 Journal of Minerals & Materials Characterization & Engineering, Vol. 11, No.4, pp.427-435, 2012 jmmce.org Printed in the USA. All rights reserved 427 Spectroscop ic Analysis of Expired and Pure Melmet R.Kayalvizhia*, M.Sri Vidhya Rubinia, A.Christy Ferdinanda, G.Meenakshib aDepartment of Physics, St.Joseph’s College of Arts & Science, Cuddalore. bDepartment of Physics, K.M.C.P.G.S, Pondicherry. *Corresponding Author: ramvizhi@yahoo.co.in ABSTRACT Spectroscopy is a perfect analyzer to find the elements of all matter. The application of spectroscopy has been used to interpret the effect of antibiotics and other medicines after its life duration. This has been studied by using the diabetics tablet melmet, expired for an year, to visualize the changes physically and chemically [1-3]. It has been observed that the occurrence of changes in color and r eduction of smell and als o the formation of new peaks and shift b y XRD and UV, FTIR characterization respectively [4]. Keywords: UV – Ultraviolet–Visible Spectroscopy , FTIR - Fourier Transform Infrared Spectroscopy, XRD – X-ray diffraction, DSC - Differential Scanning Calorimetry 1. INTRODUCTION In this new era medicine plays a vital role to human beings else for all living beings by healing diseases. In that Melmet (metformin hydrochloride) plays a vital role in treatment of diabetics and poly cystic ovary diseases. Melmet contain metformin which is used for glucose tolerance and the chances increase of fertility. It helps to control blood sugar level. PCOD (Polycystic Ovar y Diseas e) occur due to imbalance or abnormality in the hormones which can be control by Melmet [5]. 