Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 11, No.6, pp.587-595, 2012 jmmce.org Printed in the USA. All rights reserved 587 Secondary Recovery of Colum bite from Tailing Dump in Nigerian Jos Mines Field 1Ayeni*, F.A, 1Madugu, I. A, 1Sukop, P, 2Ibitoye, S. A, 2Adeleke, A. A, 3Abdulwahab, M 1National Metallurgical Development Centre (NMDC), P. M. B. 2116, Jos, Nigeria. 2Department of Materials Science and Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria 3Department of Metallurgical Engineering, Ahmadu Bello University, Zaria, Nigeria *Corresponding Author: ayeflo@yahoo.com ABSTRACT Millions of tons of tailing dump at Rayfield mine in Jos in North Central Plateau state of Nigeria have been found to contain large quantity of columbite. Initial attempts to recover columbite concentrates by local miners and mineral speculators from the columbite rich tailing dump failed due to the ineffective processing route employed. Using cone and quartering sampling method, 0.5 kg of the columbite tailing was obtained for sieve and chemical analyses. 50 kg of <1mm fraction of the sample was subjected to a first stage magnetic concentration in a three poles Dry High Intensity Magnetic Separator (DHMS) that separated the columbite in the third pole. The re-grind of the + 0.355 mm rougher concentrate fraction (containing interlocking columbite) to pass the sieve aperture was treated on the DHMS in the second stage. The rougher concentrate undersize and columbite pre-concentrate of the first stage magnetic separation were then gravity concentrated on the air float machine. The Rayfield tailings and final concentrate were assayed using ED-XRFS to obtain 12.5% and 69.6% Nb2O5, respectively. The recovery and separation efficiency were 77.95% and 77.88% in that order. The magnetic and gravity concentrations were found effective at 77.95% recovery for columbite from the Rayfield tailing dump. This study also provided database for optimum recovery of columbite from tailings of mining sites of similar composition. Key Words: Tailing dump, columbite, recovery, concentration, characterization, Magnetic, Gravity, Recovery, Assay, Flowsheet  588 Ayeni, F.A, Madugu, I. A, Sukop, P Vol.11, No.6 1. INTRODUCTION Recent economic and technological changes in mineral processing techniques have enabled old dumped tailings of columbite to become profitable to process again. Columbite contains oxide of niobium (Nb2O5) and oxide of tantalum (Ta2O5) in