 2, 20120-1, No.1, pp.1, Vol. 1gnal of Minerals & Materials Characterization & EngineerinJour jmmce.org Printed in the US A. All rights r e served 1 Parameters Affecting the Pr odu ct ion of High Carbon Ferromanganese i n Closed Submerged Arc Furnace Mamdouh Eissa*, Hoda El-Faramawy*, Azza Ahmed, Saeed Nabil and Hossam Halfa Steel and Ferroalloys Department, Central Metallurgical Research and Development Institute (CMRDI), P. O. Box 87, Helwan, Cairo, Egypt, * Corresponding Authors: h3174752@yahoo.com, mamdouh_eissa@yahoo.com ABSTRACT This study has been performed to investigate the different parameters affecting on the production of high carbon ferromanganese in closed submerged arc furnace. The analysis of industrial data revealed that using manganese ores with low Mn/Fe ratio necessitates hi gher amount of Mn-sinter in the charge. Using Mn-blend with higher Mn/Fe ratio reduces the coke consumption and this leads to reducing the electrodes consumption. The reco very of Mn ranges between 70 and 80 %. Much higher basic slag has slight effect on Mn- recovery. However, as slag basicity increases, the MnO- content of slag decreases. The manganese content of produced HCFeMn depends mainly on Mn/Fe ratio of Mn-blend. For obtaining HCFeMn alloy containing minimum 75%Mn, it is necessary to use Mn-blend with Mn/Fe ratio of higher than 6. A model for determination of the amount and composition of off-gases has been derived based on the chemical composition and material balance of the input raw materials and the produced alloy and slag. By using this model, the amount of off-gases was found to increase by increasing both Mn-blend and coke consumption. Key words: Ferromanganese, closed Furnaces, carbothermic reduction, Slag basicity 1. INTRODUCTION At Sinai Manganese Company (SMC), Abu Zenimam, standard high-carbon ferromanganese (HCFeMn) for the domestic and exported markets is smelted in 21 MVA three phase submerged electric arc furnace as closed top unit. The closed top submerged arc furnace has the following