2. EXPERIMENTAL Many people especially who are living in remote areas consuming the medicine even after the expi r y date due t o t he lack of k no wledge in t he medical fi eld . H en ce, i t i s es sen ti al t o ed ucate th e 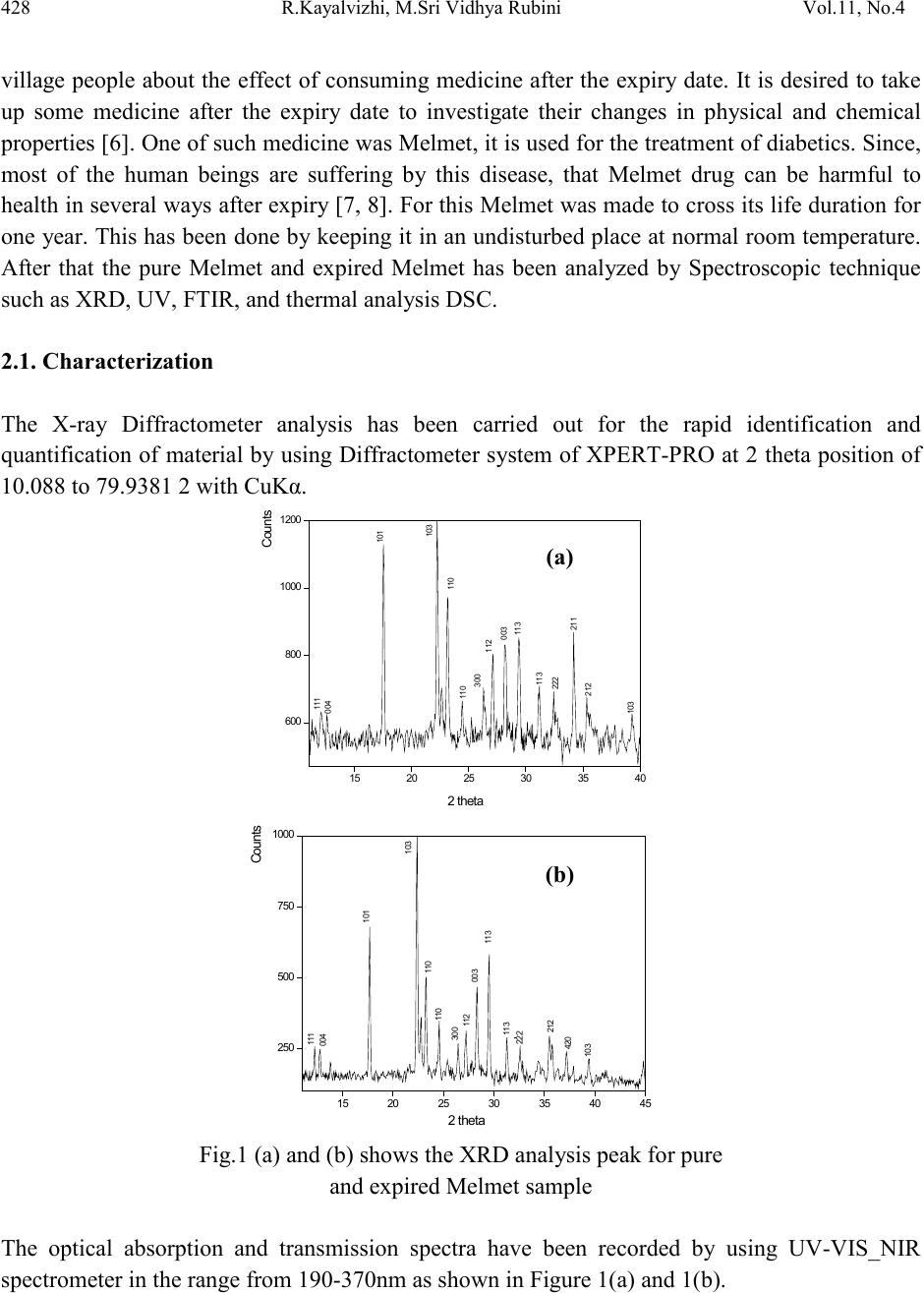 428 R.Kayalvizhi , M.S ri Vidhya Rubini Vol.11, No.4 village people about the effect of consuming medicine after the expiry date. It is desired to take up some medicine after the expiry date to investigate their changes in physical and chemical properties [6]. One of s uch m edici ne was Melmet , it is used for t he tr eatm ent o f diabet ics. Since, most of the human beings are suffering by this disease, that Melmet drug can be harmful to health i n several ways aft er expir y [7, 8]. For this Melmet was made to cross its life duration for one year. This has been done b y keeping it in an undisturbed p lace at nor mal room tem perature. After that the pure Melmet and expired Melmet has been analyzed by Spectroscopic technique such as XRD, UV, FTIR, and thermal analysis DSC. 2.1. Characterization The X-ray Diffractometer analysis has been carried out for the rapid identification and quantification of material by using Diffractometer system of XPERT-PRO at 2 theta position of 10.088 to 79.9381 2 with CuKα. 15 20 25 30 35 40 600 800 1000 1200 212 211 103 222 113 112 003 113 300 110 110 103 004 111 101 2 theta Counts 15 20 25 30 35 40 45 250 500 750 1000 103 103 420 222 212 113 113 003 112 300 110 110 101 004 111 Counts 2 theta Fig.1 (a) and (b) shows the XRD analysis peak for pure and expired Melmet sample The optical absorption and transmission spectra have been recorded by using UV-VIS_NIR spectrometer in the range from 190-370nm as shown in Figure 1(a) and 1(b). 