different proportions. When the niobium oxide is much more than the tantalum oxide, the mineral is called columbite {(Fe, Mn)Nb2O6}, while it is called tantalite {(Fe, Mn)Ta2O6}, when the tantalum oxide content is much higher. Niobium metal is best known in connection with HSLA (High Strength Low Alloy) steels, heat resistance alloys in aerospace, vehicle engines and supersonic air-crafts [1]. Columbite is one of the most important solid minerals traded in the world market. Hence, many miners and processing engineers find one way or the other to acquire mining area rich in this mineral. Millions of tons of the tailings of columbites and cassiterites mined at the Rayfield minesfield by the British Miners in the late sixties were still very rich in the mineral [2]. In the past, when mining and processing of columbite and cassiterite were done at this mines field, less attention was given to the tailings either because their uses were not well defined then or there was no appropriate method of beneficiating them. Columbite is the main important mineral form of niobium. The columbite ore dressing usually involves pre-concentrate and concentrate clean-up. In order to recover columbite, a flowsheet which combines magnetic with gravity processing has been found suitable [3]. The choice of any part of or the entire processing route would depend on the nature of the ore, particularly the content of Nb2O5 and Ta2O5 in the ore relative to its associated minerals and impurities and difference in their physical properties. The concentration processes may be carried out by wet or dry gravity, magnetic or electrostatic methods to produce concentrates containing up to 70% combined pentoxide (Nb2O5 and Ta2O5) to meet extraction requirements. However, the universally employed method for the concentration of columbite ores are magnetic and gravity [4]. Solid minerals are so important in the production of materials for different engineering applications, that today’s mineral processing engineering encourages the optimum method of beneficiating tailings for maximum secondary recovery, leaving little or no values in the final tailings. The aim of this study is to characterize and assay the columbite tailings of the Rayfield mine waste, and establish an effective and appropriate secondary recovery processing route for this tailing. 