 Journal of Minerals & Materials Characterization & Engineering, Vol. 11, No.1, pp.21-30, 2012 jmmce.org Printed in the USA. All rights reserved 21 Development of an Environmentally Friendly in-situ Pack-Cyaniding Technique 1,2*Akinluwade, K. J., 1,2Adetunji, A. R., 2Adeoye, M. O., 2Umoru, L. E., 3Kalu, P. N., 1,2Taiwo, A. T. and 1Adewoye, O. O. 1National Agency for Science and Engineering Infrastructure (NASENI), Abuja, Nigeria 2Department of Materials Science & Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria 3Department of Mechanical Engineering, Florida State University, Florida, USA *Corresponding Author: email: jakinluwade@yahoo.com; Phone: +2348080280533 ABSTRACT A safe and environmentally friendly cyaniding method has been developed to mitigate the toxic impacts of cyanide salts on the environment during conventional cya niding. The method entails in-situ diffusion of nascent cyanide from mature cassava leaves into the surface of mild steel components via pack-cyaniding. Both high-temperature in-situ diffusion into austenite and low-tempera ture in-situ diffusion into ferrite were explored. Results from light and scanning electron microscopic studies showed that surface hardness of the steel components was substantially increased. The waste product was a harmless biodegradable organic compound that posed no disposal threats. This study is important for increasing the wear resistance of ferrous parts for a longer service life in application without polluting the environment. Keywords: environmentally friendly, cassava leaves, scanning electron microscope, in-situ pack-cyaniding, biodegradable, clean technology 1. INTRODUCTION Virtually all developed and growing economies of the world are involved in production of consumer goods and services. The turnout of consumer goods is multifarious in nature— ranging from the simple everyday household utensils to the complex industrial machines. Certain parts of such machines and utensils require hardening to prevent wear. Case-  22 Akinluwade, K. J., Adetunji, A. R. Vol.11, No.1 hardening heat treatment processes are carried out either in gas chambers or in salt baths [1,2]. In small and medium scale enterprises, salt bath treatment is the