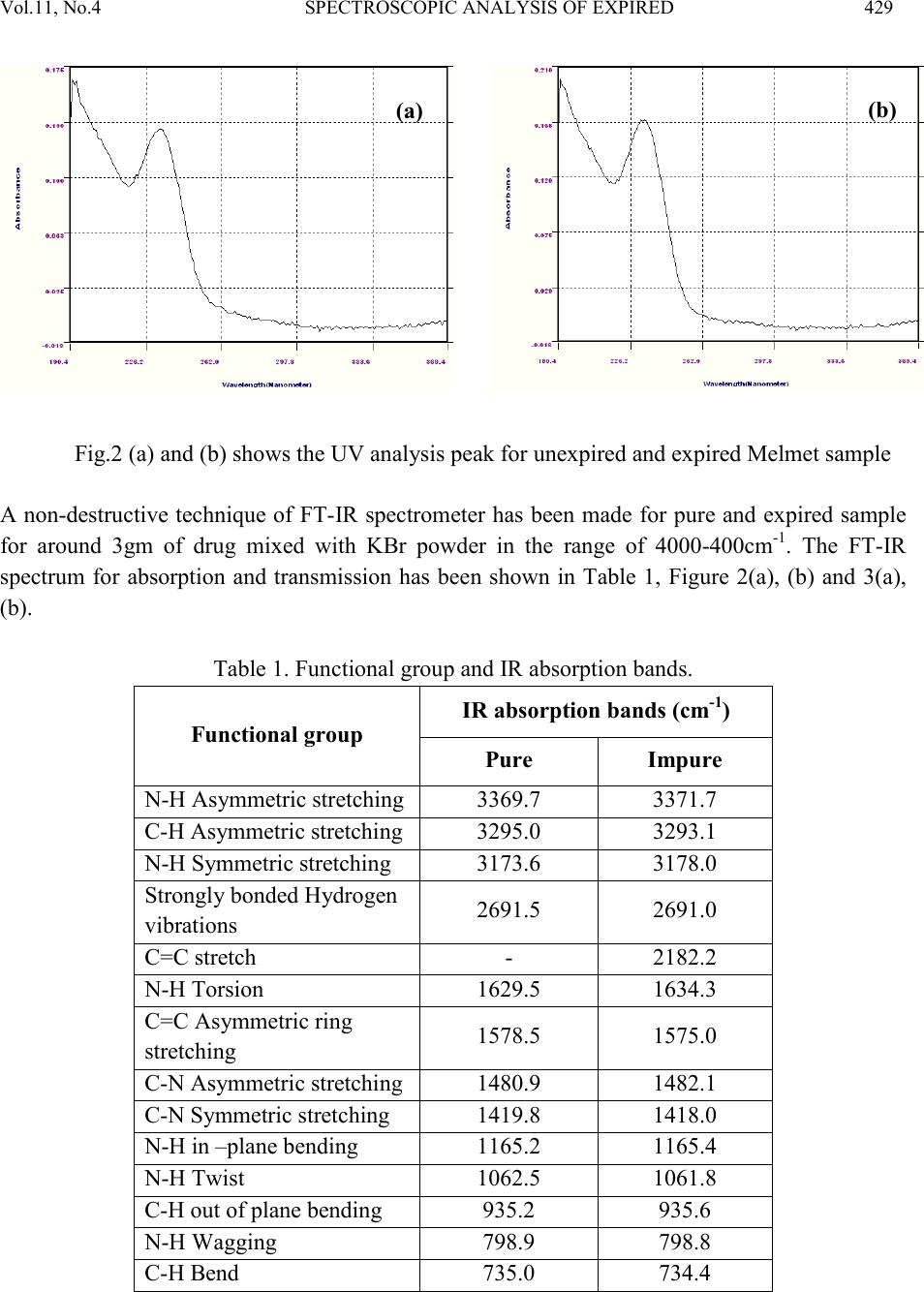 Vol.11, No .4 SPECTROSCOPIC ANALYSIS OF EXPIRED 429 Fig.2 (a) and (b) shows the UV analysis peak for unexpired and expired Melmet sample A non-destructive technique of FT-IR spectrometer has been made for pure and expired sample for around 3gm of drug mixed with KBr powder in the range of 4000-400cm-1. The FT-IR spectrum for absorption and transmission has been shown in Table 1, Figure 2(a), (b) and 3(a), (b). Table 1. Functional group and IR absorption bands. Functional group IR absorption bands (cm-1) Pur e Impure N-H Asymmetric stretching C-H Asymmetric stretching vibrations 2691.5 2691.0 stretching 1578.5 1575.0 C-N Asymmetric stretching 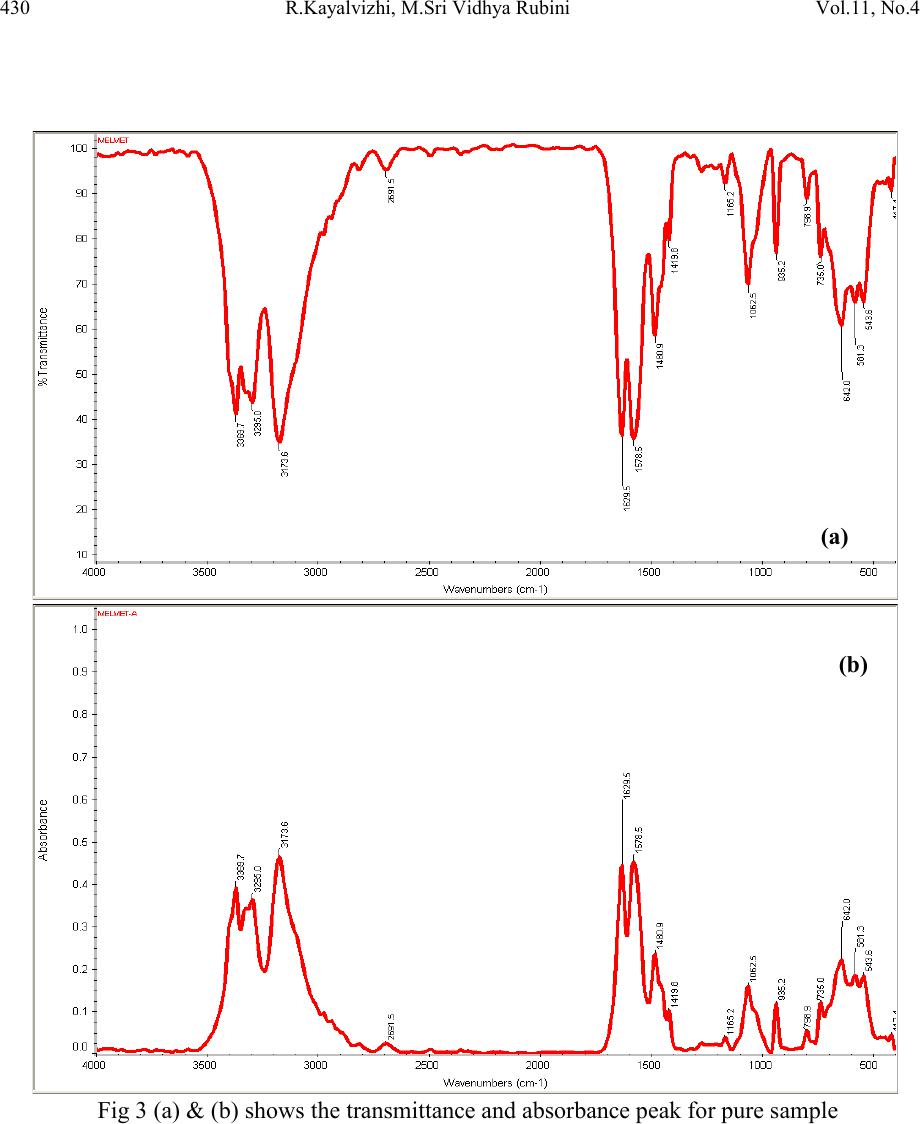 430 R.Kayalvizhi , M.S ri Vidhya Rubini Vol.11, No.4 Fig 3 (a) & (b) shows the transmittance and absorbance peak for pure sample 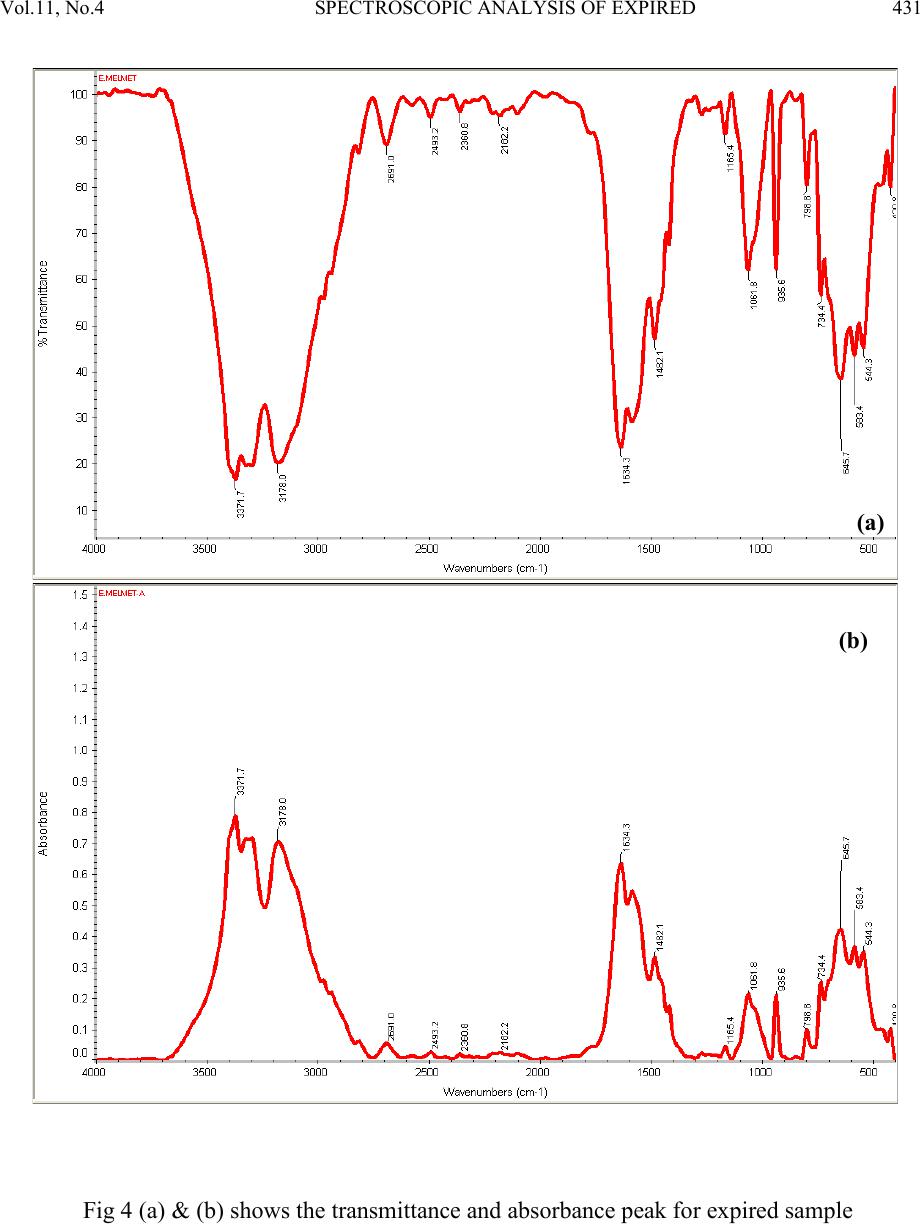 Vol.11, No .4 SPECTROSCOPIC ANALYSIS OF EXPIRED 431 Fig 4 (a) & (b) shows the transmittance and absorbance peak for expired sample 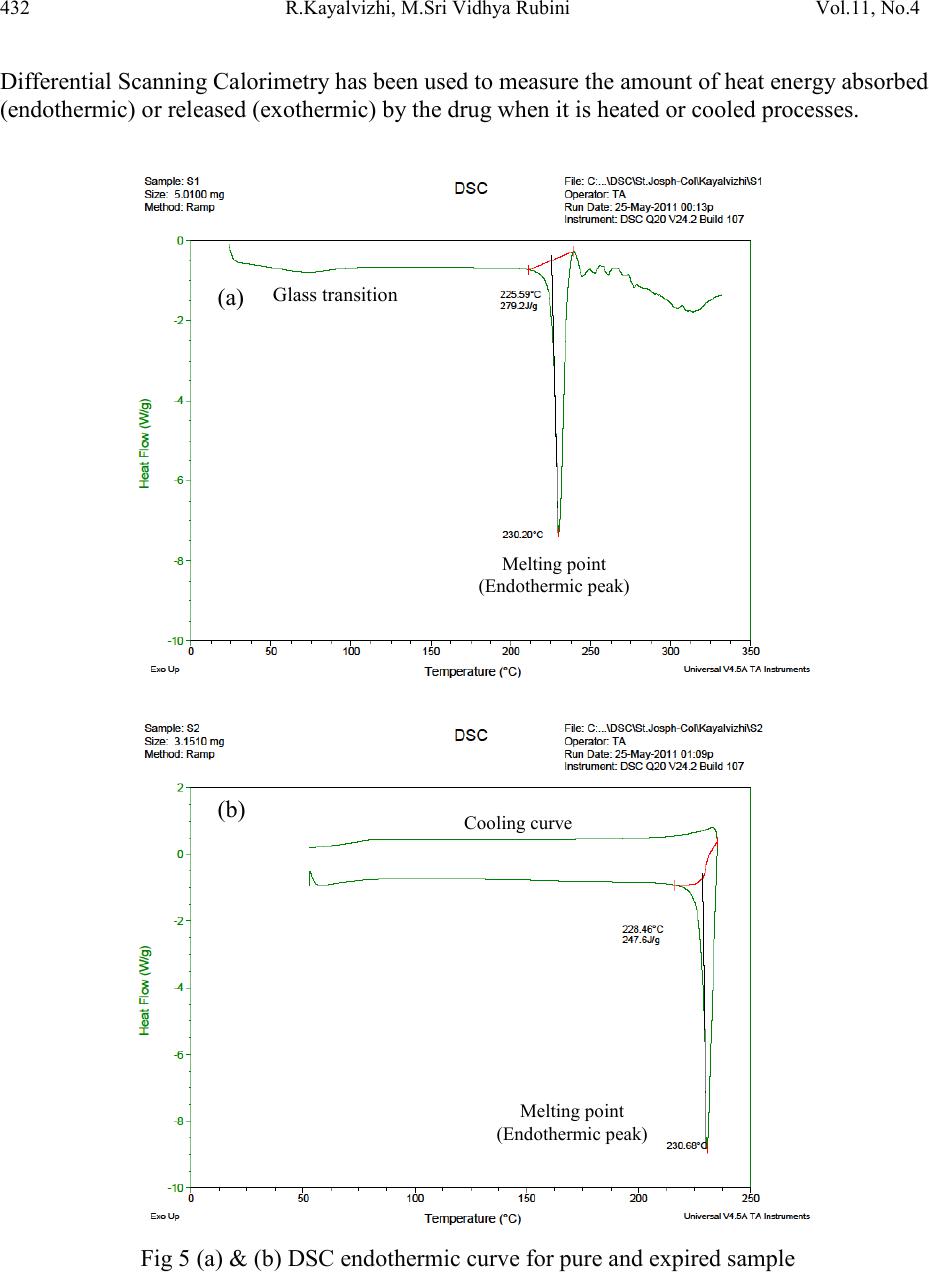 432 R.Kayalvizhi , M.S ri Vidhya Rubini Vol.11, No.4 Differential Scanning Calorimetry has been used to measure the amount of h eat energy absorbed (endothermic) or released (exother mic) by the drug when it is heated or cooled processes. Fig 5 (a) & (b) DSC endothermic curve for pure and expired sample (Endothermic peak) (Endothermic peak)  Vol.11, No .4 SPECTROSCOPIC ANALYSIS OF EXPIRED 433 3. RESULT Expired drugs can be harmful to health in several ways; they can be unpredictable in effectiveness, simply ineffective, or even toxic. According to the pharmaceutical