Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2010, 1, 97-102 doi:10.4236/msa.2010.12017 Published Online June 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA Photochemical Properties of Precipitated Solid Aerosol Produced by Burning of Titanium Microparticles under Ambient Air Valery Zakharenko, Sophia Khromova Boreskov Institute of Catalysis, Pr. Lavrentieva, Novosibirsk, Russia. Email: zakh@catalysis.ru Received February 26th, 2010; revised May 4th, 2010; accepted May 7th, 2010. ABSTRACT In order to neutralize a drastic pollution of the environment (technogenic catastrophe) it is suggested to use technogenic technologies of chemical compound decontamination. One in such technologies can be the techno logy using metal oxide solid aerosols which are active in removal of pollutant compounds and obtainable by combustion under ambient air of appropriate metal particles, for example, aluminum, magnesium, titanium and etc. It is shown that the titanium dioxide out of an solid aerosol, obtained by pyrotechnic mixture combustion containing titanium microparticles has optic, chemical and photocatalytic properties close to properties of titanium dioxide produced by a different way. The production of s uch aeros ol in direct place of a techn ogenic catastrop he can be m ade for the cleanin g of atmosphere near a pollution source. Keywords: Pyrotechnic Mixture, Precipitated Aerosol, Tio2, Photoadsorption, Photocatalysis, Troposphere 1. Introduction The Earth’s atmosphere is ability for self-cleaning from dangerous compounds because of the photocatalytic and photosorption processes on the particle aerosol surface in the troposphere. These processes are anticipated to pri- mary affect the intensity of acid rains, concentration of greenhouse and ozone- depleting g ases, as well as cleaning up harmful compounds from the atmosphere. However, in case of man-caused catastrophe, which accompany a local emission of pollutants, the decon- tamination of the atmosphere by natural aerosols may be ineffective. For the neutralization of drastic pollution of the environment can be used technogenic technologies of a chemical compound decontamination. One in such technologies can be the technology using metal oxide solid aerosols which are active in removal of pollutant compounds and obtainable by combustion under ambient air of appropriate metal particles, for example, aluminum [1], magnesium [2] and etc. It is known that magnesium oxide photosorbs efficiently halogen-containing organic compounds [3] and titanium dioxide photocatalyzes an oxidation breakdown a large number of organic com- pounds polluting atmosphere [4,5]. The pyrotechnic technology of a metal oxide prepara- tion is mobile, available and inexpensive way of a solid aerosol production near a pollution source. The technical implement may be look like on a firework, lighting flare and the like. It allows locating a contaminatin g impact of chemical compounds. For a development of such tech- nology the study of photochemical properties of the tita- nium dioxide producing by pyrotechnic mixture combus- tion under ambient air was carried out. 2. Experimental Powdery titanium dioxide was prepared by combustion pyrotechni c mixture consisting of ammo nium perchl orate, hydroxy-terminated polybutadiene (HTPB) and titanium