advantages comparing with open furnace: less power consumption, kwh/ton and higher productivity. On the other hand, the requirements for ore used in closed top  2 2 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 furnace are more restricted, e.g. free oxygen must be less than 10% and less friable ore. Otherwise, crust may be formed leading to occurrence of explosion. The proper economic production of ferromanganese performance will be improved by selecti ng the prop er raw mater ials s uitab le for t he closed top submerged arc furnace, applying the best material balance for the raw materials and enhancing the smelting condition. The result will be lower consumption of raw materials, reduced specific energy consumption, good furnace operation, higher alloy quality and lower production cost. Thus, it is of prime importance to examine the different parameters affecting on the production of high carbon ferromanganese in closed submerged arc furnace. 2. EXPERIMENTAL PROCEDURE In the high carbon ferromanganese making process at SMC, different local manganese ores and imported manganese sinter are blended along with the reducing agent (coke) and flux materials (dolomite and limestone) are mixed outside of the furnace (often called charge mix ). The different raw mat erials compo nents are weighed out bas ed on chemical an alysis of the ores, sinter, fluxes and coke and on the desired composition of alloy and slag. It is desired to obtain standard high carbon ferromanganese alloy containing at least 75% Mn and minimum content of phosphorus. The raw materials mix is transported to hoppers above the furnace from where it is fed by gravity through chutes passing through the furnace cover. In the submerged arc furnace, the electrodes are buried deep in the furnace burden and the reduction reaction takes place near the tip of the electrodes. The current flow between electrodes creates the intensive heat needed for the high temperature and energy required for the reduction reactions. Charge mix is added