2. MATERIALS AND METHODS 2.1 Materials  Vol.11, No6 Secondary Recovery of Columbite from Tailing 589 The material used for this study was collected from columbite tailing dump located in Rayfield village, about 15 km from Jos, Plateau State, Nigeria. 2.2 Methods 2.2.1 Sample collection The sample of the Rayfield tailings dump was collected from the site using grab sampling method. Fifty five kilogram (55 kg) of the columbite tailings was picked randomly within a short period of time as the representative from the tailing dump. 2.2.2 Sample preparation Using cone and quartering method, fifty five kilogram sample (55kg) of the tailing was poured into a conical heap, flattened and divided into four identical parts using a metal cutter. Two opposite corners were taken as sample; the other two corners were kept aside. The portion chosen as the sample was further coned and quartered and this continued until a sample of 0.50 kg of the columbite tailing was obtained for sieve and chemical analyses. 2.2.3 Particle size distribution analysis The columbite tailing was subjected to screen distribution analysis on a set of sieve arranged using geometric progression based on √2. The 500 g prepared sample of the tailing was placed on the topmost screen and the nest of sieve was automatically vibrated for 20 minutes. The weight of ore retained on each sieve was then determined [5]. 2.2.4 Energy dispersion-X Ray fluorescence spectrometry (ED-XRF) analysis Twenty grams of the sample was finely ground to pass through a 200-250 mesh sieve. It was dried in a Genlab oven, model MINO/50 and type Y6D144 at 105ºC for at least 1 hour and cooled. Thereafter, the sample was intimately mixed with a binder in the ratio of 5:1 sample(s) to cellulose flakes binder and pelletized at a pressure of about 19.4 kg/m2 in a pelletizing machine. At this stage, the pelletized sample(s) were stored in a dessicator for analysis. The machine, ED- XRFS, was switched on and allowed to stabilize for 2 hours. It was set at the default mode to analyze the compositions of the Rayfield columbite tailings and products from each processing unit, in form of oxides of the elements present [6]. The results of analysis was reported in percentage (%). 2.2.5 Concentration method  590 Ayeni, F.A, Madugu, I. A, Sukop, P Vol.11, No.6 Fifty kilograms of the columbite tailing was screened using the 1 mm sieve. The oversize portion was fed into rod milling machine to reduce the size and the mill product was recycled to the 1 mm sieve. The milling was repeated until the 50 kg lot passed through the sieve. The 50 kg undersize of 1 mm was thereafter fed into an optimum efficiency calibrated Dry High-intensity Magnetic Separator (Rapid) Model 4-3-15 OG, operating at a feed rate of 250 kg/hr and 0.5, 0.2 and 1.0 A for magnetite, hematite and columbite; respectively. The equipment was switched on and the sample was processed for about 15 minutes. 