commonest method probably because of its relatively low cost and reduced treatment time. Unfortunately, substantial amount of highly toxic, corrosive and environmentally unfriendly gases are liberated from the fused salt mixture into the atmosphere. The disposal of the spent salt mixture is also hazardous to both the flora and fauna of the environment. Since the mechanical properties of steels are sensitive to the content of carbon [3], manufacturers often find it cheaper to augment the level of hardening species (such as carbon and nitrogen) in mild steel components through diffusion. Liquid salt bath nitriding in cyanide-cyanate baths tends to release toxic greenhouse gases like CO, CO 2, HCN, HCl and so on into the atmosphere. Both the salt composition and by-products are very toxic [4]. Pack-c yanidin g of mil d steel usin g cassav a leaves and t he charact eriz ation of the cas e formed have been reported [5,6,7]. The presence of cyanogenic glucoside in cassava plant could be the accumulation of products of catabolism of amino acids [8] or a mechanism for deterring predators [9]. Cassava contains some amount of cyanide that is often removed as waste during processing. This study aims at converting this wanton cyanide waste to engineering value via a clean technology technique making it of benefit to human use thereby promoting the nation’s health and economy. 2. MATERIALS AND METHODS 2.1. Preparation of Cassava Powder Fresh cassava leaves of specie Manihot esculenta (bitter local variety) were collected, oven- dried, pulverized and subjected to sieve analysis to produce 1.00, 0.85, 0.60, 0.30 and 0.125 mm particle sizes. Each particle size was divided into two: A and B. Group A was mixed with BaCO3 salt by combining 4 volumes of cassava powder with 1 volume of BaCO3 salt and then divided into five portions. This was repeated for group B powder but using BaCl2 salt. In all, 50 portions of cassava leaves powder samples comprising 2 groups and 10 batches were prepared. 