particles (particle size from 60 to 90 m) under ambient air. The highly dispersed titanium dioxide powder had the specific surface area of 6 m2g-1. After a treatment of the oxide surface at temperature 625 K for 30 min in air the specific surface area was decreased to 4 m 2g-1. The crystal structures of rutile and anatase were confirmed by X-ray diffraction of the prepared titanium oxide powders. A suspension of TiO2 in the distilled water was sup- ported on the internal wall of a quartz glass reactor, whic h was transparent for the light with wav elength longer than 185 nm. The TiO2 layer was dried at room temperature in air for a week. Then, without any additional treatment, the reactor was sealed to the high vacuum system for the  98 Photochemical Properties of Precipitated Solid Aerosol Produced by Burning of Titanium Microparticles under Ambient Air photoadsorption experiments. The reactor was evacuated to 10-5 Pa at room temperature for seve ral hours. Freon 134a (asymmetric tetrafluoroethane, CH2FCF3) was provided by the State Scientific Center of Applied Chemistry (St. Petersburg). Freon 22 (CHF2Cl) was pro- duced by the Ural factory “Halogen”. Before experiments this compounds were cleaned additionally by freezing. The chemical composition of the freons during the course of the experiment was monitored with a mass spec- trometer via their sampling through a leak valve. The pressure into the reactor volume was measured with the Pirani gage, which was calibrated with oxygen. A necessary correction was introduced for the sensitively with respect to Freons. The composition of the gas phase was measured with a mass spectrometer made from an monopol e analyzer APDM-1. The ki netics of the observed processes were registered by measuring the alteration in amplitude of the most intensive peak in the mass spectra of the corresponding substances. A DRSh-250 high pressure mercury lamp illuminated the reactor through heat water and interference filters. An RTN-20S thermopile was used to measure the radiation intensity. In some experiments, the unfiltered radiation of the mercury lamp at > 300 nm was used; for this purpose the interference filter was replaced by colored glass UV filter. The total density of the radiation flux was 1 mW cm-2 through this filter. The effective quantum yield was calculated as the ratio of the number of photoadsorption or photodesorption molecules to the number of quanta penetrated through the external wall of the reactor. The diffuse reflectance spectra were taken in air on a SPECORD M40 spectrophotometer. In the studies a powder-like magnesium oxide was used as the reference standard. 3. Results and Discussion 3.1 Optical Properties of Powdered Titania Samples We studied the absorption properties of dispersed titania after storage in air for a long period of time and after activation by heating at 625 K in air for 30 min with re- spect t o the light from diff erent parts of the solar spectrum. The experiments with the samples contacted with the air for a long time are especially important for evaluating theprospects of titania prepared by this method for appli- cation in processes leading to purification of the environ ment during various man-caused catastrophes. There are three stable crystalline modifications of tita- nia encountered in the nature