industry, there are certain medications, such as nitroglycerine (used by heart patients for chest pain), insulin (used by diabetics to control blood sugar), and liquid medications such as antibiotics that degrade more quickly than other medications. This means that some of the effectiveness of the medication is lost, if it is used after the expiry date. In some cases risk with heart disease, diabetes, or serious infection. And while much of the original potency remains in other medications if used beyond their expiry date, their effectiveness depends in large part upon how the medication is stored. Factors shorten the lifespan of drug are moisture, increased temperature, manufacturing impurities, and, for some drugs, light and so on. Here the expired sample and unexpired sample were characterized by using XRD and UV to clarify why they are harmful after crossing the expiry date. The excitation of X-ra y in the life time crossed melmet (Fig 1a & 1b) has been found that in an expired sample some elements were newly raised. While comparing unexpired sample with expired drug the 2 theta values were incr eased slightl y and correspondin gly th e d-spacing range also decr eased in fr action. This is due to the incorporation of some other m aterial in the original sample. The formation of new 211 planes has been seen in expired drug. The pyramidal plane 113 where dominated 112 pyramidal plane and 003 basal more in expired drug. i.e 112 and 003 planes have been compressed and 110 planes too. The decrease d-spacing values resulting that the drug molecule getting compress. And so it is not easy to get it diluted soon. Because of this condition it is contaminate