2.2 In-situ Pack-cyaniding  Vol.11, No.1 Development of an Environment 23 Cyaniding boats of 6 cm × 6 cm × 6 cm were constructed using mild steel plates. A firm 1fireclay luting was provided at the slits between each boat and its cover plate. Mild steel bolt and nut were completely embedded in each member of a batch of group A in a cyaniding boat and loaded into a muffle furnace at room temperature. The furnace was hea ted to 950 oC and held for 5 hours. All the samples were cooled in air. The process was repe ated for other four batches but at soaking times of 4, 3, 2 and 1 hour respectively. Group B samples were pack- cyanided in the same way but at 550 oC. 2.3 Metallography and Microscopy Each specimen was roughly ground across the cross-section on 60, 120, 180, 240, 320, 400 and 600 SiC grit papers. Rough polishing was carried out on 800 and 1200 grit papers while final polishing was accomplished with alumina pastes of 6, 1, and 0.6 µm, respectively. Case depths were measured using the Olympus BH-2 Advanced Optical Microscope with a Daheng Imavision HV Camera and a calibrated eyepiece. A Cam Scan Series 2 scanning electron mi cros cope was used t o r eveal th e mi cros tru ctu ral det ail s wh il e s urface h a rdnes s w as measured using LECO ASTM E384 Microhardness tester. 3. RESULTS AND DISCUSSION 3.1 High-Temperatu re Pac k -Cyaniding at 950 oC The starting material for this experiment was a mild steel bolt with carbon content less than 0.25 wt%. The micrograph of its cross section is as shown in Plate 1. Each paired micrographs of Plates 2—5 was obtained from the optical microscope, they show distinctly formed cases for samples treated at 950 oC. The micrograph of the starting material is displayed at Plate 1 and it shows relatively fine grains. Air-cooling method was used to obtain abruptness in change from high to low carbon in the microstructure making it convenient to employ light microscopy in case depth measurement. 1 Fireclay absorbs escaping fumes that would otherwise contaminate the atmosphere. 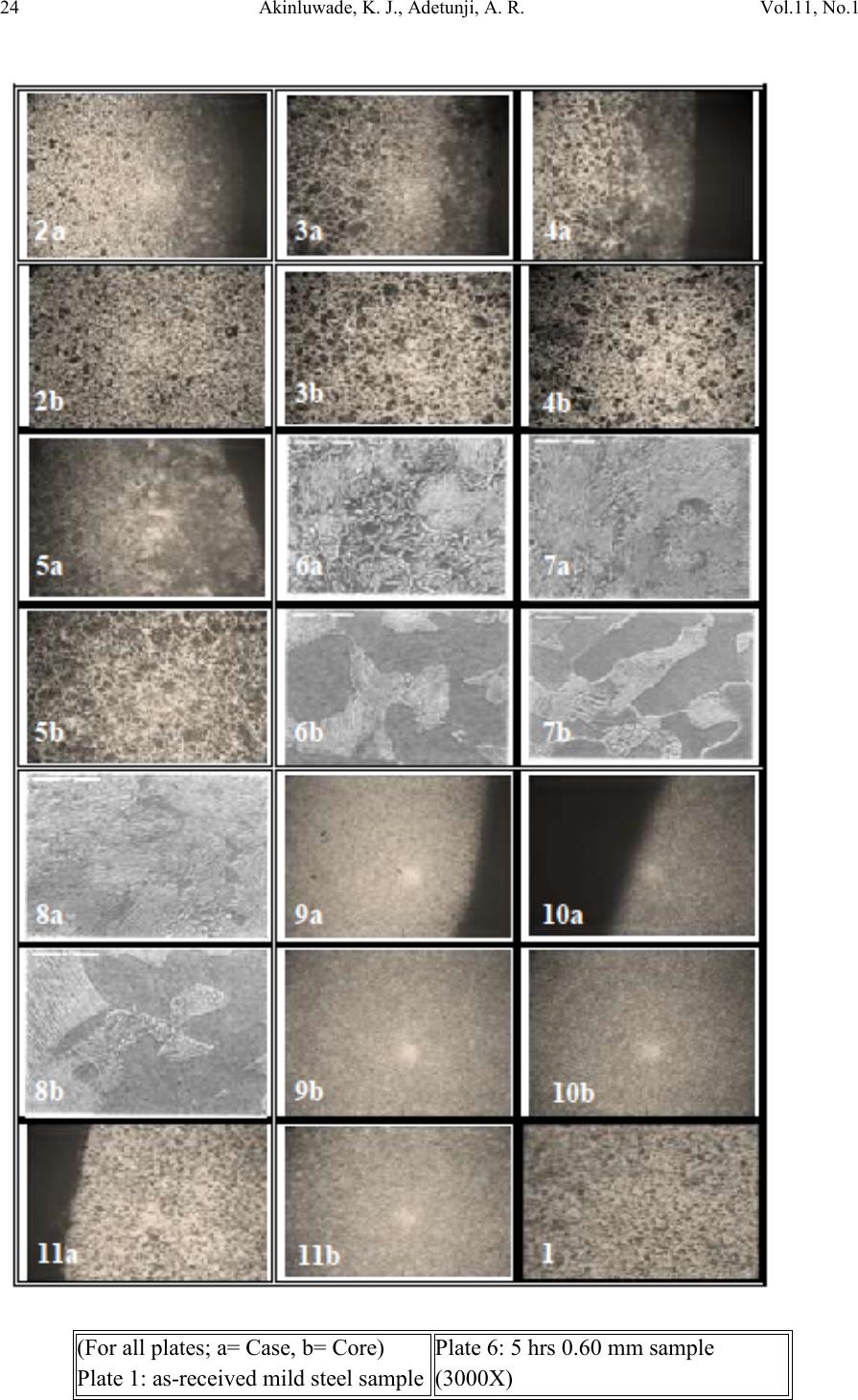 24 Akinluwade, K. J., Adetunji, A. R. Vol.11, No.1 (For all plates; a= Case, b= Core) Plate 1: as - received mild steel sample Plate 6: 5 hrs 0.60 mm sample (3000X)  Vol.11, No.1 Development of an Environment 25 3.1.1 Variation of case depth with cassava leaves particle size and soaking time Fig 1 shows the dependence of case depth on cassava leaves particle size for high