 Journal of Minerals & Materials Characterization & Engineering, Vol. 11, No.5, pp.461-469, 2012 jmmce.org Printed in the USA. All rights reser ved 461 Hydrom etallurgi cal Purif ication of Some Clay Deposits for High Temperature Applications 1,2D.O. FOLORUNSO, 1 S. ARIBO, 1,2,3 P. O. OLUBAMBI and 1,2J.O. BORODE 1Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, Nigeria 2Africa Materials Science And Engineering Networks: A Carnegie-IAS (RISE) Networks 3Department of Chemical and Metallurgical Engineering, Tshwane University of Technology, Pretoria West, Pretoria, South Africa. Corresponding Author: stdavies4ever@yahoo.com Phone Number: +2348038204252 ABSTRACT The characterization of five different Nigerian clay deposits has been carried out by employing three different characterization techniques (X-ray Diffraction, X-ray Fluorescence and Scanning Electron Microscopy/EDX). The clays obtained from the various deposits were carefully prepared for the analyses and the results obtained were compared to confirm the consistency and reliability of the different methods employed. The results revealed the various desirable (SiO2 and Al2O3) and deleterious (Fe3O4, K2O, MgO, MnO and Na2O) elements and compounds contained in the clay samples in the various proportions and the appropriate purification technique required for preparing the clays for high temperature applications. Consequent upon the identification of the deleterious impurities, oxalic acid at different concentrations was used to leach the impurities out