in the kidney and developing a serious risk such as heart disease, diabetes, or serious infection. From UV absorption pattern (Fig 2a & 2b) it is found that in an unexpired sample the two absorption peaks have been seen. First peak is around at 191nm with an absorbance range of 0.166 and the second peak is around 235nm with an absorbance range of 0.133. Whereas in an expired sample the absorbance range is slightly shifted above to 0.200 and 0.166 absorbance range respectively. The complexity of infrared spectra in 1450 to 600 cm-1 (Fig 4 a & 4 b) region makes it difficult to assign all the absorption bands, and because of the unique patterns found there, it is oft en called the fingerprint region. Absorption bands in the 4000 to 1450 cm-1 region are usually due to stretching vibrations of diatomic units, and this is sometimes called the group frequency region. A symmetric is a fashion at a wave number of 3173.6 cm-1, 3178.0cm-1, 1419.8cm-1, 1418.0cm-1, in which both bonds lengthen and contract together (in-phase), and the other in an asymmetric  434 R.Kayalvizhi , M.S ri Vidhya Rubini Vol.11, No.4 fashion, in which one bond shortens while the other lengthens. The asymmetric stretch at a wave number of 3369.7cm-1, 3371.7cm-1, 3295.0 cm-1, 3293.1 cm-1, 1480.9 cm-1, 1482.1 cm-1 is infrared active because there is a change in the molecular dipole moment during this vibration. (To be "active" means that absorption of a photon to excite the vibration is allowed by the rules of quantum mechanics.) [9]. Bond stretching 2182.2 cm-1 and bond bending 735.0 cm-1, 734.4 cm-1, more complicated molecules vibrate in rocking and twisting modes, which arise from combinations