periodically and the metal and slag are collected during tapping at appropriate intervals (often at 30 Mw). Produced slag and metal are tapped from the same tap-hole. 3. RESULTS & DISCUSSION 3.1 Statistical Analysis of Collected Data The real operating data for producing high carbon ferromanganese at SMC were collected and statistical analysis of collected data has been conducted to evaluate the different parameters affectin g on the production process. The material balance for producing one ton high carbon ferromanganese has been calculated and an example of the material balance is summarized in Table 1. 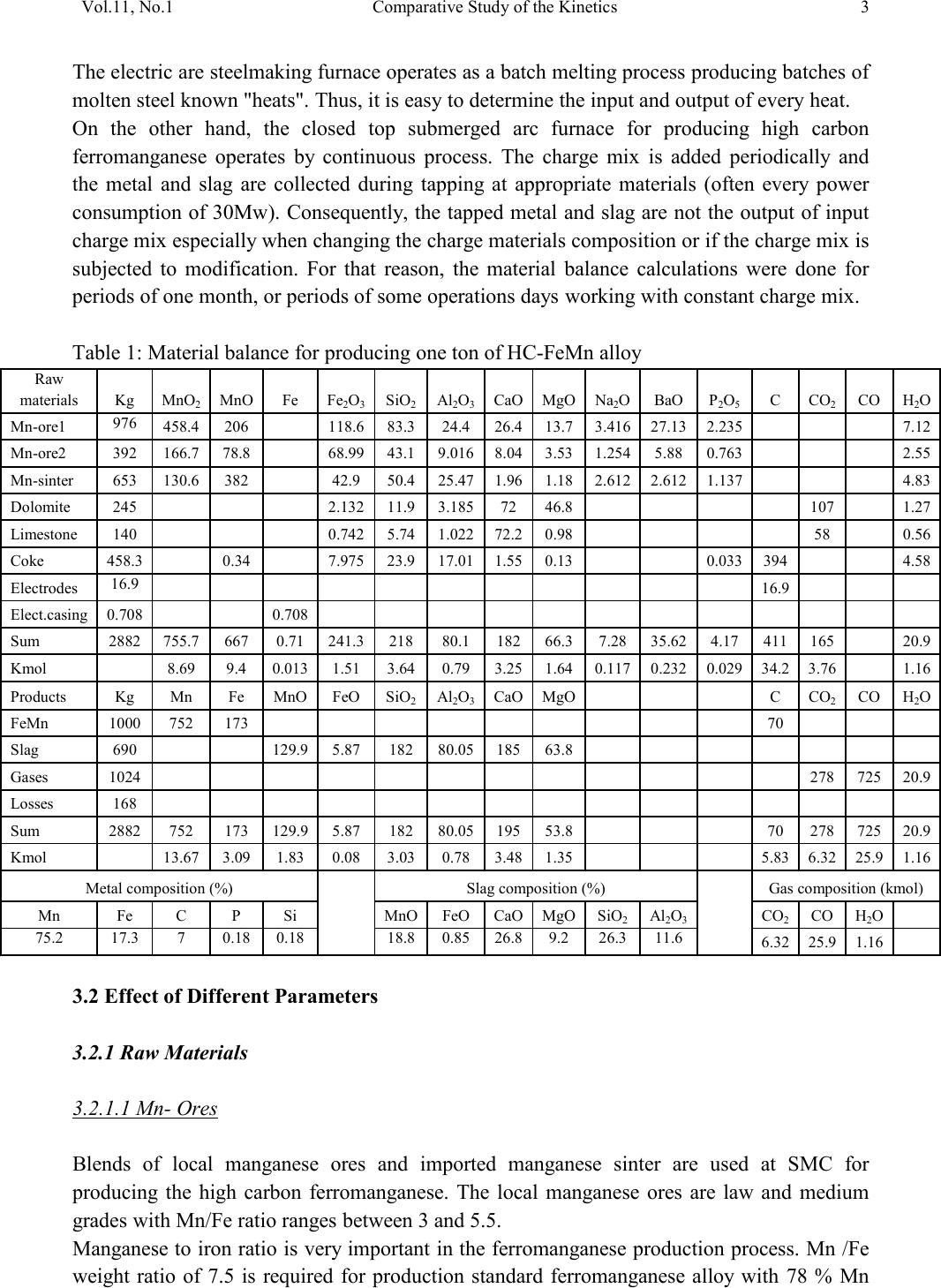 Vol.11, No.1 Comparative Study of the Kinetics 3 The electric are s teelmak ing furnace operat es as a batch m elti ng process prod ucing batches of molten steel known "heats". Thus, it is easy to determine the input and output of every heat. On the other hand, the closed top submerged arc furnace for producing high carbon ferromanganese operates by continuous process. The charge mix is added periodically and the metal and slag are collected during tapping at appropriate materials (often every power consumption of 30Mw). Consequently, the tapped metal and slag are not the output of input charge mix esp ecial l y when changing the charge materials composition or if the charge mix is subjected to modification. For that reason, the material balance calculations were done for periods of one month, or periods of some operations days working with constant charge mix. Table 1: Material balance for producing one ton of HC-FeMn alloy materials Kg MnO MnO Fe Fe O SiO Al O CaO MgO Na O BaO P O C CO CO H O Mn-ore1 458.4 206 118.6 83.3 24.4 26.4 13.7 3.416 27.13 2.235 7.12 Mn-ore2 392 166.7 78.8 68.99 43.1 9.016 8.04 3.53 1.254 5.88 0.763 2.55 Mn-sinter 653 130.6 382 42.9 50.4 25.47 1.96 1.18 2.612 2.612 1.137 4.83 Dolomite 245 2.132 11.9 3.185 72 46.8 107 1.27 Limestone 140 0.742 5.74 1.022 72.2 0.98 58 0.56 Coke 458.3 0.34 7.975 23.9 17.01 1.55 0.13 0.033 394 4.58 Electrodes 