in the form of rutile, anatase and brookite crystals. Titania samples of all these crys talline modifications with stoichiometric chemical com- position are white powers. The absorption of light with energies below the band gap of titan ia in rutile modifica- tion (Eg = 3.05 eV) is minimal by all such powders (Figure 1, inserts a) and b)). This absorp tion cor respo nds to the impurity absorption bands for single crystals or surface absor p tion for f in e ly dispersed powders. According to these data, there is a bend on the absorp- tion edge curve for powdered titania samples consisting from a mixture of anatase and rutile modifications (Fig- ure 1, insert s a) and b ), cu rv es A, B, and C) . Su ch a b end is not observed for pure anatase (Figure 1, inserts c), curve D) or rutile (Figure 1, insert c), curve E) samples. Titania produced by Degussa contains rutile, an atase and some amorphous phase [6]. The absorption band edge of anatase powder is shifted by 0.1-0.2 eV to shorter wave- lengths compared to that of rutile powder (3.0 and 2.8-2.9 eV, respect i v e ly). If the titania stoichiometry is changed due to partial reduction of Ti4+ to Ti3+ and formation of lattice oxygen vacancies du ring the oxide synt hesis, the pow ders becom e colored and absorption in the surface band of the oxide appears. Figure 1 (insert c), curve E) shows the diffuse reflectance spectrum of TiO2 sample prepared at Bore- skov Institute of Catalysis, which had substantially non- stoichiome t ri c composition and gree n color. Acco r di n g t o the results of the XRD studies, such sample contained titania in rutile modification. The greenish titania sample studied by us consisted of TiO2 in rutile modification mixed with ana tase. It was shown by EPR t hat this sample (which was use d i n the phot oa dso rpt ion e xpe ri m ents) was not stoichiometric and contained Ti3+ ions. The meas- urements of diffuse reflectance optical density (Figure 1, insert c), curve F) also proved that of sample was not stoichiometric. The titania sample was not subjected to any treatment during registration of the spectra. A similar sample was used for deposition on the reactor walls. The diffuse reflectance optical density spectrum of titania heated in air at 620 K for 30 min is shown in Figure 1 (insert d), curve H). Its spectrum is compared with that of the titania sampled from the gas mixture after combustion of the pyrotechnic mixture using a vacuum impactor (Figure 1, insert d), curve G). Thus, the absorption of titania powders prepared by combustion of pyrotechnic mixture containing titanium microparticles in the region before the TiO2 intrinsic ab- sorption edge (corresponding to the region of the solar troposphere irradiation spectrum) depends on the synthe- sis method and after-treatment of the samples. 3.2 Chemical Properties of Titania Prepared by Combustion of Titanium Microparticles in Air When the reactor with deposited TiO2 was evacuated at room temperature, water and carbon dioxide were the ain products released from the surface of the titania m Copyright © 2010 SciRes. MSA 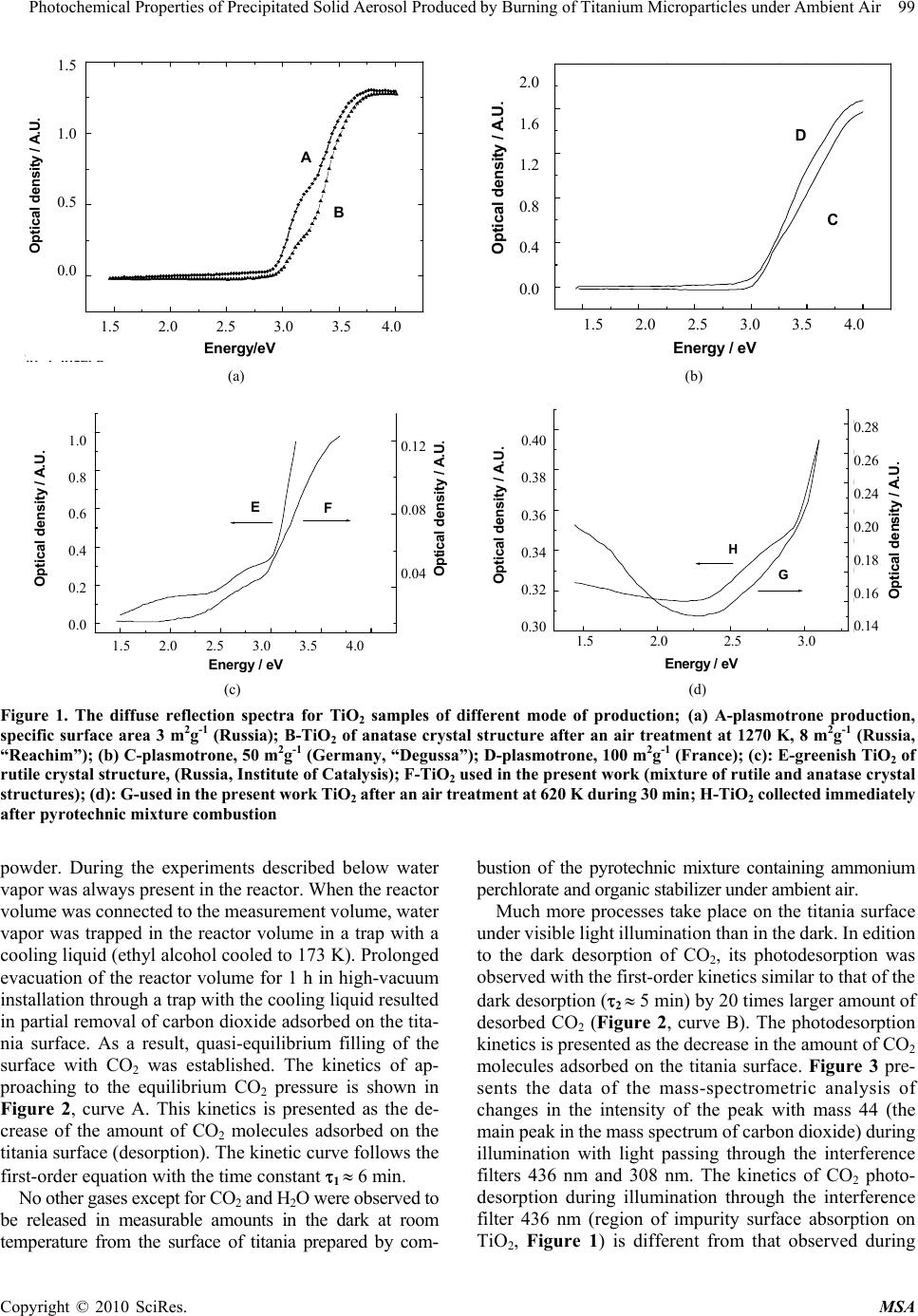 Photochemical Properties of Precipitated Solid Aerosol Produced by Burning of Titanium Microparticles under Ambient Air 99 Copyright © 2010 SciRes. MSA 1,5 2,0 2,5 3,0 3,5 4,0 0,0 0,5 1,0 1,5 Fig1inseta B A Ener gy/e V Optical density 1.5 1.0 0.5 0.0 1.5 2.0 2.5 3.0 3.5 4.0 Opt ic a l d e ns i ty / A.U . 