of bond bending in adjacent portions of a molecule. Torsions at 1629.5 cm-1, 1634.3 cm-1 involve changes in dihedral angles. This type of mode is analogous to twisting the lid off the top of a jar. No bonds are stretched, and no bond angles change, but the spatial relationship between the atoms attached to each of two adjacent atoms will ch ange. [ 10] Stretching frequencies are higher than corresponding bending frequencies. (It is easier to bend a bond than to stretch or compress it.)At a wavenumber of 2691.5 cm-1, 2691.0 cm-1, a strong bond to hydrogen have higher stretching frequencies than those to heavier atoms. Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds. In a rocking, wagging or twisting coordinate the bond lengths within the groups involved do not change which is at 798.9 cm-1, 798.8 cm-1. The angles do. Rocking is distinguished from wagging by the fact that the atoms in the group stay in the same plane. [11] Fig (5a & 5b) shows that the thermal analysis of pure and expired medicine. Pure melmet has been started to characterize from 10°C to 500°C for an amount of 5mg in Ramp method. The endothermic peak has been start at 225.59°C after the Glass Transition and exists its melting point at 230.20°C. After endothermic peak its starts to dispose in the form of brown colour. The same endothermic peak has been found in expired melmet but at high heat flow level. Cooling curve were tried to find its exothermic process but there is no result since it’s not a crystalline material. 