temperat ure pack cyanidi ng. It w as gener ally obs erved th at case d epth in creases wi th part icle size at constant soaking temperature—this is the general trend displayed by the curves. The variation of case depth with soaking time is provided in Fig 2. The curves exhibit a gentle rise followed by a slight decline especially in 0.60 and 0.85 mm particle sizes and then a gentle rise beginning from 4 hrs. In general, case depth increases with soaking time. Fig 1: Case depth as a function of particle size for high temperature pack cyaniding 3.1.2 Variation of case hardness with cassava leaves particle size and soaking time The curves generally increase with particle size. The curves of Fig 3 have a slight gradient from 0.125 mm to 0.30 mm. Between 0.30 mm and 0.60 mm the curves are almost linear (100X) Plate 2: 0.60 mm particle size soak 3 hrs (100X) Plate 3: 1.0 mm particle size soak 3 hrs (100X) Plate 4: 0.85 mm particle size soak 1 hr (100X) Plate 5: 0.125 mm particle size soak 5 hrs (100X) Plate 7: 3 hrs 0.60 mm sample (3000X) Plate 8: 4 hrs 0.85 mm sample (3000X) Plate 9: 0.125 mm particle size soak 1 hr (100X) Plate 10: 0.60 mm particle size soak 4 hrs (100X) Plate 11: 0.30 mm particle size soak2 hrs (100X) 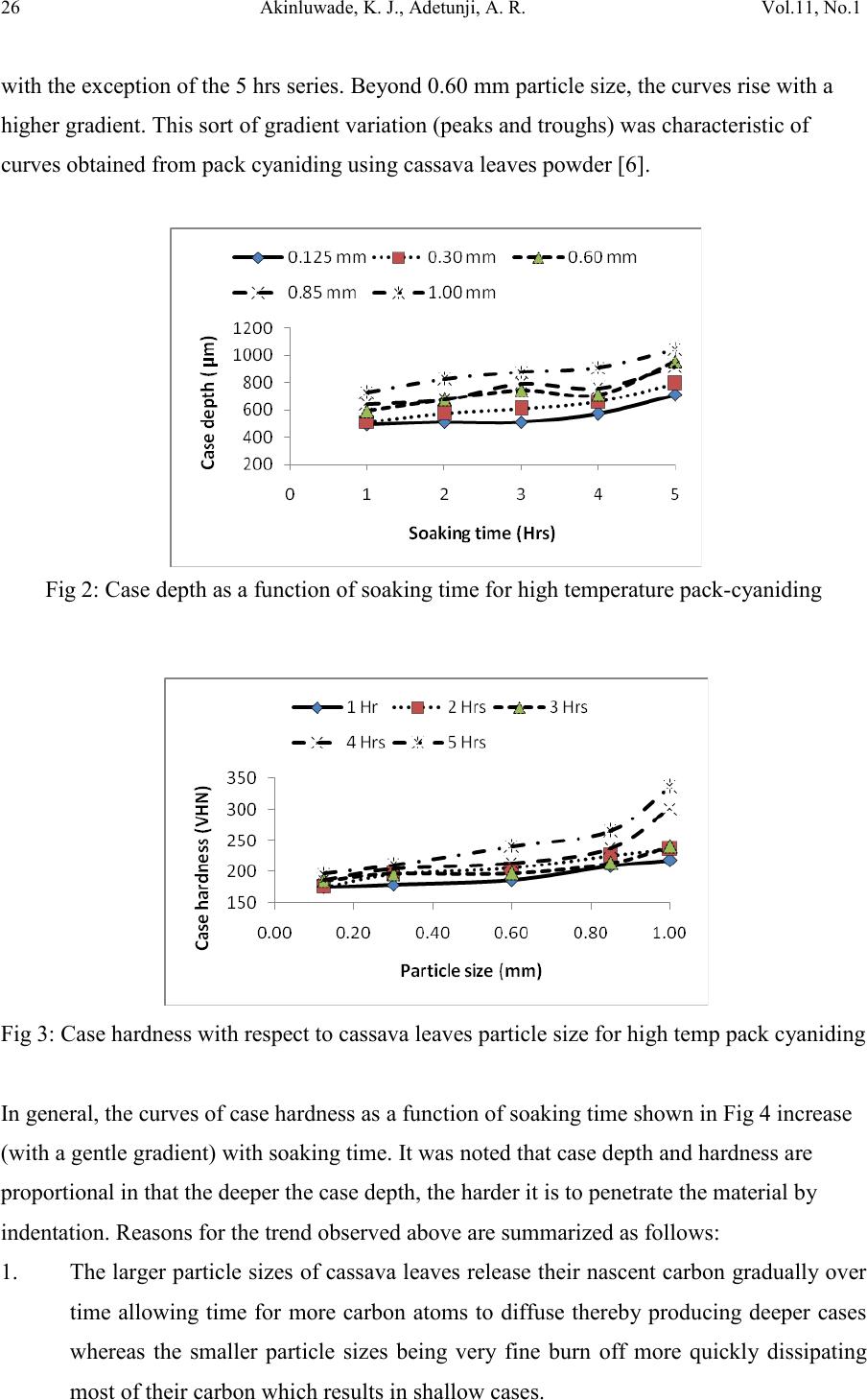 26 Akinluwade, K. J., Adetunji, A. R. Vol.11, No.1 with the exception of the 5 hrs series. Beyond 0.60 mm particle size, the curves rise with a higher gradient. This sort of gradient variation (peaks and troughs) was characteristic of curves obtained from pack cyaniding using cassava leaves powder [6]. Fig 2: Case depth as a function of soaking time for high temperature pack-cyaniding Fig 3: Case hardness with respect to cassava leaves particle size for high temp pack cyaniding In general, the curves of case hardness as a function of soaking time shown in Fig 4 increase (with a gentle gradient) with soaking time. It was noted that case depth and hardness are proportional in that the deeper the case depth, the harder it is to penetrate the material by indentation. Reasons for the trend observed above are summarized as follows: 1. The lar ger particl e sizes o f cassava l eaves releas e their nas cent carbon gradual ly over time allowing time for more carbon atoms to diffuse thereby producing deeper cases whereas the smaller particle sizes being very fine burn off more quickly dissipating most of their carbon which results in shallow cases. 