of the various clays. However, three out of the five dep osit s were chos en for purification because they possess the least quantities of the most deleterious (Fe 2O3) of the impurities. Keywords: Hydrometallurgy, Purification, High Temperature, Clay and Characterization.  462 D.O. FOLORUNSO, S. ARIBO, P. O. OLUBAMBI Vol.11, No.5 1. INTRODUCTION Refractori es are materials that can withstand high temperature and chemical attack in the severe conditions of working [1,2]. They are thermal insulators and therefore used for making furnace linings, kilns, nozzles for pouring molten metal, heat exchangers and driers, to mention a few [3,4]. Applications in furnaces include anything from primary metal melting, through to heat treatment, glass production and processing, ceramic component manufacture, many forms of chemical processing and testing [5,6] . The term "clay" refers to a naturally occurring material composed primarily of fine-grained minerals, which is generally plastic at appropriate water contents and will harden when dried or fired [7]. Although clay usually contains phyllosilicates, it may contain other materials that impart pla sti city a nd har de n when dr ied or fire d . Earlier works on various Nigerian refractory clay deposits have shown many of them to be unsuitable for refractory works in the as-mined states. They are either high or low in one or more of the important refractory properties desired for good refractory works, or they are completely lacking in them [8]. The unsuitability of the local clay deposits for refractory works in the as- mined states has therefore prompted the need for this work, which is principally aimed at characterizing and subsequently purifying them in readiness for the production of materials for high temperature applications. 2. MATERIALS AND METHOD Research Materials • Clays from: Ifon in Ondo State (sample A) Igb a r a -odo in Ekiti State, (B) Ipetumodu in Osun State (C) Is an -Ekiti in Ekiti State (D) Iseyin in Oyo State, (E) A K U R E C E . B A D 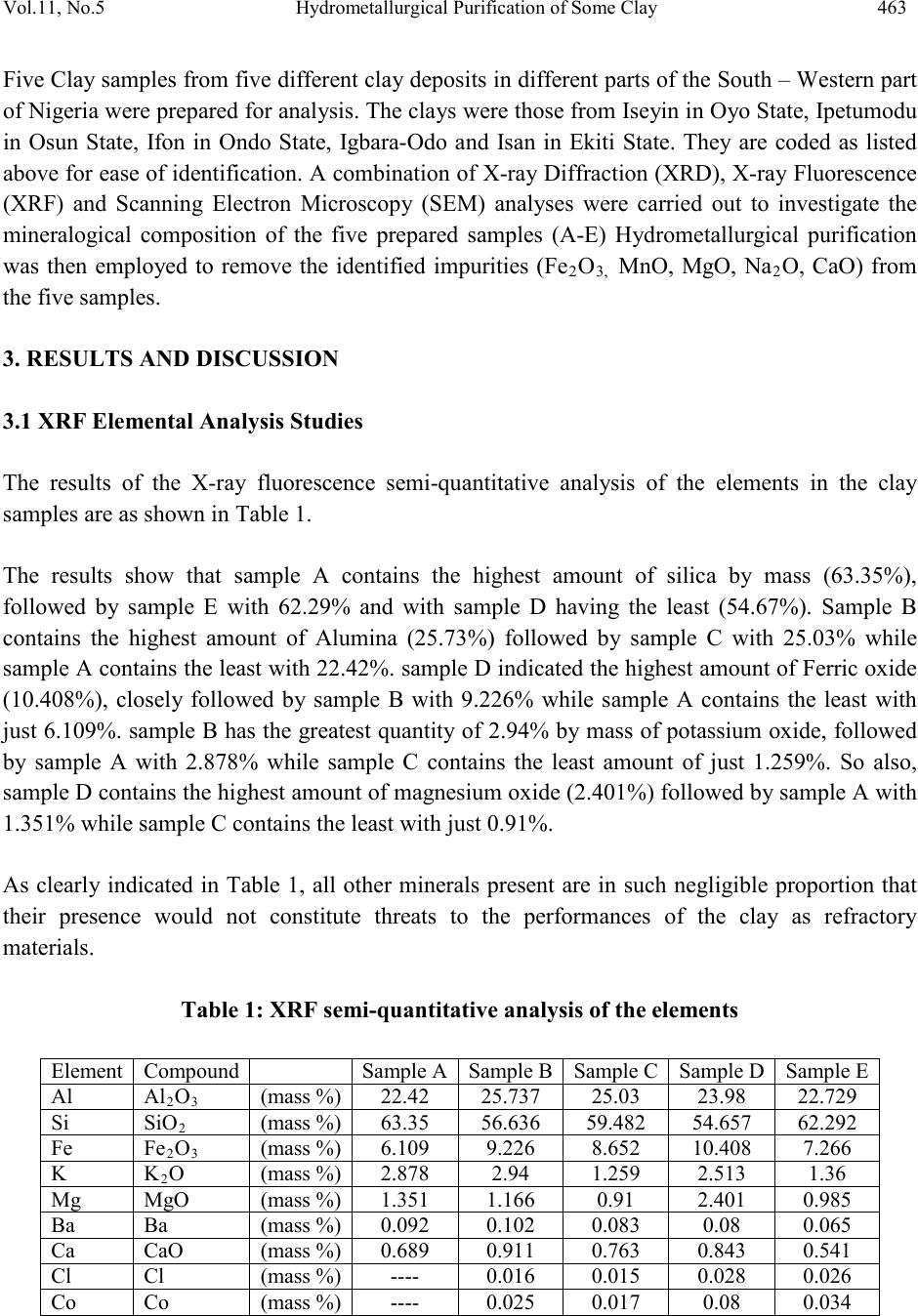 Vol.11, No .5 Hydrometallurgical Purification of Some Clay 463 Five Clay samples from five different clay deposits in different parts of the South – Western part of Nigeria were prepared for anal ysis. The clays were those f rom Ise yin in O yo State, Ipetumodu in Osun State, Ifon in Ondo State, Igbara-Odo and Isan in Ekiti State. They are coded as listed above for ease of identification. A combination of X-ray Diffracti on (XRD), X-ray Fluores cence (XRF) and Scanning Electron Microscopy (SEM) analyses were carried out to investigate the mineralogical composition of the five prepared samples (A-E) Hydrometallurgical purification was then employed to remove the identified impurities (Fe2O3, MnO, MgO, Na2O, CaO) from the five samples. 3. RESULTS AND DISCUSSION 3.1 XRF Elemental Analysis Studies The results of the X-ray fluorescence semi-quantitative analysis of the elements in the clay samples are as shown in Table 1. The results show that sample A contains the highest amount of