4. CONCLUSION The experimental data indicates that the medicine which crossed its life time develops some new element which causes the chan ges in its ph ysical and chemical condition. Using Xrd it’s clearly found that the incorporation of elements in the form of new peak and shift in absorption peak explains its molecular contraction after expiry. And the small changes in its Stretching condition using FTIR and the shift in Heat Flow during Endothermic process explain the changes in its molecular bonding strength.  Vol.11, No .4 SPECTROSCOPIC ANALYSIS OF EXPIRED 435 REFERENCES [1] Hahnemann S., 1833, “The Organon of the Healing Art” Ed 5, aphorisms 5 and 217, ISBN 0879832282. [2] Lord JM, Flight IHK, Norman RJ. “Metformin in polycystic ovary syndrome: systematic review and meta-analysis.” BMJ. 2003;327(7421):951– 3. doi:10.1136/bmj.327.7421.951. PMID 14576245. PMC 259161. [3] Mishra. TK., 2005, Journal, Indian Academy of Clinical Medicine Volume 6, No. 4 . [4] Lee J, Thompson E., 2007, "X-ray drug picture", The Homeopath (Northampton: The Society of Homeopaths) 26 (2): 43–48, ISSN 0263-3256. [5] Ching-Ling Cheng, 2004, European Journal of Pharmaceutical Sciences, V o lume 22, Issue 4, pp297-304 [6] Paul Nicklin, 1996, International Journal of Pharmaceutics, Volume 128, Issues 1-2, 29, pp 155-162 [7] Arma gan Onal, 2009, European Journal of Medicinal Chemistry, Volume 44, Issue 12, pp 4998-5005 [8] I.I. Hamdan, 2010, Journal of Pharmaceutical and Biomedical Analysis, Volume 53, Issue 5, 15, Pages 1254-1257 [9] Schwartz A. T., et al., 1994, Chemistry in context DC, American chemical society, Washington [10] Bratman, MD, Steven (1997). The Alternative Medicine Sourcebook. Lowell House. p. 7. ISBN 978-1-56565-626-0. [11] G. Derosa, 2011, Di abetes R esearch and Clini cal Pract ice, Volum e 91, Issue 3, Pages 265- 270.
|