50 kg of the Rayfield tailing (-1.00 mm) was fed through the hopper of the rapid and the shutter was opened slightly to allow even and gradual spread of the feed on the magnetic belt. The magnetic belt conveyed the feed through the three discs of the rapid. The ferromagnetic material (magnetite) in the feed was separated from the feed at the first disc, the second disc separated rougher (hematite, escaped magnetite and some interlocking columbite), the third disc removed columbite and the non-magnets are collected in front of the belt. For maximum recovery, the rougher was processed further by sieving using 0.355 mm sieve size. The use of this sieve size was based on the results of the sieve analysis which showed that 0.355 mm size fraction had the highest number of grains reported. The oversize particles of 0.355 mm was crushed using the rod mill until all passed through the sieve. The undersize was recycled to the Rapid for further separation. The undersize of the 0.335 mm sieve was further reprocessed using pneumatic (air) floating table, Kipp Kelly model MY. The products of the air floating table were rougher concentrate, middling, and tailings. The middling was recycled to the table while the tailing made up the final tailing. The pre-concentrate from the rapid which consisted mainly of columbite, small quantity of silica and hematite was further processed using air floating machine operated at a tilted angle of 60°, speed of 2.75 stroke/s. motor speed 1425 rpm and feed rate of 250 kg/hr. The products of the air flotation were final concentrate (columbite), middling and tailing. The air floatation procedure was repeated for the middling and tailing while the resulting tailing was added to the tailing of the rougher process to form the final tailing of the process. Finally, samples were taken from products of each processing unit for assaying using ED-XRFS machine (Energy Dispersion-X Ray Fluorescence spectrometry) Minipal 4 Model 20. 