1,5 2,02,5 3,0 3,5 4,0 0,0 0,4 0,8 1,2 1,6 2,0 b) D C Energy / eV Optical density / A.U. 2.0 1.6 1.2 0.8 0.4 0.0 1.5 2.0 2.5 3.0 3.5 4.0 (a) (b) 1,5 2,0 2,53,0 3,5 4,0 0,0 0,2 0,4 0,6 0,8 1,0 Optical density / A.U. Energy / eV 0,04 0,08 0,12 c) Optical density / A.U . F E 1.0 0.8 0.6 0.4 0.2 0.0 0.12 0.08 0.04 1.5 2.0 2.5 3.0 3.5 4.0 1,5 2,0 2,5 3,0 0,30 0,32 0,34 0,36 0,38 0,40 Optical density / A.U. Energy / eV 0,14 0,16 0,18 0,20 0,22 0,24 0,26 0,28 d) Optical density / A.U. G H 0.40 0.38 0.36 0.34 0.32 0.30 0.28 0.26 0.24 0.20 0.18 0.16 0.14 1.5 2.0 2.5 3.0 (c) (d) Figure 1. The diffuse reflection spectra for TiO2 samples of different mode of production; (a) A-plasmotrone production, specific surface area 3 m2g-1 (Russia); B-TiO2 of anatase crystal structure after an air treatment at 1270 K, 8 m2g-1 (Russia, “Reachim”); (b) C-plasmotrone, 50 m2g-1 (Germany, “Degussa”); D-plasmotrone, 100 m2g-1 (France); (c): E-greenish TiO2 of rutile crystal structure, (Russia, Institute of Catalysis); F-TiO2 used in the present work (mixture of rutile and anatase crystal structures); (d): G-used in the present work TiO2 after an air treatment at 620 K during 30 min; H-TiO2 collected immediately after pyrotechnic mixture combustion powder. During the experiments described below water vapor was always present in the reactor. Wh en the reactor volum e was conn ecte d t o th e mea surement vol ume, water vapor was trapped in the reactor volume in a trap with a cooling liquid (eth yl alcohol cooled to 173 K). Prolong ed evacuation of the reactor volume for 1 h in high-vacuum installation through a trap with the co oling liqu id resulted in partial removal o f carbon diox ide adsorb ed on the tita- nia surface. As a result, quasi-equilibrium filling of the surface with CO2 was established. The kinetics of ap- proaching to the equilibrium CO2 pressure is shown in Figure 2, curve A. This kinetics is presented as the de- crease of the amount of CO2 molecules adsorbed on the titania surface (desorption). The kinetic curve follows the first-order equation with the time constant 1 6 min. No other gases except for CO2 and H2O were observed to be released in measurable amounts in the dark at room temperature from the surface of titania prepared by com- bustion of the pyrotechnic mixture containing ammonium perchlorate and organic stabilizer under ambient air. Much more processes take place on the titania surface under visible light illumination than in the dark. In edition to the dark desorption of CO2, its photodesorption was observed with the first-order kinetics similar to that of the dark desorption (2 5 min) by 20 times larger amount of desorbed CO2 (Figure 2, curve B). The photodesorption kinetics is pres ented as t he decrease i n the amount of C O 2 molecules adsorbed on the titania surface. Figure 3 pre- sents the data of the mass-spectrometric analysis of changes in the intensity of the peak with mass 44 (the main peak i n the m ass spect rum of car bon di oxi de) duri ng illumination with light passing through the interference filters 436 nm and 308 nm. The kinetics of CO2 photo- desorption during illumination through the interference filter 436 nm (region of impurity