16.9 Elect.casing 0.708 0.708 Sum 2882 755.7 667 0.71 241.3 218 80.1 182 66.3 7.28 35.62 4.17 411 165 20.9 Kmol 8.69 9 .4 0.013 1.51 3.64 0.79 3.25 1.64 0.117 0.232 0.029 34.2 3.76 1.16 Products Kg Mn Fe MnO FeO SiO Al O CaO MgO C CO CO H O FeMn 1000 752 173 70 Slag 690 129.9 5.87 182 80.05 185 63.8 Losses 168 Sum 2882 752 173 129.9 5.87 182 80.05 195 53.8 70 278 725 20.9 Kmol 13.67 3.09 1.83 0.08 3.03 0.78 3.48 1.35 5.83 6.32 25.9 1.16 Metal composition (%) Slag composition (%) Gas composition (kmol) Mn Fe C P Si MnO FeO CaO MgO SiO Al O CO CO H O 6.32 25.9 1.16 3.2 Effect of Different Parameters 3.2.1 Raw Materials 3.2.1.1 Mn- Ores Blends of local manganese ores and imported manganese sinter are used at SMC for producing the high carbon ferromanganese. The local manganese ores are law and medium grades with Mn/Fe ratio ranges between 3 and 5.5. Manganese to iron ratio is very important in the ferromanganese production process. Mn /Fe weight ratio of 7.5 is required for production standard ferromanganese alloy with 78 % Mn  4 4 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 [1]. Furthermore, the local manganese ores vary widely in their content of manganese, iron, silicon, alumina, lime, magnesia, potassium and sodium oxides, barium oxide and phosphorus. Mixtures of two Mn- ores are usually used to be blend with Mn-si nter. The charged Mn-ores mixture amounts to 890 - 1890 kg per ton produced alloy (average 1375 kg/ton) with Mn/Fe ratio ranges between 3.3 and 5.5 (average 4.08). 3.2.1.2 Mn-Sinter Mn-sinter is suited for use in ferromanganese furnaces, since it is mechanically strong and thermally stable, allowing the gas to disperse evenly throughout the preheating and pre- reduction zone. Moreover, the requirements for ore used in closed top furnace are more restricted, i.e. excess oxygen must be less than 10 %. To adjust this vital parameter in closed top furnaces, excess oxygen, Mn-sinter is used in the charge mix. Excess ox ygen is defined as the difference between the actual amount of