silica by mass (63.35%), followed by sample E with 62.29% and with sample D having the least (54.67%). Sample B contains the highest amount of Alumina (25.73%) followed by sample C with 25.03% while sample A contains the least with 22.42%. sample D indicated the highest amount of Ferric oxide (10.408%), closely followed by sample B with 9.226% while sample A contains the least with just 6.109%. sample B has the greatest quantity of 2.94% by mass of potassium oxide, followed by sample A with 2.878% while sample C contains the least amount of just 1.259%. So also, sample D contains the hi ghest amount of ma gnesium ox ide (2.401%) followed by sample A with 1.351% while sample C contains the least with just 0.91%. As clearly indicated in Table 1, all other minerals present are in such negligible proportion that their presence would not constitute threats to the performances of the clay as refractory materials. Table 1: XRF s e mi -quantitative analysis of the elements 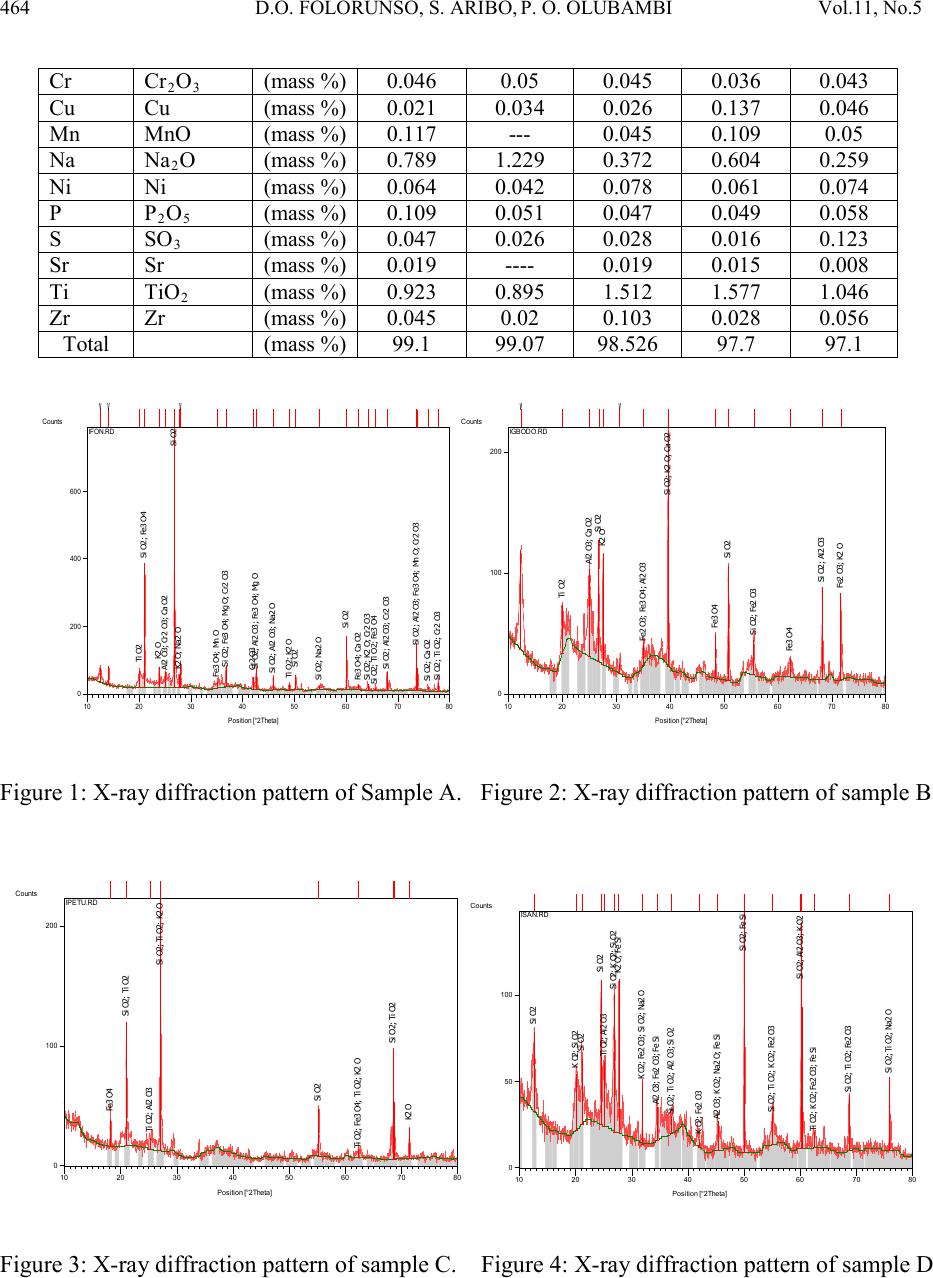 464 D.O. FOLORUNSO, S. ARIBO, P. O. OLUBAMBI Vol.11, No.5 Position [ ° 2Theta] 10 20 3040 5060 7080 C o unt s 0 200 400 600 Ti O2Si O2; Fe3 O4 K2 O Al2 O3; C r2 O3; C a O 2Si O2 K2 O ; Na 2 O Fe3 O4; M n O Si O2; Fe3 O4; Mg O; Cr2 O3 Cr2 O3 Si O2; Al2 O3; Fe 3 O 4; Mg O Si O2; Al2 O3; Na2 O Ti O2; K2 O Si O2 Si O2; Na2 O Si O2 Fe3 O4; Ca O2 Si O2; K2 O; Cr2 O3 Si O2; Ti O2; Fe3 O4 Si O2; Al2 O3; Cr2 O 3 Si O2; Al2 O3; Fe 3 O 4; Mn O ; Cr2 O 3 Si O2; Ca O2 Si O2; Ti O2; Cr2 O3 IFON.RD Position [ ° 2Theta] 10 20 