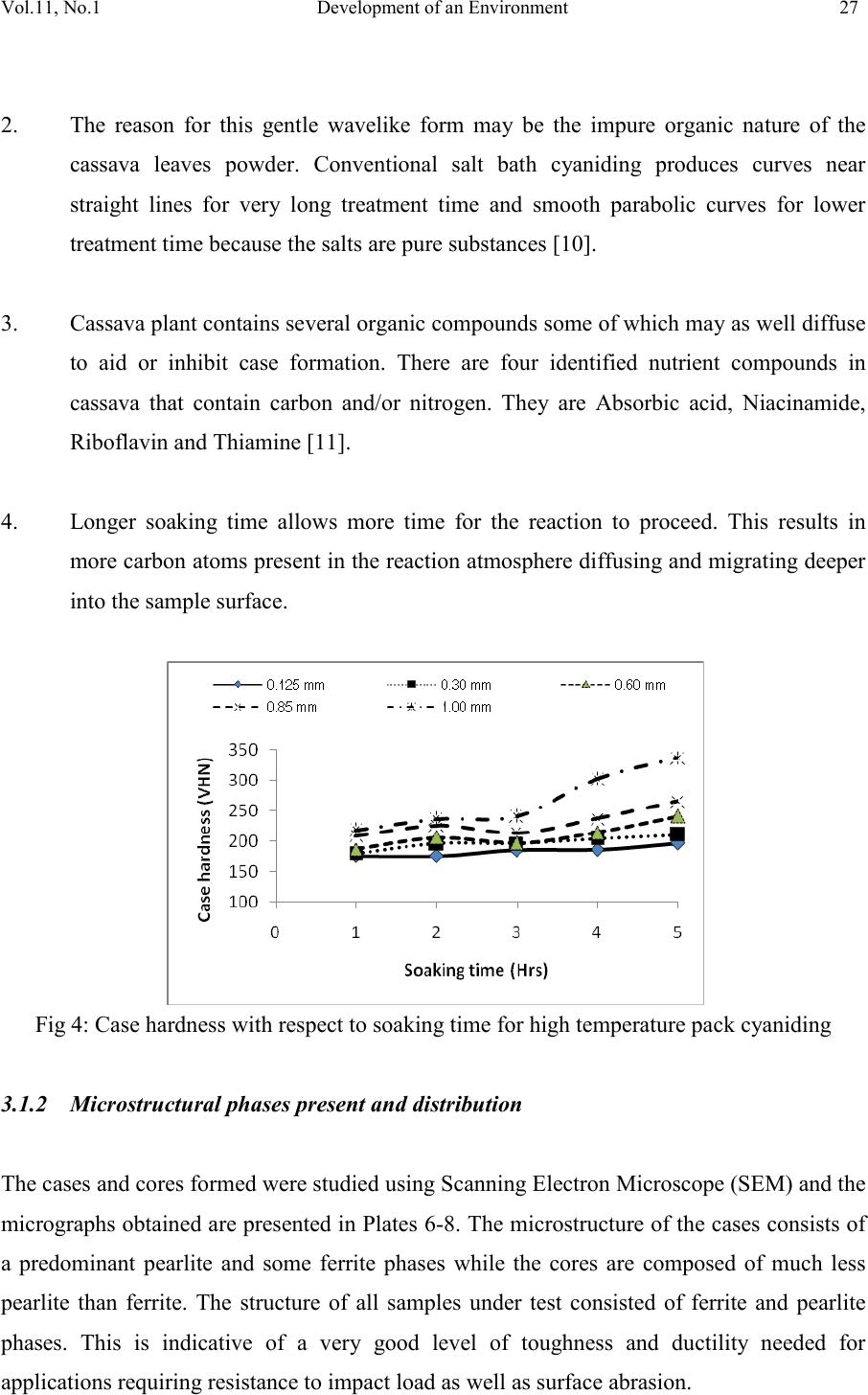 Vol.11, No.1 Development of an Environment 27 2. The reason for this gentle wavelike form may be the impure organic nature of the cassava leaves powder. Conventional salt bath cyaniding produces curves near straight lines for very long treatment time and smooth parabolic curves for lower treatment time because the salts are pure substances [10]. 3. Cassava plant contains several organic compounds some of which may as well diffuse to aid or inhibit case formation. There are four identified nutrient compounds in cassava that contain carbon and/or nitrogen. They are Absorbic acid, Niacinamide, Riboflavin and Thiamine [11]. 4. Longer soaking time allows more time for the reaction to proceed. This results in more carbon atoms present in t he reaction atmosphere diffusing and migrating deeper into the sample surface. Fig 4: Case hardness with respect to soaking time for high temperature pack cyaniding 3.1.2 Microstructural phases present and distribution The cases and cores formed were studied using Scanning Electron Microscope (SEM) and the micrographs obtained are presented in Plates 6-8. The microstructure of th e cases consists of a predominant pearlite and some ferrite phases while the cores are composed of much less pearlite than ferrite. The structure of all samples under test consisted of ferrite and pearlite phases. This is indicative of a very good level of toughness and ductility needed for applications requiring resistance to impact load as well as surface abrasion. 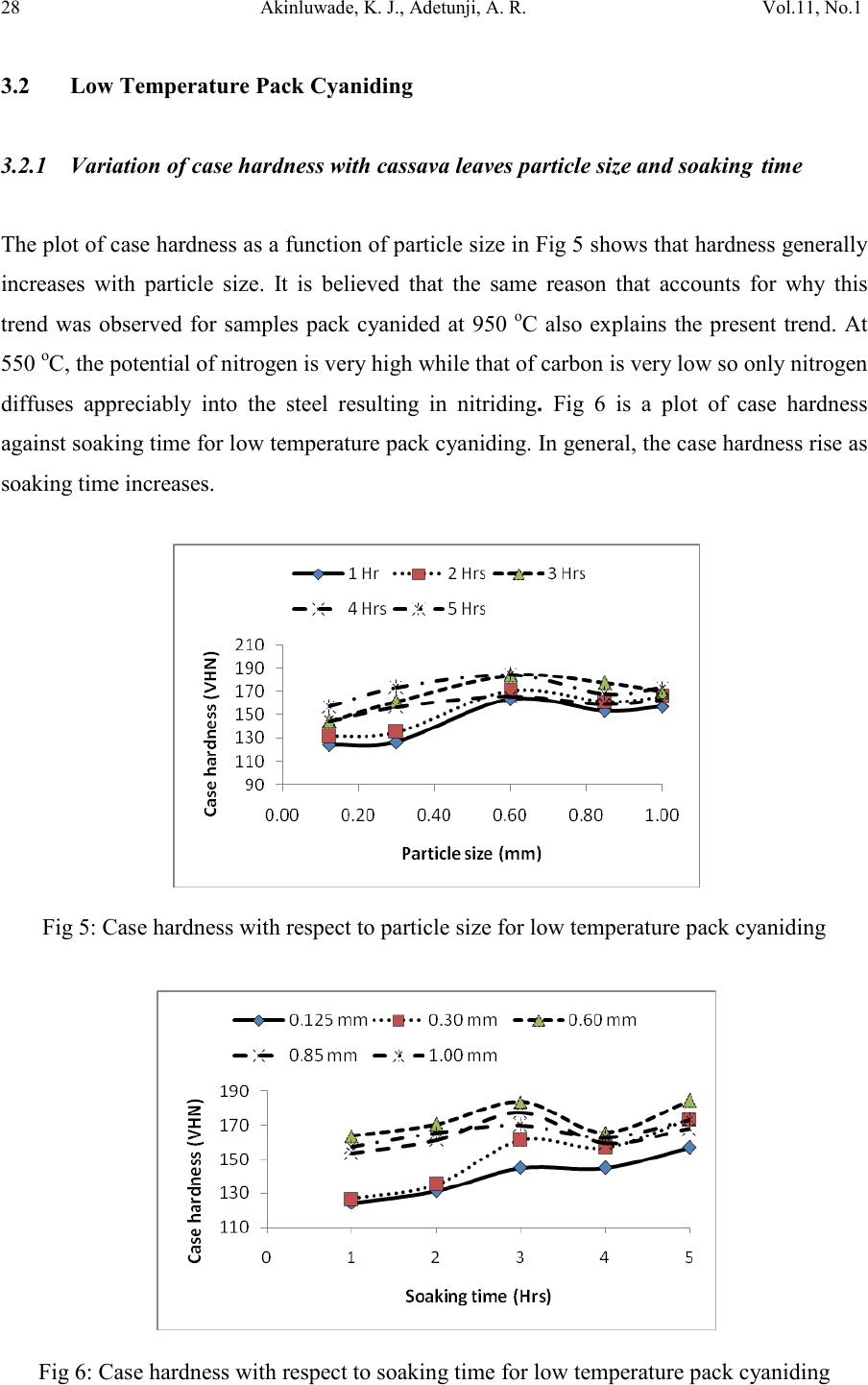 28 Akinluwade, K. J., Adetunji, A. R. Vol.11, No.1 3.2 Low Temperature Pack Cyaniding 3.2.1 Variation of case hardness with cassava leaves particle size and soaking time The plot of case hardness as a function of particle siz e in Fig 5 shows that hardness generally increases with particle size. It is believed that the same reason that accounts for why this trend was observed for samples pack cyanided at 950 oC also explains the present trend. At 550 