oxygen chemically bounded to manganese and the theoretically amount of oxygen assuming the total amount of manganese being present in the monoxide MnO state. At SMC, Mn-sinter is used to increase the Mn-Fe ratio of the blend and reduce the excess oxygen. Mn-sinter has a higher Mn /Fe ratio ranges between 9.3 and 12.6, and the charged amount ranges between 385 and 870 kg per one ton produced ferromanganese alloy (average 642 kg/ton). This amount represents 22 to 45% of the Mn- blend (average 32.2%). By adding Mn –sinter into the Mn–ores mixture, the Mn /Fe ratio of Mn-blend increases to 4.5 – 6.4 (average 5.4) and the Mn-blend amounts to 1745 - 2430 kg per ton produced ferromanganese alloy (average 1998 kg/ton). Furthermore, addition of Mn-sinter reduces the excess oxygen, Figure1. As Mn/Fe ratio of local Mn-ores increases, the Mn-sinter weight per one ton produced HCFeMn decreases, Figure 2, and Mn-sinter% in the Mn-blend decreases, Figu re 3 . 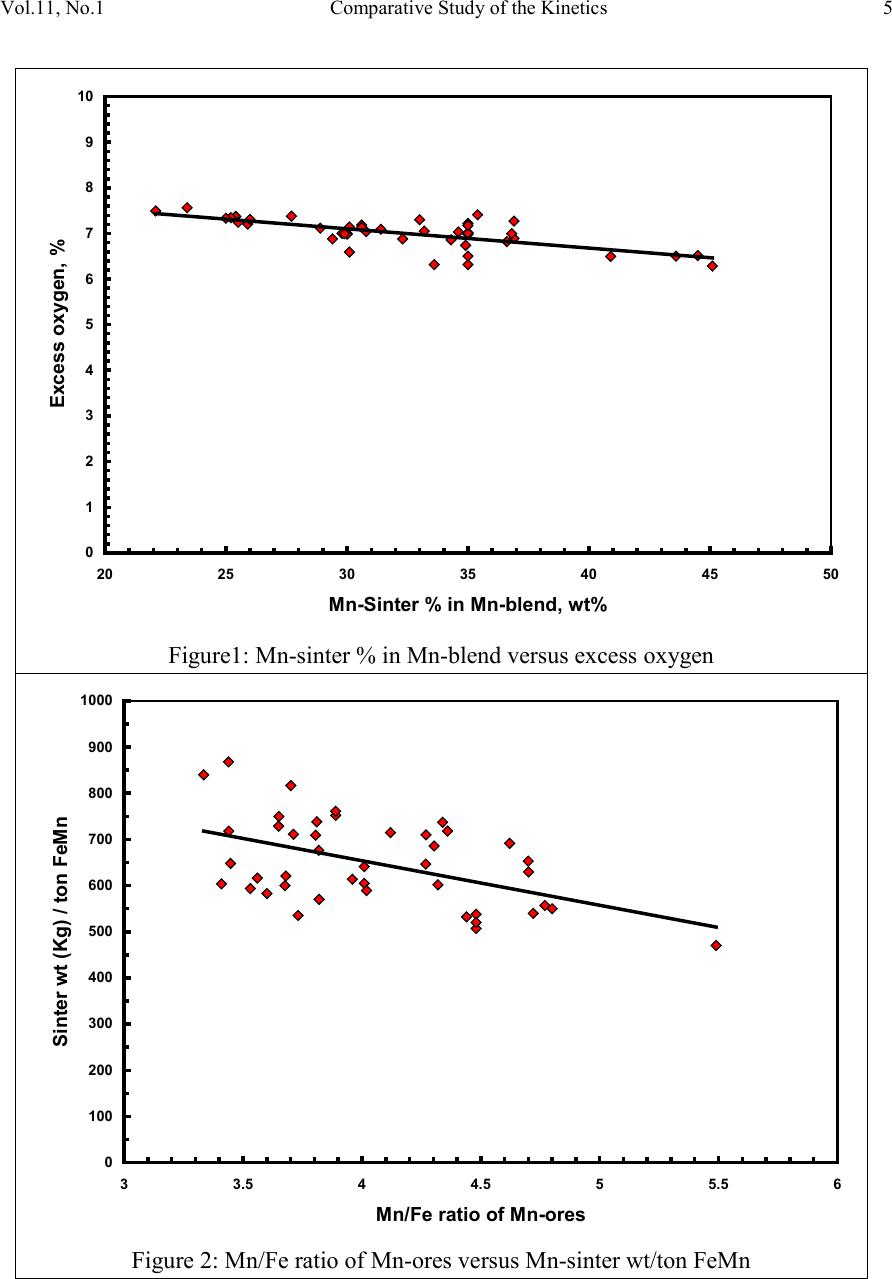 Vol.11, No.1 Comparative Study of the Kinetics 5 0 1 2 3 4 5 6 7 8 9 10 20 25 30 35 40 45 50 Mn-Sinter % in Mn-blend, w t% Figure1: Mn-si nt er % in Mn-blend versus excess oxygen 0 100 200 300 400 500 600 700 800 900 1000 33.5 44.5 55.5 6 Sin ter wt (Kg ) / ton FeMn Figure 2: Mn/Fe ratio of Mn-ores versus Mn-sinter wt/ton FeMn  6 6 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 0 10 20 30 40 50 33.5 44.5 55.5 6 Mn-sinter % in Mn-blend, wt% Figure 3: Mn/Fe ratio of Mn-ores versus Mn-sinter% in Mn-blend 3.2.1.3 Reducing Agent Coke is added as a source of carbon for ore reduction. The interior of a furnace producing high carbon ferromanganese consists of two main zones with different characteristics: the low temperature pre-reduction zone, and the high temperature coke bed zone. As the raw materials move down in the pre-reduction zone, the higher oxides of manganese are pre- reduced in solid state to Mn3O4 and preferably further to MnO by CO gas formed in the crater zone. The extent of the simultaneously running Boudouard