemp- ferol Cause Inhibition of Inducible Nitric Oxide Synthase, Cyclooxygenase-2 and Reactive C-Protein, and Down- Regulation of the Nuclear Factor Kappab Pathway in Chang Liver Cells,” European Journal of Pharmacology, Vol. 557, No. 2-3, 2007, pp. 221-229. [57] M. Comalada, D. Camuesco, S. Sierra, I. Ballester, J. Xaus, J. Galvez and A. Zarzuelo, “In Vivo Quercitrin Anti-Inflammatory Effect Involves Releases of Quercetin, Which Inhibits Inflammation through Down-Regulation of NF-Kappab Pathway,” European Journal of Immu- nology, Vol. 35, 2005, pp. 584-592. [58] M. P. Nair, S. Mahajan, J. L. Reynolds, R. Aalinkeel, H. Nair, S. A. Schwartz and C. Kandaswami, “The Flavon- oid Quercetin Inhibits Proinflammatory Cytokine (Tumor Ne-Crosis Factor Alpha) Gene Expression in Normal Pe- ripheral Mononuclear Cells via Modulation of the NF-ΚB System,” Clinical and Vaccine Immunology, Vol. 13, 2006, pp. 319-328. [59] K. Muraoka, K. Shimizu, X. Sun, T. Tani, R. Izumumi, K. Miwa and K. Yamamoto, “Flavonoids Exert Diverse In- hibitory Effects on the Activation of NF-kB,” Transplan- tation Proceedings, Vol. 34, 2002, pp. 1335-1340. [60] C. A. Musonda and J. K. Chipman, “Quercetin Inhibits Hydrogen Peroxide (H2O2)-Induced NF-kappaB DNA Binding Activity and DNA Damage in HepG2 Cells,” Carcinogenesis, Vol. 19, No. 9, 1998, pp. 1583-1589. [61] S. Martínez-Flórez, M. B. Gutiérrez S. Sánchez-Campos, J. González-Gallego and M. J. Tuñón, “Quercetin Pre- vents Nitric Oxide Production and Nuclear Factor Kappa B Activation in Interleukin-1Β-Activated Rat Hepato- cytes,” Journal of Nutrition, Vol. 135, 2005, pp. 1359- 1365. [62] J. Chen, F. M. Ho, P. L. Chao, C. P. Chen, K. G. Jeng, H. B. Hsu, S. T. Lee, W. Wen Tung and W. W. Lin, “Inhibi- tion of iNOS Gene Expression by Quercetin Is Mediated By the Inhibition of IΚB Kinase, Nuclear Factor-Kappa B and STAT1, and Depends on Heme Oxygenase-1 Induc- tion in Mouse BV-2 Microglia,” European Journal of Pharmacology, Vol. 521, No. 1-3, 2005, pp. 9-20. T. Banerjee, A. Van der Vliet and V. A. Ziboh, “Down- Regulation of COX-2 and iNOS by Amentoflavone and Quercetin in A549 Human Lung Adenocarcinoma Cell Line,” Prostaglandins Leukot Essent Fatty Acids, Vol. 66, No. 5-6, 2002, pp. 485-492. [64] J. Duarte, R. Jiménez, F. O'Valle, M. Galisteo, R. Pérez- Palencia, F. Vargas, F. Pérez-Vizcaíno, A. Zar-Zuelo and J. Tamargo, “Protective Effects of the Flavonoid Quercetin in Chronic Nitric Oxide Deficient Rats,” Journal of Hy- pertension, Vol. 20, No. 9, 2002, pp. 1843-1854. [65] T. L. Wadsworth and D. R. Koop, “Effects of Ginkgo Biloba Extract (EGb 761) and Quercetin on Lipopoly- saccharide-Induced Release of Nitric Oxide,” Chemico- Biological Interaction, Vol. 137, No. 1, 2001, pp. 43-58. [66] T. L. Wadsworth, T. L. McDonald and D. R. Koop, “Ef- fects of Ginkgo Biloba Extract (EGb 761) and Quercetin on Lipopolysaccharide-Induced Signaling Pathways In- volved in the Release of Tumor Necrosis Factor-Alpha,” Biochemical Pharmacology, Vol. 62, No. 7, 2001, pp. 963-974. [67] H. M. Abd El-Gawad and A. E. Khalifa, “Quercetin, Coenzyme Q10, and L-canavanine as Protective Agents against Lipid Peroxidation and Nitric Oxide Generation in Endotoxin-Induced Shock in Rat Brain,” Pharmacol- ogical Research, Vol. 43, No. 3, 2001, pp. 257-263. [68] S. C. Shen, W. R. Lee, H. Y. Lin, H. C. Huang, C. H. Ko, L. L. Yang and Y. C. Chen, “In Vivo and In Vitro In- hibitory Activities of Rutin, Wogonin and Quercetin on Lipopolysaccharide-Induced Nitric Oxide and Prostaglan- Din E(2) Production,” European Journal of Pharmacol- ogy, Vol. 446, No. 1-3, 2002, pp. 187-194. [69] O. Coskun, M. Kanter, A. Korkmaz and S. Oter, “Quercetin, a Flavonoid Antioxidant, Prevents and Pro- tects Streptozotocin-Induced Oxidative Stress and Beta- Cell Damage in Rat Pancreas,” Pharmacological Re- search, Vol. 51, No. 2, 2005, pp. 117-123. [70] Z. Shutenko, Y. Henry, E. Pinard, J. Seylaz, P. Potier, F. Berthet, P. Girard and R. Sercombe, “Influence of the Antioxidant Quercetin in Vivo on the Level of Nitric Copyright © 2010 SciRes. CM  E. ROSTOKA ET AL. Copyright © 2010 SciRes. CM 17 Oxide Determined by Electron Paramagnetic Resonance in Rat Brain During Global Ischemia and Reperfusion,” Biochemical Pharmacology, Vol. 57, No. 2, 1999, pp. 199-208. [71] J. Da Silva, S. M. Hermann, V. Heuser, W. Peres, N. Marroni, J. González-Gallego and H. Erdtmann, “Evalua- tion of the Genotoxic Effect of Rutin and Quercetin by Comet Assay and Micronucleus Test,” Food and Chemi- cal Toxicology, Vol. 40, No. 7, 2002, pp. 941-947. [72] M. Číž, M. Pavelková, L. Gallová, J. Králová, L. Kubala and A. Lojek, “The Influence of Wine Polyphenols on Reactive Oxygen and Nitrogen Species Production by Murine Macrophages RAW 264.7,” Physiological Re- search, Vol. 57, 2008, pp. 393-402. [73] S. Bastianetto, W. H. Zheng and R. Quirion, “Neuropro- tective Abilities of Resveratrol and Other Red Wine Con- stituents against Nitric Oxide-Related Toxicity in Cul- tured Hippocampal Neurons,” British Journal of Phar- macology, Vol. 131, No. 4, 2000, pp. 711-720. |