surface absorption on TiO2, Figure 1) is different from that observed during 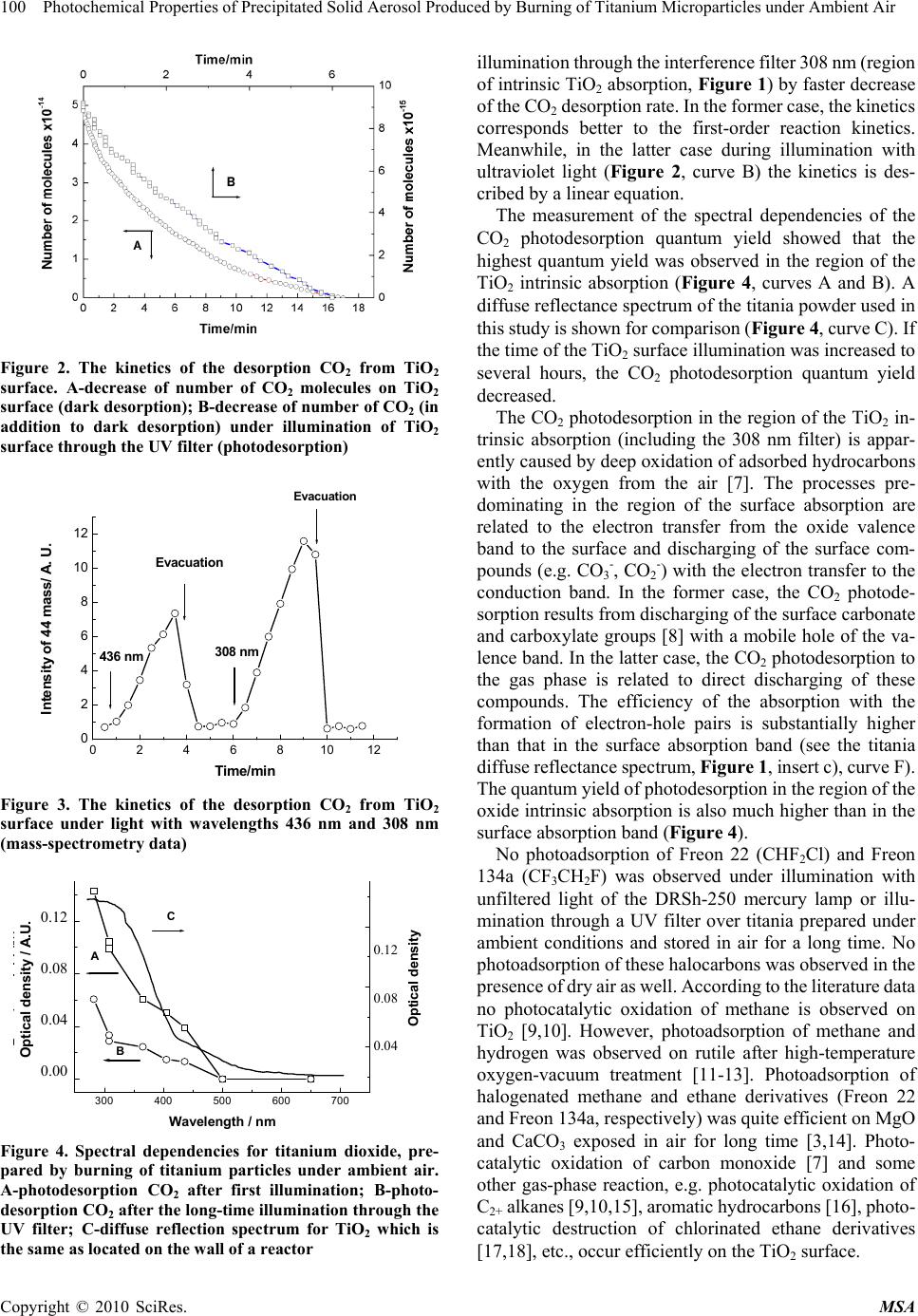 100 Photochemical Properties of Precipitated Solid Aerosol Produced by Burning of Titanium Microparticles under Ambient Air Figure 2. The kinetics of the desorption СО2 from TiO2 surface. A-decrease of number of СО2 molecules on TiO2 surface (dark desorption); B-decrease of number of СО2 (in addition to dark desorption) under illumination of TiO2 surface through the UV filter (photodesorption) 024681012 0 2 4 6 8 10 12 Evacuation Evacuation 308 nm 436 nm Intens ity of 44 mass/ A. U. Time/min Figure 3. The kinetics of the desorption СО2 from TiO2 surface under light with wavelengths 436 nm and 308 nm (mass-spectrometry data) 300 400 500 600 700 0,00 0,04 0,08 0,12 C B A Optical density Q uan t um y i e ld (%) Wa vele ngt h / nm 0,04 0,08 0,12 0.12 0.08 0.04 0.00 0.12 0.08 0.04 Optical density / A.U. Figure 4. Spectral dependencies for titanium dioxide, pre- pared by burning of titanium particles under ambient air. A-photodesorption СО2 after first illumination; B-photo- desorption СО2 after the long-time illumination through the UV filter; C-diffuse reflection spectrum for TiO2 which is the same as located on the wall of a reactor illumination through the interference filter 308 nm (region of intrinsic TiO2 absorption, Figure 1) by faster decrease of the CO2 desorption rate. In the former case, the kinetics corresponds better to the first-order reaction kinetics. Meanwhile, in the latter case during illumination with ultraviolet light (Figure 2, curve B) the kinetics is des- cribed by a linear equation. The measurement of the spectral dependencies of the CO2 photodesorption quantum yield showed that the highest quantum yield was observed in the region of the TiO2 intrinsic absorption (Figure 4, curves A and B). A diffuse reflectance spectrum of the titan ia powder used in this study is shown for comparison (Figure 4, curve C). If the time of the TiO2 surface illumination was increased to several hours, the CO2 photodesorption quantum yield decreased. The CO2 photodesorption in the region of the TiO2 in- trinsic absorption (including the 308 nm filter) is appar- ently caused by deep oxidation of adsorbed hydrocarbons with the oxygen from the air [7]. The processes pre- dominating in the region of the surface absorption are related to the electron transfer from the oxide valence band to the surface and discharging of the surface com- pounds (e.g. CO3-, CO2-) with the electron transfer to the conduction band. In the former case, the CO2 photode- sorption results from discharging of the surface carbonate and carboxylate groups [8] with a mobile hole of the va- lence band. In the latter case, the CO2 photodesorption to the gas phase is related to direct discharging of these compounds. The efficiency of the absorption with the formation of electron-hole pairs is substantially higher than that in the surface absorption band (see the titania diffuse reflectance spectrum, Figure 1, inser t c), c urve F). The quantum yi eld of p hoto deso rptio n in t he regi on o f the oxide intrinsic absorp tion is also much higher than in th e surface absorption band (Figure 4). No photoadsorption of Freon 22 (CHF2Cl) and Freon 134a (CF3CH2F) was observed under illumination with