reaction (CO2+C = 2CO) is responsible for the variation in carbon. After further reheati ng, th e pre-reduced ore and added fluxes start melting at temperatures of about 1250oC to 1300oC. The coke remains solid, so below this area there is a permanent coke bed [2]. The melting together of ores and fluxes and reduction of MnO dissolved in the slag phase take place in the coke bed. The coke bed starts approximately at the tip of the submerged electrodes. It constitutes a permanent reservoir of coke. The relative amount of coke in the charge mix determines whether the coke bed increases, decr eases or stab le in siz e. In addition to being the ch emical reduct ant it i s also th e heating element of the process where the electric current runs and ohmic energy is produced. The coke consumption ranges between 400 - 550 kg/ton HCMnFe (average 462 kg/ton). The coke consumption increases as the Mn-blend weight increases, Figure 4, and Mn/Fe of the Mn-bl end decreas es, Figure 5. 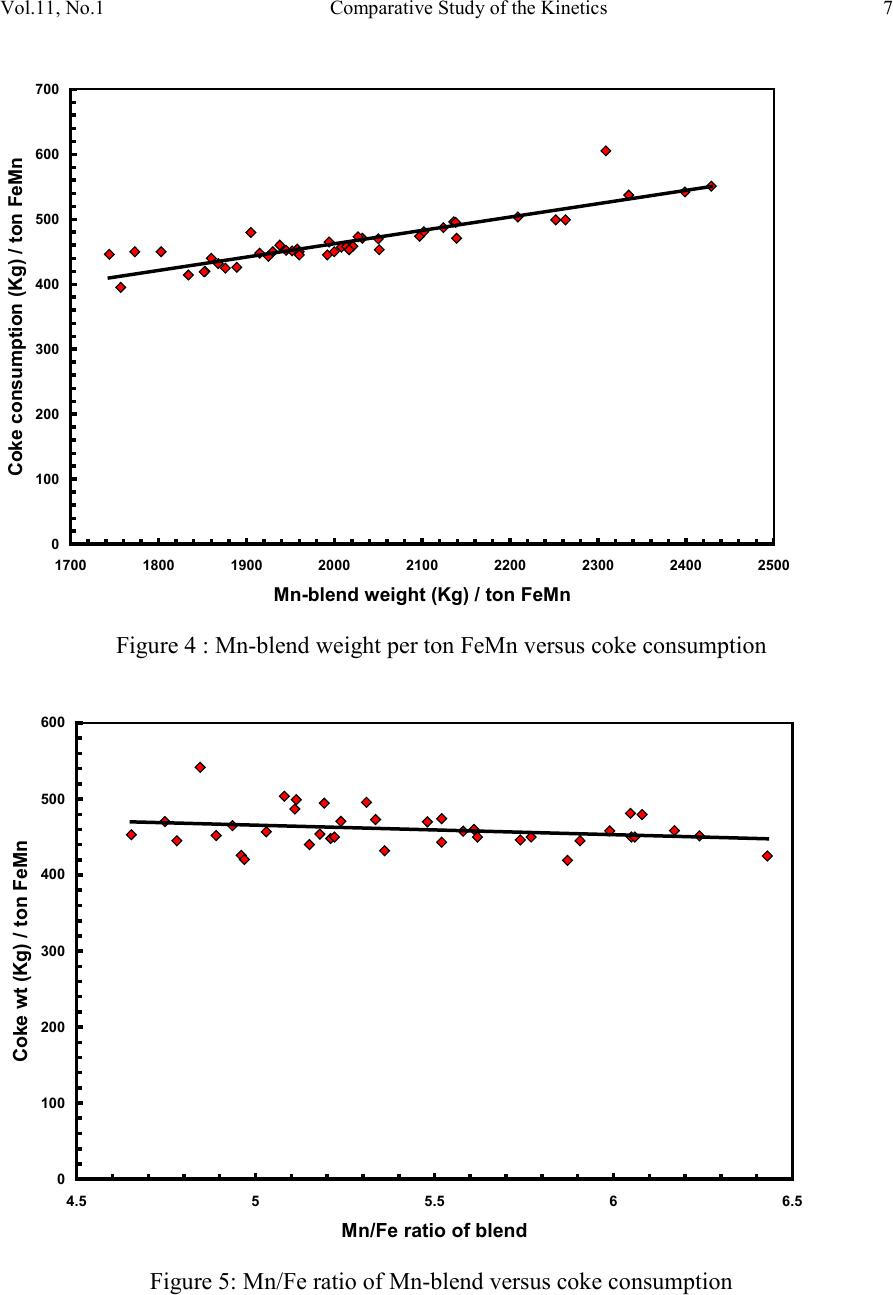 Vol.11, No.1 Comparative Study of the Kinetics 7 0 100 200 300 400 500 600 700 1700 1800 1900 2000 2100 2200 23002400 2500 Mn-blend weight (Kg) / ton FeMn Coke consumption (Kg) / ton FeMn Figure 4 : Mn-blend weight per ton FeMn versus coke consumption 0 100 200 300 400 500 600 4.5 55.5 66.5 Figure 5: Mn/Fe ratio of Mn-blend versus coke consumption 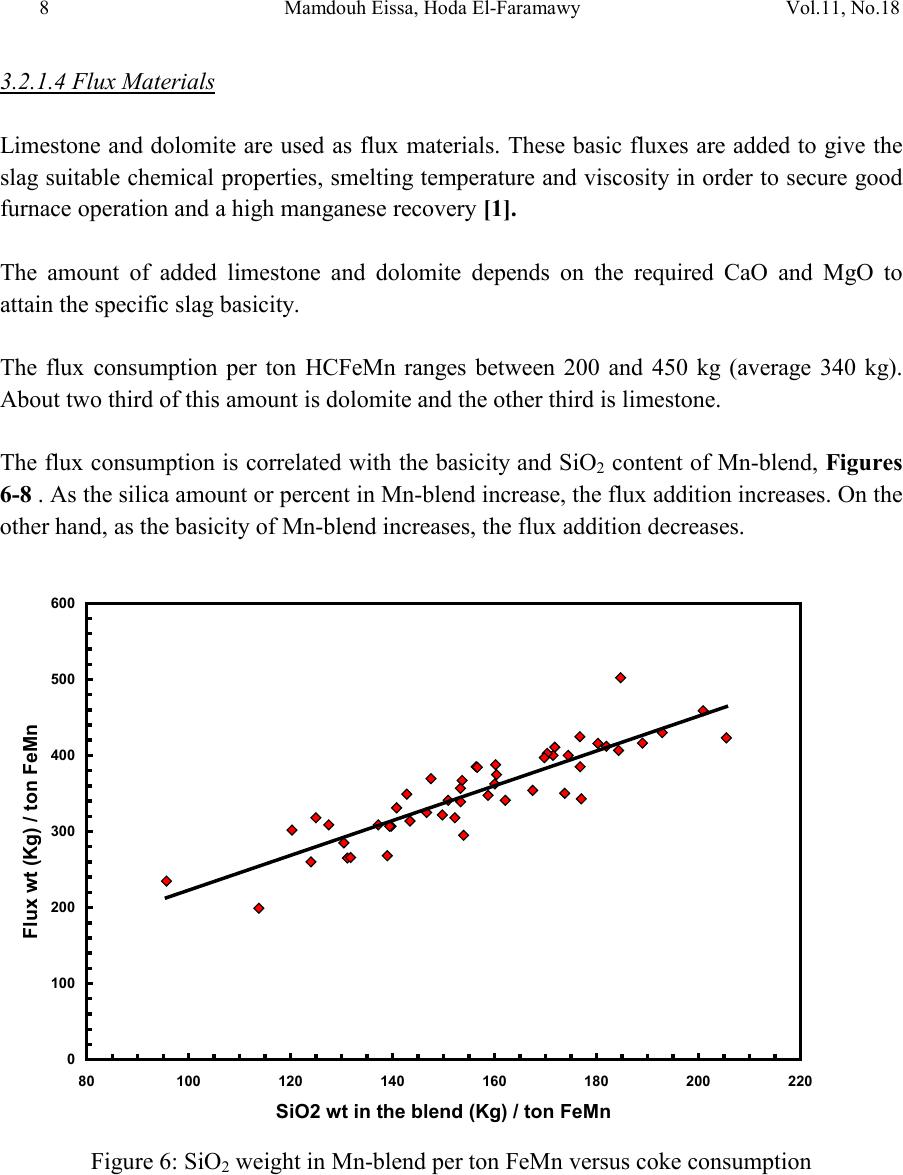 8 8 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 3.2.1.4 Flux Materials Limestone and dolomite are used as flux materials. These basic fluxes are added to give the slag suitable chemical properties, smelting temperature and viscosity in order to secure good furnace operation and a high manganese reco very [1]. The amount of added limestone and dolomite depends on the required CaO and MgO to attain the specific slag basicity. The flux consumption per ton HCFeMn ranges between 200 and 450 kg (average 340 kg). About two third of this amount is dolomite and the other third is limestone. The flux consumption is correlated with the basicity and SiO2 content of Mn-blend, Figures 6-8 . As the silica amount or percent in Mn-blend increase, the flux addition increases. On the other hand, as the basicity of Mn-blend increases, the flux addition decreases. 