3. RESULTS AND DISCUSSION 3.1 Results The results obtained on the particle size and chemical composition analyses are presented in Tables 1 and 2 respectively, while that of mass and metallurgical balances, recovery and separation efficiency for the process are presented in Tables 3 to 5. The secondary recovery processing flowsheet and the particle size distributions curve are presented in Figures 1 and 2 respectively.  Vol.11, No6 Secondary Recovery of Columbite from Tailing 591 Table 1: Grain size distribution of Rayfield columbite tailing Sizes (m) Weight (g) Weight Percentage (%) Nominal aperture size (m) Cumulative weight (%) undersize Cumulative weight (%) oversize Nb2O5 Ta2O5 +1000 6.04 1.2 1000 98.8 1.2 0.3 0.0 −1000+850 17.87 3.6 850 95.2 4.8 2.5 0.4 −850+710 49.79 10.0 710 85.2 14.8 3.8 0.7 −710+500 75.41 15.1 500 70.1 29.9 8.2 1.1 −500+355 101.55 20.3 355 49.8 50.2 11.4 1.5 −355+250 137.47 27.5 250 22.3 77.7 16.8 1.9 −250+180 78.22 15.7 180 6.6 93.4 17.2 2.1 −180+125 23.29 4.7 125 1.9 98.1 16.1 2.1 −125+90 7.46 1.5 90 0.4 99.6 8.4 1.7 −90 2.06 0.4 Total 499.16 Table 2: Chemical analysis of Rayfield columbite tailing and concentrate using ED – XRF Parameters (%) Rayfield tailing Pre – conc. Rougher Concentrate Tailing Al2O3 4.70 1.30 0.22 0.43 4.84 SiO2 40.3 7.00 9.30 4.51 54.60 TiO2 0.74 0.90 0.14 0.56 0.54 Fe2O3 12.90 12.36 1.17 11.18 3.48 ZrO2 7.80 1.31 76.26 1.13 18.90 Nb2O5 12.50 63.50 2.16 69.60 2.91 Ag2O 1.90 ND ND ND 0.62 Ta2O5 1.90 4.59 0.22 4.88 0.31 ThO2 2.78 0.27 0.33 0.19 1.42 Table 3: Mass balance for the process Item Quantity(kg) Feed 50 Concentrate 7 Rougher concentrate 19 Tailing 23.7 Loss 0.3 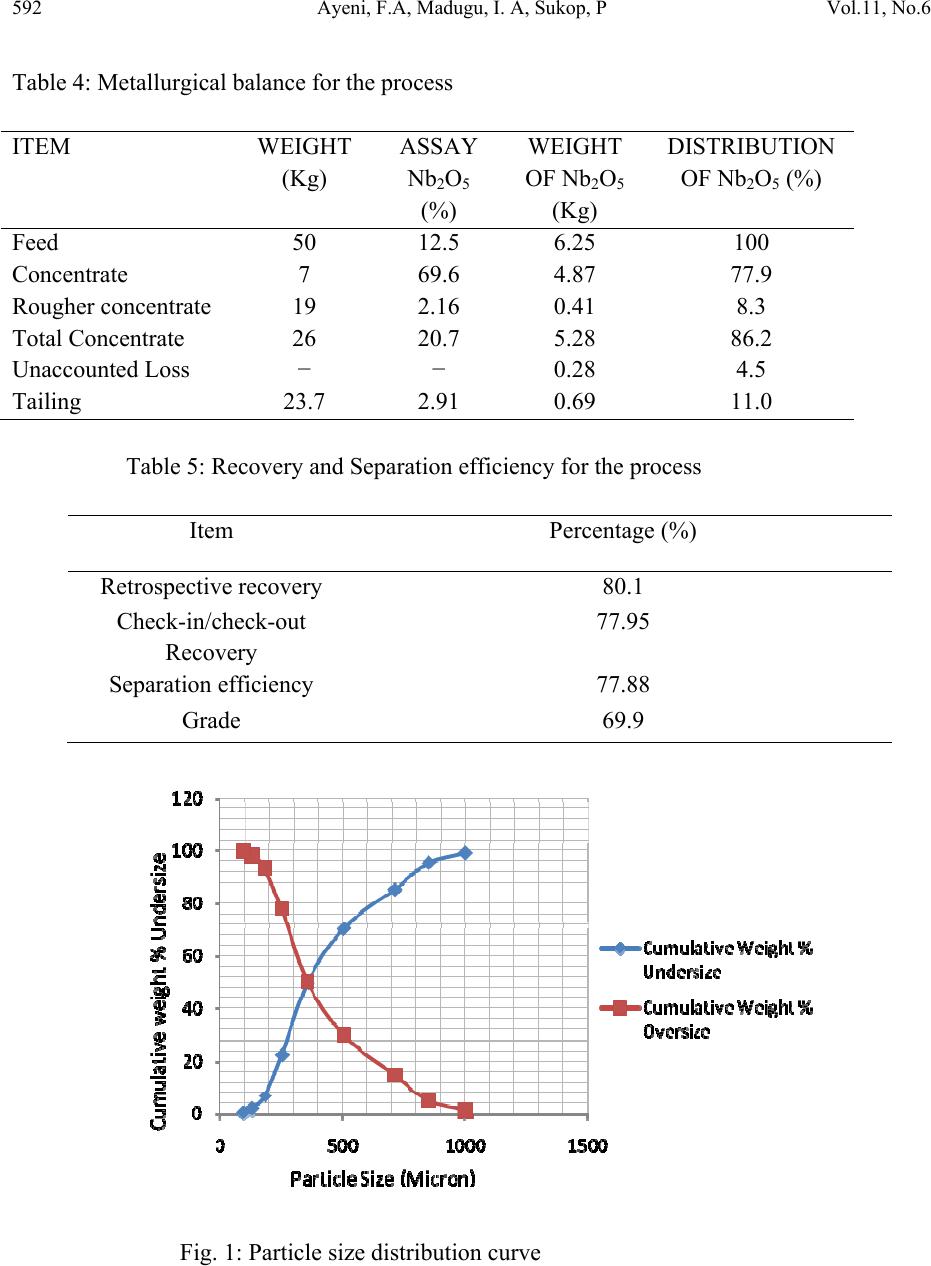 592 Ayeni, F.A, Madugu, I. A, Sukop, P Vol.11, No.6 Table 4: Metallurgical balance for the process ITEM WEIGHT (Kg) ASSAY Nb2O5 (%) WEIGHT OF Nb2O5 (Kg) DISTRIBUTION OF Nb2O5 (%) Feed 50 12.5 6.25 100 Concentrate 7 69.6 4.87 77.9 Rougher concentrate 19 2.16 0.41 8.3 Total Concentrate 26 20.7 5.28 86.2 Unaccounted Loss − − 0.28 4.5 Tailing 23.7 2.91 0.69 11.0 Table 5: Recovery and Separation efficiency for the process Item Percentage (%) Retrospective recovery 80.1 Check-in/check-out Recovery 77.95 Separation efficiency 77.88 Grade 69.9 Fig. 1: Particle size distribution curve 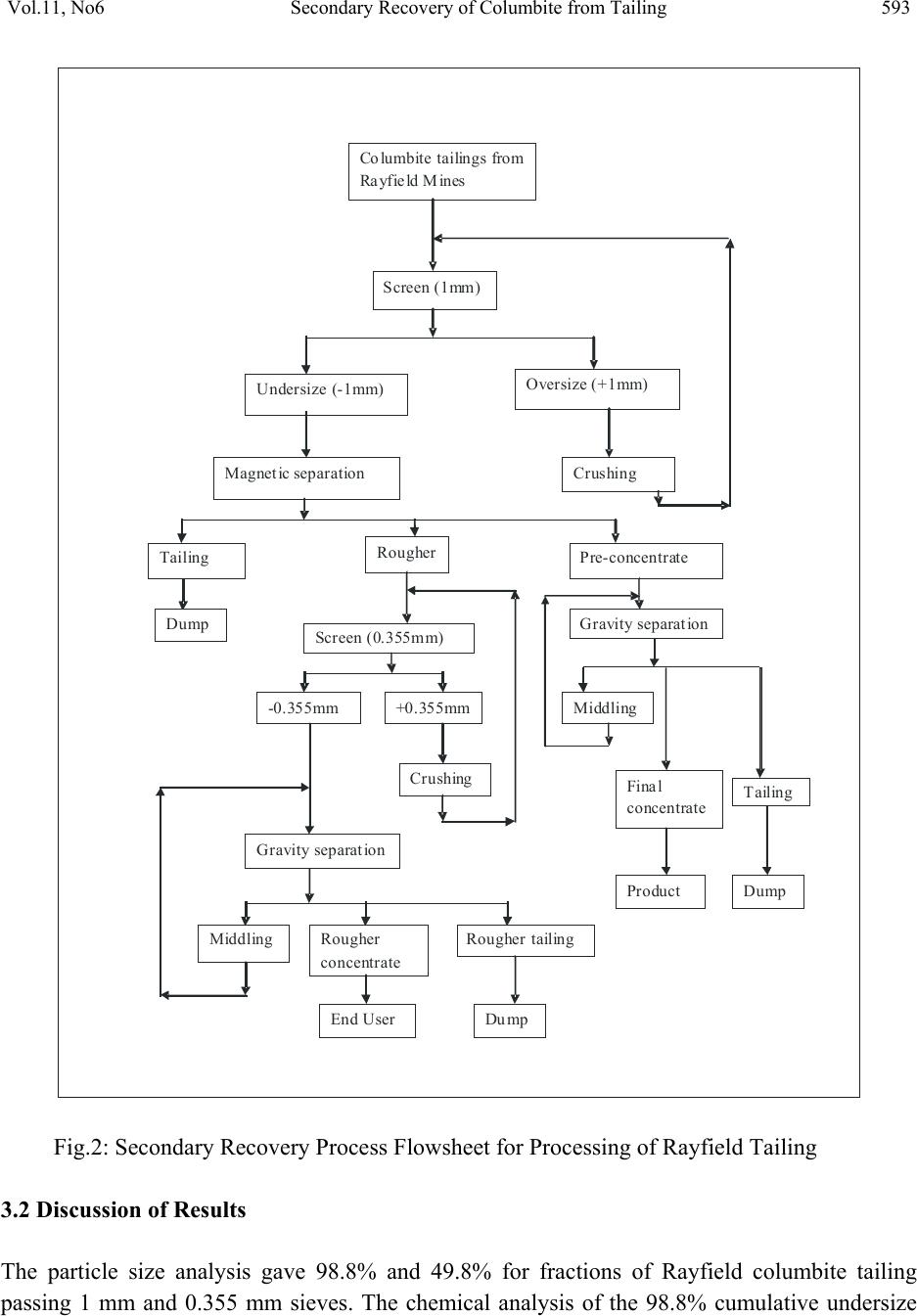 Vol.11, No6 Secondary Recovery of Columbite from Tailing 593 Co lumbitetailingsfrom Ra yfie ldMines Screen(1mm) Undersize (-1mm)Oversize (+1mm) MagneticseparationCrushing Tailing Rougher P re-co ncent r ate Screen(0.355mm)Gravity separation -0.355mm +0.355mm Crushing Middling Final concentrate Tailing Gravity