unfiltered light of the DRSh-250 mercury lamp or illu- mination through a UV filter over titania prepared under ambient conditions and stored in air for a long time. No photoadsor pti on of t hese halocarb ons was o bserve d in the presence of dry air as well. According to the literature data no photocatalytic oxidation of methane is observed on TiO2 [9,10]. However, photoadsorption of methane and hydrogen was observed on rutile after high-temperature oxygen-vacuum treatment [11-13]. Photoadsorption of halogenated methane and ethane derivatives (Freon 22 and Freon 134a, respectively) was quite efficient on MgO and CaCO3 exposed in air for long time [3,14]. Photo- catalytic oxidation of carbon monoxide [7] and some other gas-phase reaction, e.g. photocatalytic oxidation of C2+ alkanes [9, 10,15] , arom atic hydr ocarbons [16], photo- catalytic destruction of chlorinated ethane derivatives [17,18], etc., occur efficiently on the TiO2 surface. Copyright © 2010 SciRes. MSA 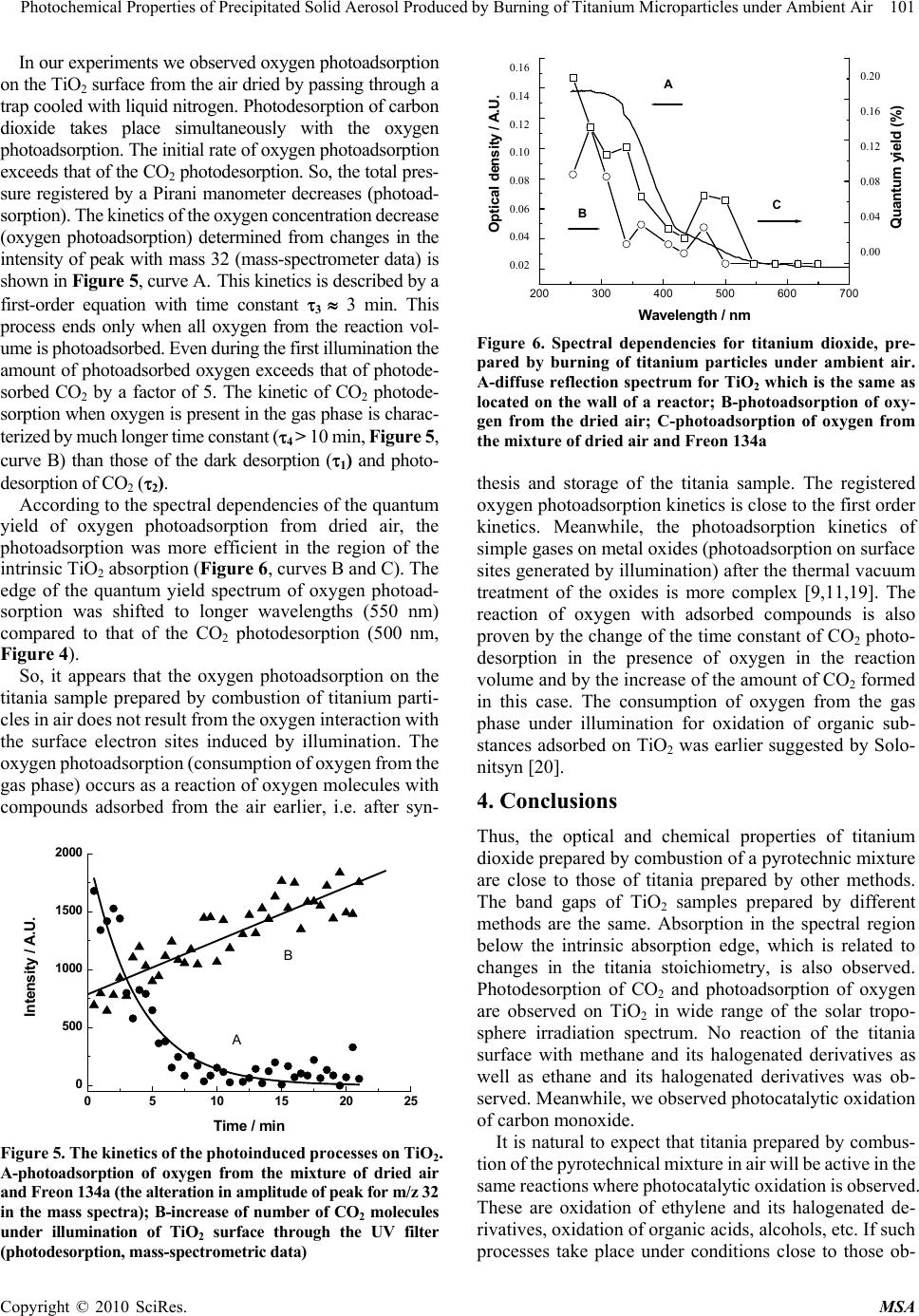 Photochemical Properties of Precipitated Solid Aerosol Produced by Burning of Titanium Microparticles under Ambient Air 101 In our experiments we observed oxygen photoadsorption on the TiO2 surface from the air dried by passing through a trap cooled with liquid nitrogen. Photodesorption of carbon dioxide takes place simultaneously with the oxygen photoadsorption. The initial rate of oxygen photoadsorption exceeds that of the CO 2 photodesorption. So, the total pres- sure registered by a Pirani manometer decreases (photoad- sorption). The kinetics of the oxygen concentration decrease (oxygen photoadsorption) determined from changes in the intensity of peak with mass 32 (mass-spectrometer data) is shown in Figure 5, curve A. This kinetics is described by a first-order equation with time constant 3 3 min. This process ends only when all oxygen from the reaction vol- ume is photoadsorbed. Even during the first illumination the amount of photoadsorbed oxygen exceeds that of photode- sorbed CO2 by a factor of 5. The kinetic of CO2 photode- sorption when oxygen is present in the gas phase is charac- terized by much longer time constant (4 > 10 min, Figure 5, curve B) than those of the dark desorption (1) and photo- desorption of CO2 (2). According to the spectral dependencies of the quantum yield of oxygen photoadsorption from dried air, the photoadsorption was more efficient in the region of the intrinsic TiO2 absorption (Figure 6, curves B and C). The edge of the quantum yield spectrum of oxygen photoad- sorption was shifted to longer wavelengths (550 nm) compared to that of the CO2 photodesorption (500 nm, Figure 4). So, it appears that the oxygen photoadsorption on the titania sample prepared by combustion of titanium parti- cles in air does not result from the oxygen interaction with the surface electron sites induced by illumination. The oxygen photoadsorption (consumption of oxygen from the gas phase) occurs as a reaction of oxygen molecu les with compounds adsorbed from the air earlier, i.e. after syn- 0510 15 20 25 0 500 1000 1500 2000 B A Intensity / A.U. Time / min Figure 5. The kinetics of the photoinduced processes on TiO2. A-photoadsorption of oxygen from the mixture of dried air and Freon 134a (the alteration in amplitude of peak for m/z 32 in the mass