0 100 200 300 400 500 600 80100 120 140 160 180 200 220 SiO2 wt in the blend (Kg) / ton FeMn Figure 6: SiO2 weight in Mn-blend per ton FeMn versus coke consumption 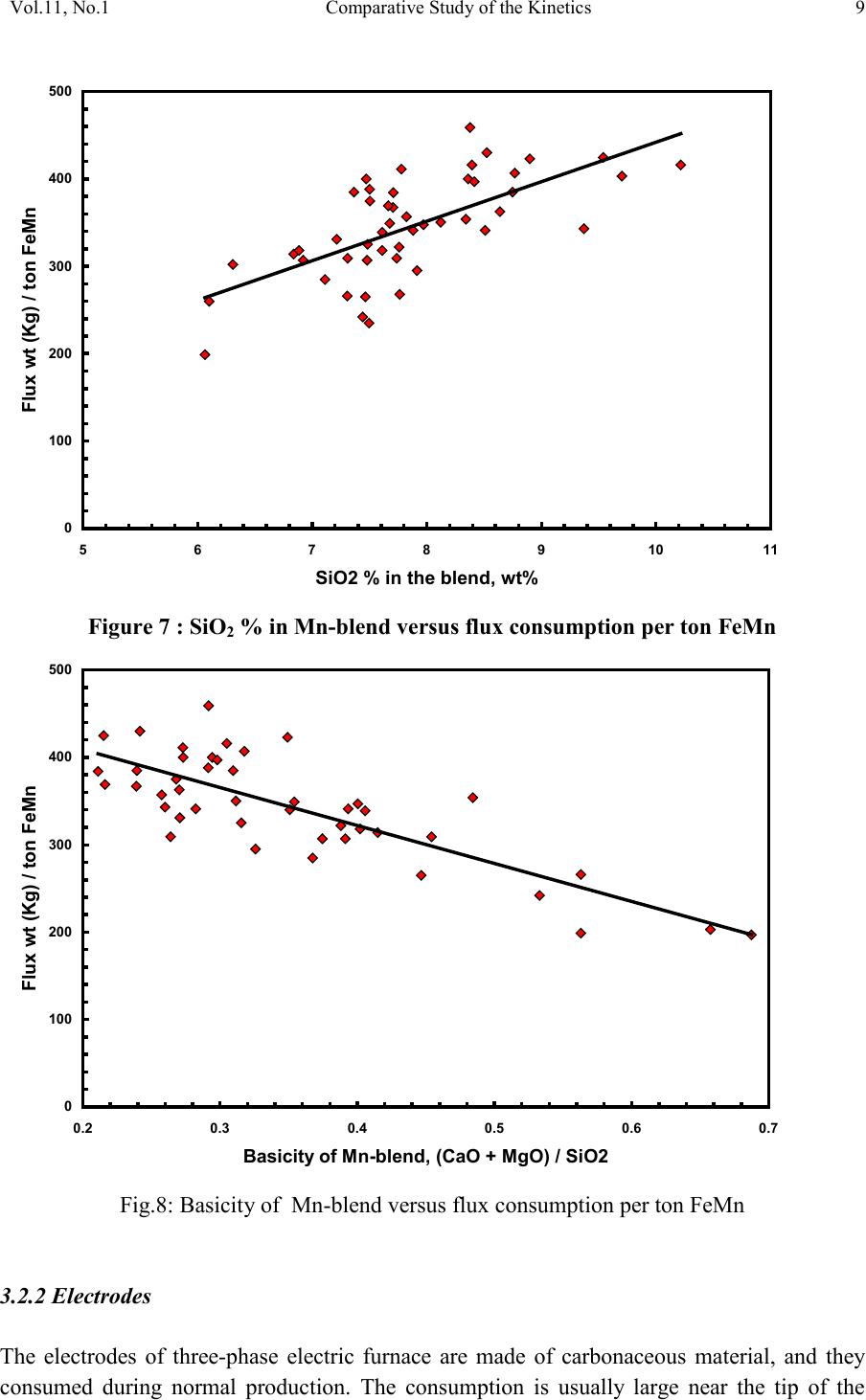 Vol.11, No.1 Comparative Study of the Kinetics 9 0 100 200 300 400 500 5678910 11 Figure 7 : SiO2 % in Mn-blend versus flux consumption per ton FeMn 0 100 200 300 400 500 0.2 0.3 0.4 0.50.6 0.7 Basicity of Mn-blend, (CaO + MgO) / SiO2 Fig.8: Basicity of Mn-blend versus flux consumption per ton FeMn 3.2.2 Electrodes The electrodes of three-phase electric furnace are made of carbonaceous material, and they consumed during normal production. The consumption is usually large near the tip of the  10 10 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 electrode where the temperature is high and the reactants are more active. To keep the electrode tip at the same position it is therefore necessary to prolong the electrode regularly. The electrodes consumption per ton HCFeMn ranges between 8 and 37 kg (average 17.5 kg). The electro des consumption increases as the coke consumption per ton HCFeMn increases, Figure 9. 3.2.3 Electrodes Casing Electrodes casing is a low carbon steel and is consumed with the consumption of electrodes. The electrodes casing consumption ranges between 0.4 and 1.3 kg / ton HCFeMn (average 0.6 kg/ton 0 5 10 15 20 25 30 35 40 350 400 450 500 550 600 650 Coke consumption (Kg) / ton FeMn Electrodes consumption (Kg) / to FeMn Figure 9: Coke consumption versus electrodes consumption per ton FeMn 3.3. Products 3.3.1 High Carbon Ferromanganese The quality of high carbon ferromanganese depends mainly on the content of manganese and phosphorus. The phosphorus content of standard HCFeMn is < 0.2 %. Most of the phosphorus in the ore remains in