separation Middling Rougher con centr at e Rougher tailing End UserDu mp Product Dump Dump Fig.2: Secondary Recovery Process Flowsheet for Processing of Rayfield Tailing 3.2 Discussion of Results The particle size analysis gave 98.8% and 49.8% for fractions of Rayfield columbite tailing passing 1 mm and 0.355 mm sieves. The chemical analysis of the 98.8% cumulative undersize  594 Ayeni, F.A, Madugu, I. A, Sukop, P Vol.11, No.6 fraction gave 12.5% Nb2O5. These results indicate that the tailing is rich and can be economically processed at this size fraction assay which is far higher than 4 to 6% Nb2O5 required for columbite tailing [7]. The size distribution also indicates that the tailing is alluvial and the form may lead to reduction in energy for comminution and hence cost of processing. The chemical analysis result of the Rayfield tailing passing 1 mm shows 40.3% silica, 12.9% iron oxide, 7.8% zirconia and 12.5% columbite as its major constituents. However, after magnetic separation the pre-concentrate has a significant reduction of silica and zirconia to 7% and 1.31% respectively and increase of columbite to 63.5%, while the iron remains almost the same at 12.36%, which may be due to interlocking with the columbite. The result of the cleaning operation of pre–concentrate using pneumatic table indicates the presence of 4.51% silica, 11.18% iron oxide, 1.13% Zirconia and 69.6% columbite in the final concentrate. These results showed that the columbite content was increased by about 82.04%, while reduction percents of 88.81, 13.33 and 85.51 were obtained for silica, iron oxide and zirconia, respectively. The results suggest that the combined magnetic/gravity concentration method produced a substantial upgrade of the tailings. The mass and metallurgical balance of the two final products from cleaning of pre – concentrate shows 7 kg and 77.9% distribution of columbite in the final concentrate assaying 69.6% respectively with a loss of 0.3 kg. Combining the final concentrate with the rougher concentrate, mass and metallurgical balance of 26 kg concentrate, 23.7 kg final tailing and 86.2% distribution of columbite assaying 20.7% respectively were obtained. This indicates that the proportion of metals in the feed that reports to concentrate may have an inverse relationship with the grade [7]. Recovery and separation efficiency of 77.95% and 77.88% respectively was obtained using this processing method. The appreciable recovery