spectra); B-increase of number of СО2 molecules under illumination of TiO2 surface through the UV filter (photodesorption, mass-spectrometric data) 200 300 400 500 600 700 0,02 0,04 0,06 0,08 0,10 0,12 0,14 0,16 Optica l densi ty Wavelength / nm 0,00 0,04 0,08 0,12 0,16 0,20 Quantum yield (%) C B A 0.16 0.14 0.12 0.10 0.08 0.06 0.04 0.02 0.20 0.16 0.12 0.08 0.04 0.00 Optical density / A. U. Figure 6. Spectral dependencies for titanium dioxide, pre- pared by burning of titanium particles under ambient air. A-diffuse reflection spectrum for TiO2 which is the same as located on the wall of a reactor; B-photoadsorption of oxy- gen from the dried air; C-photoadsorption of oxygen from the mixture of dried air and Freon 134a thesis and storage of the titania sample. The registered oxygen photoadsorp tion k inetics is close to the first order kinetics. Meanwhile, the photoadsorption kinetics of simple gases on metal oxides (photoadsorption on surface sites generated by illumination) after th e thermal vacuum treatment of the oxides is more complex [9,11,19]. The reaction of oxygen with adsorbed compounds is also proven by the change of the time constant of CO2 photo- desorption in the presence of oxygen in the reaction volume and by the increase of the amount of CO2 formed in this case. The consumption of oxygen from the gas phase under illumination for oxidation of organic sub- stances adsorbed on TiO2 was earlier suggested by Solo- nitsyn [20]. 4. Conclusions Thus, the optical and chemical properties of titanium dioxide prepared by combustion of a pyrotechnic mixture are close to those of titania prepared by other methods. The band gaps of TiO2 samples prepared by different methods are the same. Absorption in the spectral region below the intrinsic absorption edge, which is related to changes in the titania stoichiometry, is also observed. Photodesorption of CO2 and photoadsorption of oxygen are observed on TiO2 in wide range of the solar tropo- sphere irradiation spectrum. No reaction of the titania surface with methane and its halogenated derivatives as well as ethane and its halogenated derivatives was ob- served. Meanwhile, we observed pho tocatalytic oxid ation of carbon monoxide. It is natural to expect that titania prepared by combus- tion of the pyrotechnical mixture in air will be active in the same reactions where phot ocatalytic oxidat ion is obser ved. These are oxidation of ethylene and its halogenated de- rivatives, oxidation of organic acids, alcohols, etc. If such processes take place under conditions close to those ob- Copyright © 2010 SciRes. MSA  102 Photochemical Properties of Precipitated Solid Aerosol Produced by Burning of Titanium Microparticles under Ambient Air Copyright © 2010 SciRes. MSA served in the combustion zone, they can contribute to cleaning of the environment from various gaseous pol- lutants. REFERENCES [1] B. Z. Eapen, V. K. Hoffmann, M. Schoenitz and E. L. Dreizin, “Combustion of Aerosolized Spherical Aluminum Powders and Flakes in Air,” Combustion Science and Technology, Vol. 176, No. 7, 2004, pp. 1055-1069. [2] I. E. Molodetsky, E. L. Dreizin, E. P. Vicenzi and C. K. Law, “Phases Titanium Combustion in Air,” Combustion and Flame, Vol. 112, No. 4, 1998, pp. 522-532. [3] V. N. Parmon and V. S. Zakharenko, “Photocatalysis and Photosorption in the