3040 5060 7080 C o unt s 0 100 200 Ti O2 Al2 O3; Ca O2 Si O2 K2 O Fe2 O 3; Fe3 O 4; A l2 O3 Si O2; K2 O; Ca O2 Fe3 O4 Si O2 Si O2; Fe2 O3 Fe3 O4 Si O2; Al2 O3 Fe2 O 3; K2 O IGBODO.RD Figure 1: X-ray diffraction pattern of Sample A. Figure 2: X-ray diffraction pattern of sample B Position [ ° 2Theta] 10 20 30 40 50 60 70 80 C o unt s 0 100 200 Fe3 O4 Si O2; Ti O2 Ti O2; Al2 O3Si O2; Ti O2; K2 O Si O2 Ti O2; Fe 3 O 4; Ti O2; K2 O Si O2; Ti O2 K2 O IPETU.RD Position [ ° 2Th eta] 10 20 30 40 5060 70 80 C o unt s 0 50 100 Si O2 K O2; Si O2 Si O2 Si O2 Ti O2; Al2 O3Si O2; K O2; Si O2 K2 O; Fe Si K O 2; Fe 2 O 3; Si O2; Na2 O Al2 O3; Fe 2 O 3; Fe Si Si O2; Ti O2; Al2 O3; Si O2 K O2; Fe2 O3 Al2 O3; K O 2; Na2 O ; Fe Si Si O2; Fe Si Si O2; Ti O2; K O2; Fe2 O3 Si O2; Al2 O3; K O2 Ti O2; K O2; Fe 2 O 3; Fe Si Si O2; Ti O2; Fe2 O3 Si O2; Ti O2; Na2 O ISAN.RD Figure 3: X-ray diffraction pattern of sample C. Figure 4: X-ray diffraction pattern of sample D 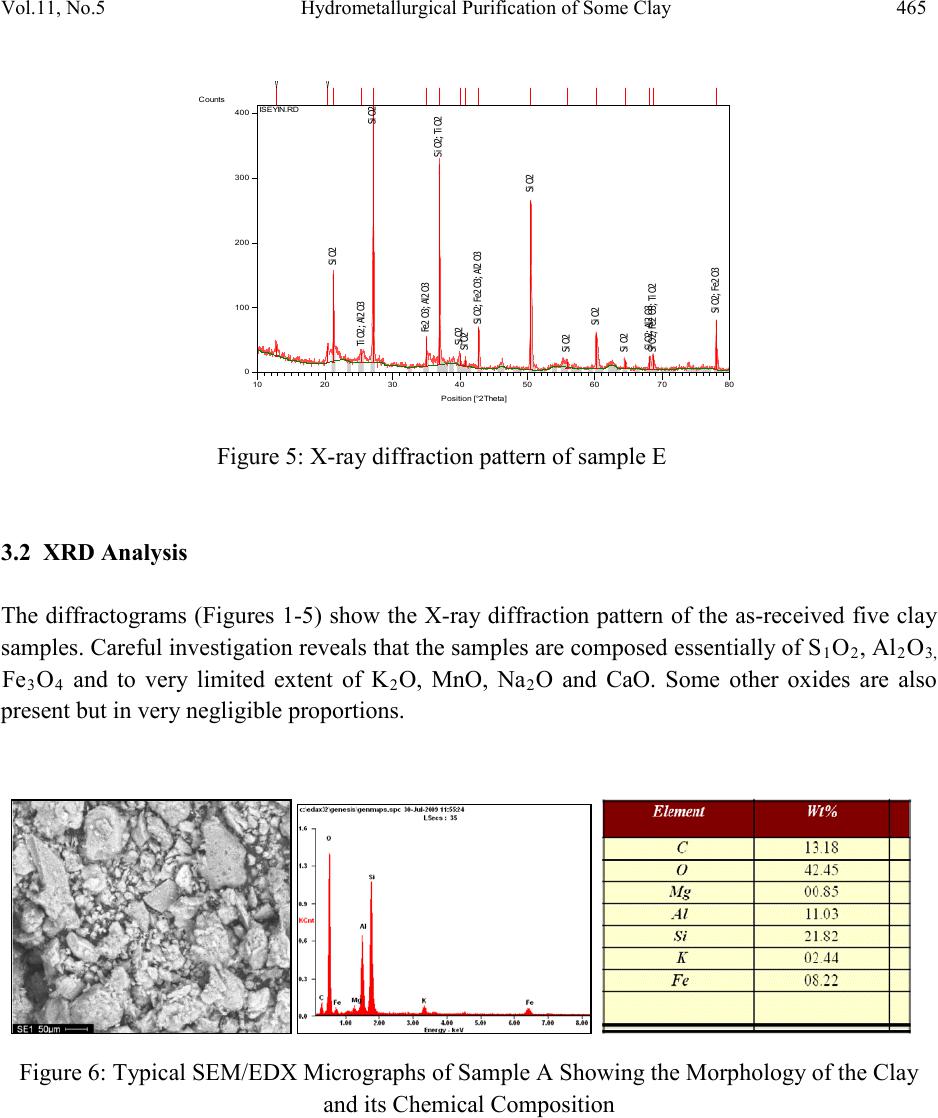 Vol.11, No .5 Hydrometallurgical Purification of Some Clay 465 Position [ ° 2Theta] 10 20 30 40 5060 70 80 C o unt s 0 100 200 300 400 Si O2 Ti O2; Al2 O3Si O2 Fe 2 O 3; Al2 O3Si O2; Ti O2 Si O2 Si O2Si O2; Fe2 O3; A l2 O3 Si O2 Si O2 Si O2 Si O2 Si O2; Al2 O3 Si O2; Fe2 O3; Ti O2 Si O2; Fe2 O3 ISEYIN.RD Figure 5: X-ray diffraction pattern of sample E 3.2 XRD Analysis The diffractograms (Figures 1-5) show the X-ray diffraction pattern of the as-received five clay samples. Careful investigation reveals that the samples are composed