and separation efficiency achieved with very little loss is an evidence of the effectiveness of the applied processing route for the Rayfield columbite tailing dump. 4. CONCLUSIONS Rayfield tailing has been characterized into particle size and composition using physical and chemical methods respectively. ED-XRF spectrometry analysis indicates that 12.5 % columbite, 40.3% silica, 7.8% zirconia, 12.9% iron, alumina and other associated minerals form the constituents of the tailing. The tailing has been successfully beneficiated to a higher columbite content of 69.6% and lower silica, zirconia, iron contents of 4.51%, 1.13% and 11.18% in that order using magnetic and gravity concentration methods. Recovery and separation efficiency using these concentration methods or process route are established to be 77.95% and 77.88% respectively. This is an evidence of the effectiveness of the processing route applied for the tailing dump. 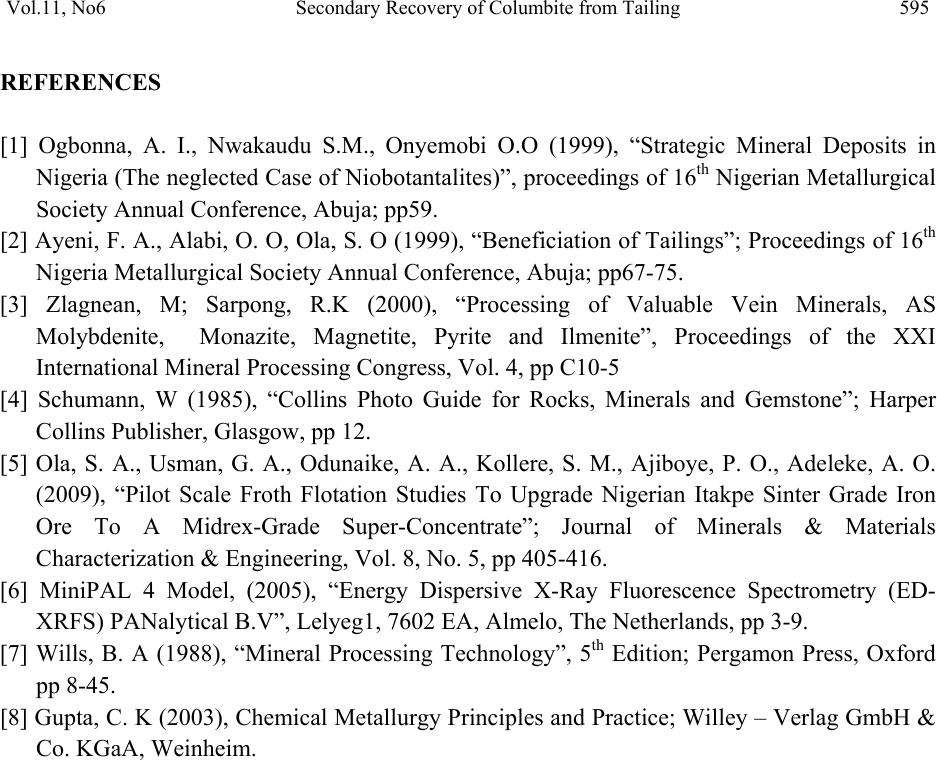 Vol.11, No6 Secondary Recovery of Columbite from Tailing 595 REFERENCES [1] Ogbonna, A. I., Nwakaudu S.M., Onyemobi O.O (1999), “Strategic Mineral Deposits in Nigeria (The neglected Case of Niobotantalites)”, proceedings of 16th Nigerian Metallurgical Society Annual Conference, Abuja; pp59. [2] Ayeni, F. A., Alabi, O. O, Ola, S. O (1999), “Beneficiation of Tailings”; Proceedings of 16th Nigeria Metallurgical Society Annual Conference, Abuja; pp67-75. [3] Zlagnean, M; Sarpong, R.K (2000), “Processing of Valuable Vein Minerals, AS Molybdenite, Monazite, Magnetite, Pyrite and Ilmenite”, Proceedings of the XXI International Mineral Processing Congress, Vol. 4, pp C10-5 [4] Schumann, W (1985), “Collins Photo Guide for Rocks, Minerals and Gemstone”; Harper Collins Publisher, Glasgow, pp 12. [5] Ola, S. A., Usman, G. A., Odunaike, A. A., Kollere, S. M., Ajiboye, P. O., Adeleke, A. O. (2009), “Pilot Scale Froth Flotation Studies To Upgrade Nigerian Itakpe Sinter Grade Iron Ore To A Midrex-Grade Super-Concentrate”; Journal of Minerals & Materials Characterization & Engineering, Vol. 8, No. 5, pp 405-416. [6] MiniPAL 4 Model, (2005), “Energy Dispersive X-Ray Fluorescence Spectrometry (ED- XRFS) PANalytical B.V”, Lelyeg1, 7602 EA, Almelo, The Netherlands, pp 3-9. [7] Wills, B. A (1988), “Mineral Processing Technology”, 5th Edition; Pergamon Press, Oxford pp 8-45. [8] Gupta, C. K (2003), Chemical Metallurgy Principles and Practice; Willey – Verlag GmbH & Co. KGaA, Weinheim. |