Earth’s Atmosphere,” CatTech, Vol. 5, 2001, pp. 96-115. [4] M. R. Hoffman, S. T. Martin, W. Choi and D. W. Bah- neman, “Environmental Application of Semiconductor Photocatalysis,” Chemical Review, Vol. 95, No. 2, 1995, pp. 69-96. [5] X. Chen and S. S. Mao, “Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications,” Chemical Review, Vol. 107, No. 1, 2007, pp. 2691-2959. [6] R. I. Bickley, T. G. Carreno, J. S. Lees, L. Palmisano and R. J. D. Tilley, “Structural Studies of P-25 Titanium Ox- ide,” Journal of Solid State Chemistry, Vol. 92, No. 17, 1991, pp. 178-186. [7] V. S. Zakharenko, “Photoadsorption and Photocatalytic Oxidation on the Metal Oxides—Components of Tropo- spheric Solid Aerosols under the Earth’s Atmospheric Conditions,” Catalysis Today, Vol. 39, No. 3, 1997, pp. 243-349. [8] P. Jackson a nd G. D. Par fitt, “Infr ared Study o f the Sur face Properties of Rutile,” Journal of Chemical Society, Faraday Transaction I, Vol. 68, No. 1 , 1972, pp . 896-906. [9] M. Formenti, F. Juillet, P. Meriaudeau, S. J. Teichner and P. Vergnon, “Preperation in a Hydr ogen -Ox ygen Flam e o f Ultrafine Metal Oxide Particles. Oxidative Properties to- ward Hydrocarbons in the Presence of Ultraviolet Radia- tion,” Journal of Colloid and Interface Science, Vol. 39, No. 1, 1972, pp. 79-89. [10] A. Mills and S. J. Le Hunte, “An Overview of Semicon- ductor Photocatalysis,” Journal of Photochemistry and Photobiology A , Vol. 108, No. 1, 1997, pp. 1-35. [11] R. J. Bickley and F. S. Stone, “Photoadsorption and Photocatalysis at Rutile Surfaces,” Journal of Catalysis, Vol. 31, No. 31, 1973, pp. 389-397. [12] S. L. Kaliaguine, B. N. Shelimov and V. B. Kazansky, “Reactions of Methane and Ethane with Hole Centers O-,” Journal of Catalysis, Vol. 55, No. 3, 1978, pp. 384-399. [13] A. Fujishima, N. R. Tata and D. A. Tryk, “Titanium Di- oxide Photocatalysis,” Journal of Photochemistry and Photobiology C, Vol. 1, No. 1, 2000, pp. 1-21. [14] V. S. Zakharenko and V. N. Parmon, “Photoadsorption and Photocatalysis Processes Influencing on Earth’s At- mosphere Composition,” Kinetics and Catalysis, Vol. 41, No. 6, 2000, pp. 756-759. [15] N. Djeghri, M. Formenti, F. Juillet and S. J. Teichner, “Photointeraction on the Surface of Titanium Dioxide between Oxygen and Alkanes,” Faraday Discussion, Chemical Society, Vol. 58, No. 1, 1974, pp. 185-193. [16] V. A. Isidorov, E. M. Klokova, V.G. Povarov and S. Klokova, “Photocatalysis on Atmospheric Aerosols: Ex- perimental Studies and Modeling,” Catalysis Today, Vol. 39, No. 3, 1997, pp. 233-242. [17] M. R. Nimies, W. A. Jacoby, D. M. Blake and T . A. Milne, “Gas-Phase Photodestruction over TiO2 Powders of Various Chlorinated Ethylene,” Environmental Science and Technology, Vol. 27, No. 3, 1993, pp. 732-739. [18] M. D. Driessen, A. L. Goodman, T. M. Miller, G. A. Za- harias and V. U. Grassian, “Gas-Phase Photooxidation of Trichloroethylene on TiO2,” Journal of Physical Chemis- try B, Vol. 102, No. 3, 1998, pp. 549-556. [19] A. Lisebigler, G . Lu and J. T. Yates, “Photocatalysis on the TiO2 Surfaces: Principles, Mechanisms and Selected Re- sults,” Chemical Review, Vol. 95, No. 3, 1995, pp. 735- 758. [20] A. N. Terenin and Y. P. Solonitsin, “Illumination Influ- ence over Gas Adsorption b y Solids,” Discussion Faraday Society, Vol. 28, No. 28, 1959, pp. 28-31. |