es sen t i ally of S1O2, Al2O3, Fe3O4 and to very limited extent of K2O, MnO, Na2O and CaO. Some other oxides are also present but in very negligible proportions. Figure 6: Typical SEM/EDX Micrographs of Sample A Showing the Morphology of the Clay and its Chemical Composition 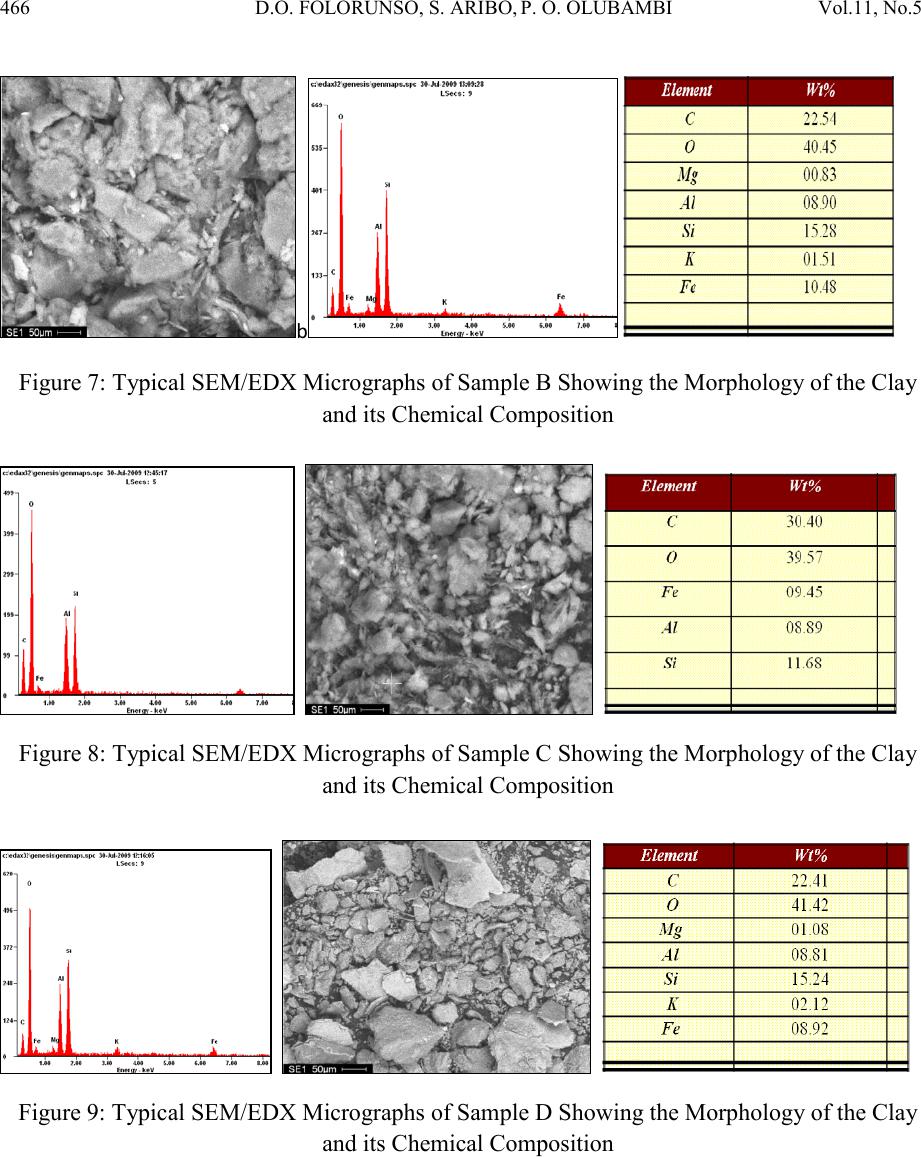 466 D.O. FOLORUNSO, S. ARIBO, P. O. OLUBAMBI Vol.11, No.5 b Figure 7: Typical SEM/EDX Micrographs of Sample B Showing the Morphology of the Clay and its Chemical Composition Figure 8: Typical SEM/EDX Micrographs of Sample C Showing the Morphology of the Clay and its Chemical Composition Figure 9: Typical SEM/EDX Micrographs of Sample D Showing the Morphology of the Clay and its Chemical Composition 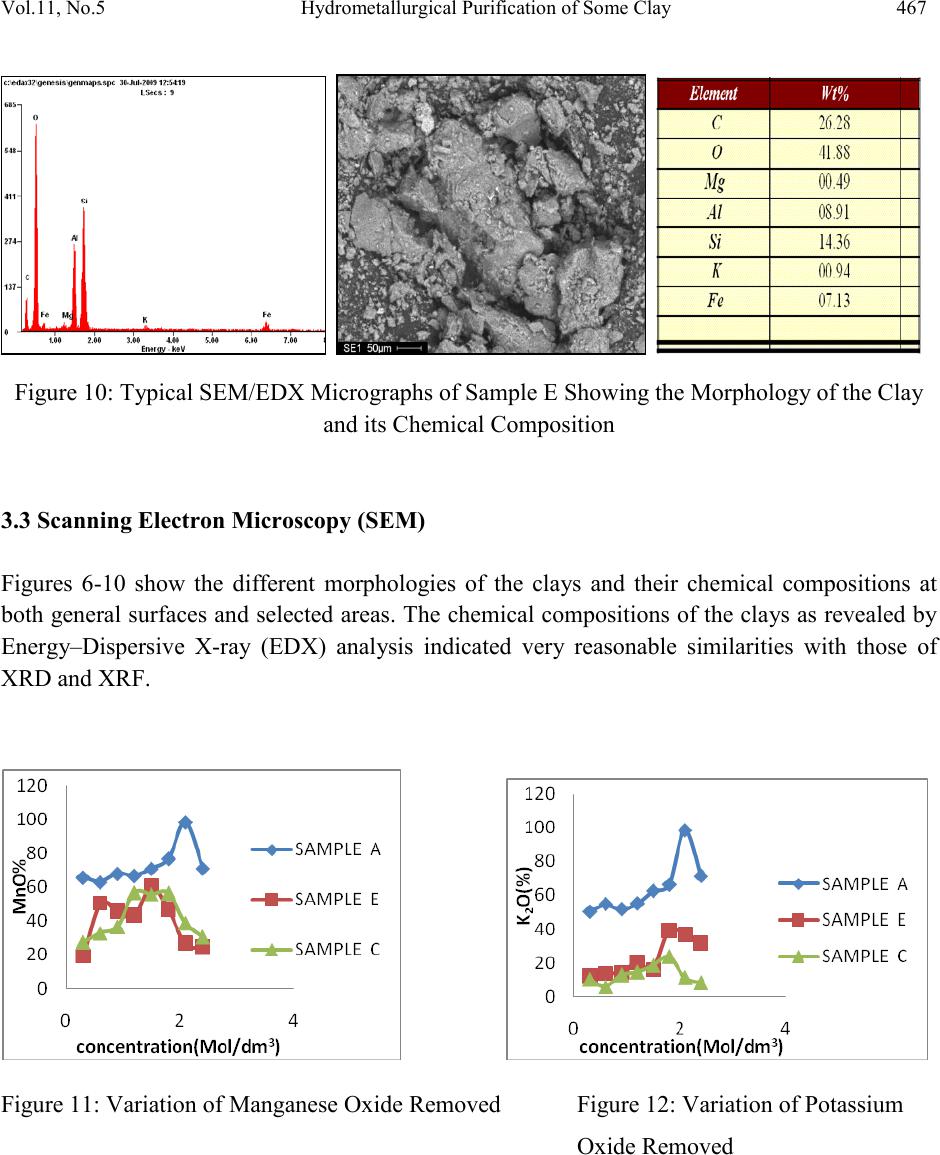 Vol.11, No .5 Hydrometallurgical Purification of Some Clay 467 Figure 10: Typical SEM/EDX Micrographs of Sample E Showing the Morphology of the Clay and its Chemical Composition 3.3 Scanning Electron Microscopy (SEM) Figures 6-10 show the different morphologies of the clays and their chemical compositions at both general surfaces an d selected areas. The chemical compositions of the clays as revealed by Energy–Dispersive X-ray (EDX) analysis indicated very reasonable similarities with those of XRD and XRF. Figure 11: Variation of Manganese Oxide Removed Figure 12: Variation of Potassium Oxide Removed 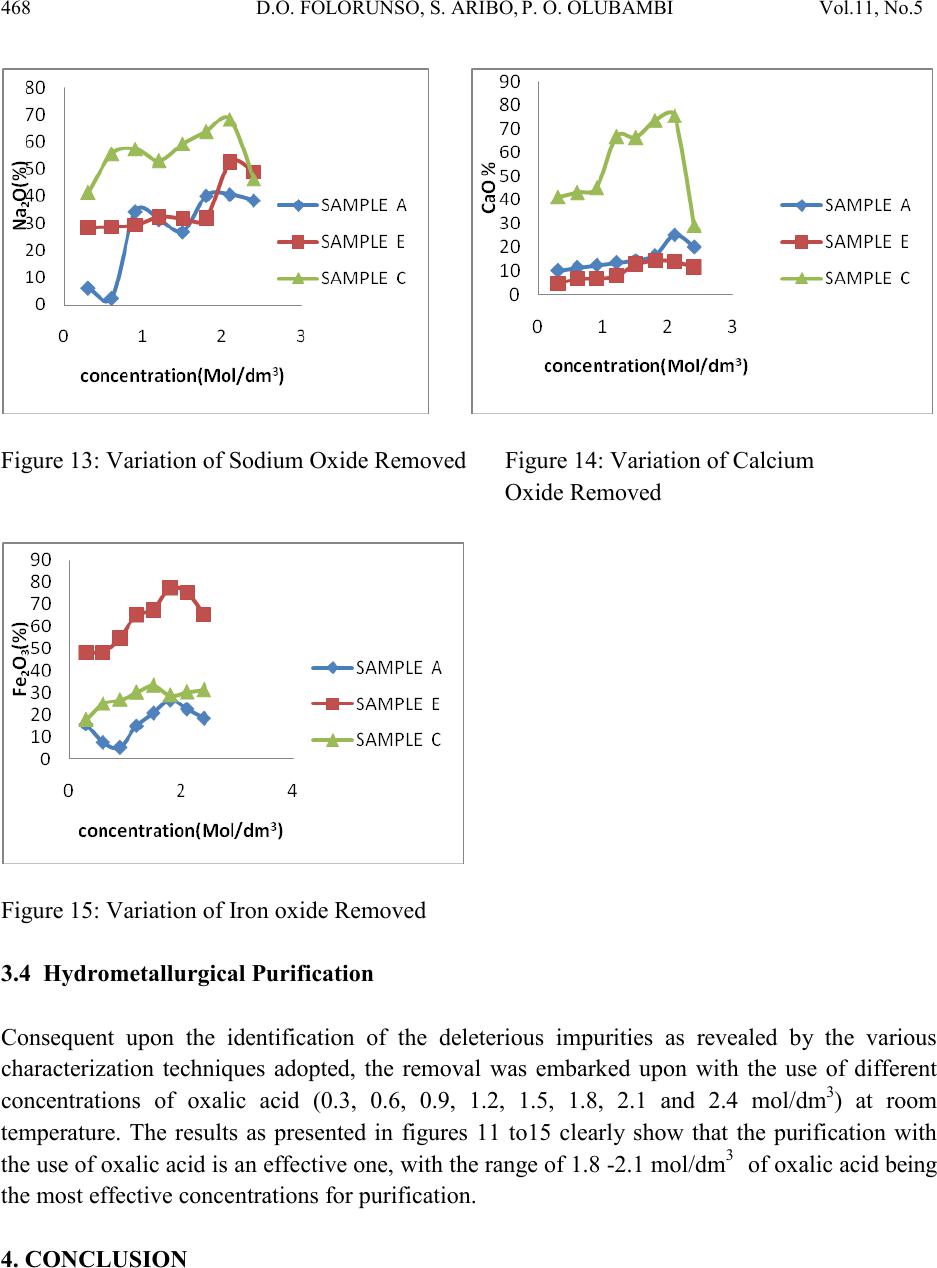 468 D.O. FOLORUNSO, S. ARIBO, P. O. OLUBAMBI Vol.11, No.5 Figure 13: Variation of Sodium Oxide Removed Figure 14: Variation