oC, the potential of nitrogen is very high while that of carbon is very low so only nitrogen diffuses appreciably into the steel resulting in nitriding. Fig 6 is a plot of case hardness against soaking time for low temperature pack c yaniding. In general, the case hardness rise as soaking time increases. Fig 5: Case hardness with respect to particle size for low temperature pack cyaniding Fig 6: Case hardness with respect to soaking time for low temperature pack cyaniding  Vol.11, No.1 Development of an Environment 29 3.2.2 Microstructural constituents and phases At 550oC, the potential of nitrogen is very high while that of carbon is very low so only nitrogen diffuses appreciably into the steel resulting in nitriding. In addition, the atomic radius of N (0.071 nm) is small relative to that of C (0.077 nm). From the Fe-Fe3C phase diagram, the iron component of mild steel is in the ferritic phase and only nitrogen can diffuse into the steel. During conventional salt bath nitriding, a white layer of Fe4N (γ` nitride), and Fe3N (ε nitride) forms near the outer layer of steel surface. Nitrogen has partial solubility in iron. It can form a solid solution with ferrite at nitrogen contents up to about 6% where a compound called gamma prime (γ`), with a composition Fe4N, is formed. At nitrogen contents greater than 8%, the equilibrium reaction product is the epsilon compound ε, with composition Fe3N. Nitrided cases are stratified, the outermost surface can be all γ`. If this is so, it is referred to as the white la yer [10]. The white layer is undesirable because it is so hard that it may spall in service. Results showed that appreciable cases were formed in samples treated at 950 oC while only a thin hard layer case was formed at the outer surface of samples treated at 550 oC. The cases varied in both hardness and depth with cassava leaves particle size and soaking time. 5. CONCLUSIO N The following conclusion can be drawn from the study. 1. A locally developed clean technology for the metallurgical industry that reduces the toxic impact of cyanide on the personnel and environment has been developed. 2. This method is capable of increasing the wear resistance of ferrous parts for a longer service life in application. 