of Calcium Oxide Removed Figure 15: Variation of Iron oxide Removed 3.4 Hydrometallurgical Purification Consequent upon the identification of the deleterious impurities as revealed by the various characterization techniques adopted, the removal was embarked upon with the use of different concentrations of oxalic acid (0.3, 0.6, 0.9, 1.2, 1.5, 1.8, 2.1 and 2.4 mol/dm3) at room temperature. The results as presented in figures 11 to15 clearly show that the purification with the use of oxalic acid is an effective one, with the range of 1.8 -2.1 mol/dm3 of oxali c acid being the most effective concentrations for purification. 4. CONCLUSION  Vol.11, No .5 Hydrometallurgical Purification of Some Clay 469 The combination of the three characterization techniques adopted in this study (XRD, XRF & SEM/EDX) showed consistency in the revelation of the quantities of the desired elements (Al203 and Si02) and the deleterious impurities (Fe203, K20, Mg0, Na2O, CaO and MnO) contained in the samples. The application of oxalic acid in the purification process also showed a very reasonable degree of effectiveness, with the range 1.8-2.1 mol/dm3 of the acid being the most effective concentrations for purification. The clays after purification have the potential for application in the production of acidic refractories. ACKNOWLEDGEME NT The authors wish to express their p rofound gratitude to the following bodies for their support for the research work; • Federal University of Technology, Akure (F.U.T.A.) • Africa Materials Science and Engineering Network (AMSEN) • Regional Initiatives in Science and Education(RISE) • Science Init i ati ve Gro up (SIG) REFERENCES [1] Hassan, S.B.; “Effect of silicon carbide on some refractory properties of Kankara clay” (2001), Proceedings of Nigerian Metallurgical Society. [2] Azom, T.; “Refractories- An overview” (2008), Journal of materials online at www.azom.com [3] Dogan, C.P., Kwong, K.S., Bennet, J.P.; “Improved refractory materials for Slagging coal Gasifiers,“ (2002), in proceedings from the 27th International conference on coal utilization and fuel systems, Clearwater, Florida. [4] Ahmed, K.S.; “Development of Phosphate Bonded Fireclay Refractory Castable”,(1986) . Unpublished M.Sc (Chem.) Thesis, Chemical Engineerin g Dept., Ahmadu Bello Universit y, Zaria, Nigeria. [5] Hassan, S.B.;“Refractory Properties of Bauchi and Onibode Clays of Nigeria for Furnace Linning”(2000), African Journal of Science and Technology, Vol.1. No. 1, Pp. 33-41. [6] Bakker, W.T.; “Refractories for present and future electric power plants”(1993), Key Engineering Materials, Trans Tech Publications, Vol. 88, Pp. 41-70. [7] Ndaliman, M.B.; ”Effect of certain additives on refractory properties of termite hill for furnace lining”(2000), Proc.2nd Ann. Engin. Conf. Federal University of Technology, Minna, Nigeria. 4-6 Sept. 2000, Pp.139-144. [8] Borode, J.O., Onyemaobi, O.O. and Omotoyinbo, J.A.;Suitability of some Nigerian Clays as refractory raw materials(2000). Nigerian Journal of Engineering Management. Vol.3. Pp. 14-18.
|