3. The study also showed that pack-cyaniding is capable of converting some agricultural wastes to wealth without releasing toxic fumes into the air.  30 Akinluwade, K. J., Adetunji, A. R. Vol.11, No.1 4. The study has shown that pack-cyaniding is feasible with cassava leaves and has the potential to boost the economic viability of the plant for a developing economy. REFERENCES 1. Rajan, T. V., Sharma, C. P. and Sharma, A. (1988) ‘Heat Treatment: Principles and Techniques’. Prentice Hall of India Private Limited, New Delhi. 2. Hutchings, I. M. (1992) ‘Tribology: Friction and Wear of Engineering Materials’. Edward Arnold, London 3. Fathi, H. (1997) Handbook of Extractive Metallurgy. Vol.1, The Metal Industry, Ferrous Metals. Wilhelm Oswald & Co., Wilhelm. 4. Mehrkam, Q. D., Easterday, J. R., Payne, B. R., Foreman, R. W., Vukovich, D. and Godding, A. D., (1991) “Liquid Nitriding of Steels”. In: Heat Treating—ASM Handbook (Revised vol. 4). Stephen M. Copley, editor. USA., pp. 923-943 5. Akinluwade, K. J. (2010) Low and High Temperature Pack-cyaniding of Mild Steel in Pulverized Cassava Leaves. M. Sc. Thesis of Obafemi Awolowo University, Ile-Ife, Nigeria. 6. Adetunji, A. R., AttahDaniel, B. E., Adeoye, M. O., Umoru, L. E, Adeyinwo, C. E., Pelemo, D. A., Olasupo, O. A. and Adewoye, O. O. (2008). “Metallographic Studies of Pack Cyanided Mild Steel Using Cassava Leaves.” Materials and Manufacturing Processes. 23(4), pp. 385-390 7. Ibironke, O. J., Falaiye, A., Ojumu, T. V., Odo, E. A. and Adewoye, O. O. (2004) Case-Depth Studies of Pack Cyaniding of Mild Steel Using Cassava Leaves; Materials and Manufacturing Processes. 19(5) pp. 899-905 8. Conn, E. E. (1973) “Cyanogenic Glucosides: Their Occurrence, Biosynthesis and Function”. In: Chronic Cassava Toxicity. (Nestel, B. and MacIntyre, R., Eds). Conference Proceedings IDRC, Ottawa, Canada., pp.55-63 9. Hosel, W. (1981) The Enzymatic Hydrolysis of Cyanogenic Glucosides. B. Vennesland, E. E. Conn, C. J. Knowles, J. Westley, & F. Wissing (Eds.). Cyanide in Biology., pp. 217–232 Academic Press, London. 10. Foreman, R. W. (1991) Pack Carburizing of Steel. In: Heat Treating—ASM Handbook (Revised vol. 4). Stephen M. Copley, editor. USA, pp. 749-755
|