the finished product. The recovery of phosphorus is high (average 98%) and decreases with increasing the slag basicity as shown in Figure 10. However, due to the relatively low phosphorus content of Mn-blend, the phosphorus content in the produced HCFeMn is low of average 0.18 %. The recovery of Mn ranges between 70 and 80 % (average 75 %). In smelting of high carbon ferromanganese, manganese recovery was found to increase b y increas in g s lag bas ici ty [3-10] and decreasing slag viscosity [5,6]. 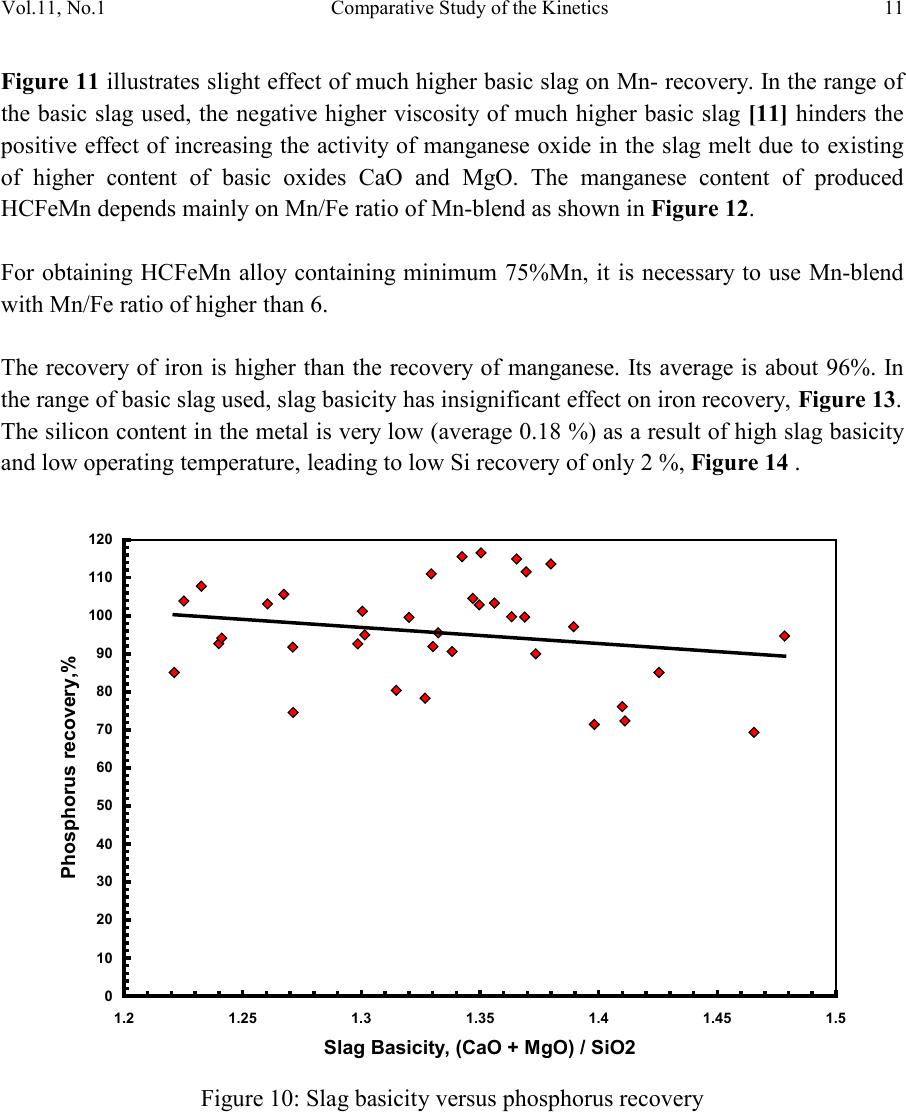 Vol.11, No.1 Comparative Study of the Kinetics 11 Figure 11 illustrates slight effect of much higher basic slag on Mn- recovery. In the range of the basic slag used, the negative higher viscosity of much higher basic slag [11] hinders the positive effect of increasing the activity of manganese oxide in the slag melt due to existing of higher content of basic oxides CaO and MgO. The manganese content of produced HCFeMn depends mainly on Mn/Fe ratio of Mn-blend as shown in Figure 12. For obtaining HCFeMn alloy containing minimum 75%Mn, it is necessary to use Mn-blend with Mn/Fe ratio of higher than 6. The recovery of iron is higher than the recovery of manganese. Its average is about 96%. In the range of basic slag used, slag basicity has insignificant effect on iron recovery, Figu re 13. The silicon content in the metal is very low (average 0.18 %) as a result of high slag basicity and low operating temperature, leading to low Si recovery of only 2 %, Figu re 14 . 0 10 20 30 40 50 60 70 80 90 100 110 120 1.2 1.25 1.3 1.35 1.4 1.45 1.5 Slag Basicity, (CaO + MgO) / SiO2 Phosph o ru s r eco very,% Figure 10: Slag basicity versus phosphorus recovery  12 12 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 0 10 20 30 40 50 60 70 80 90 100 1.2 1.25 1.3 1.35 1.4 1.45 1.5 Slag Basicity, (CaO + MgO) / SiO2 Figure11: Slag basicity, (CaO + MgO) / SiO2, versus manganese recovery Mn Content in FeMn Alloy,% Figure12: Mn / Fe ratio of Mn-blend versus manganese content in FeMn 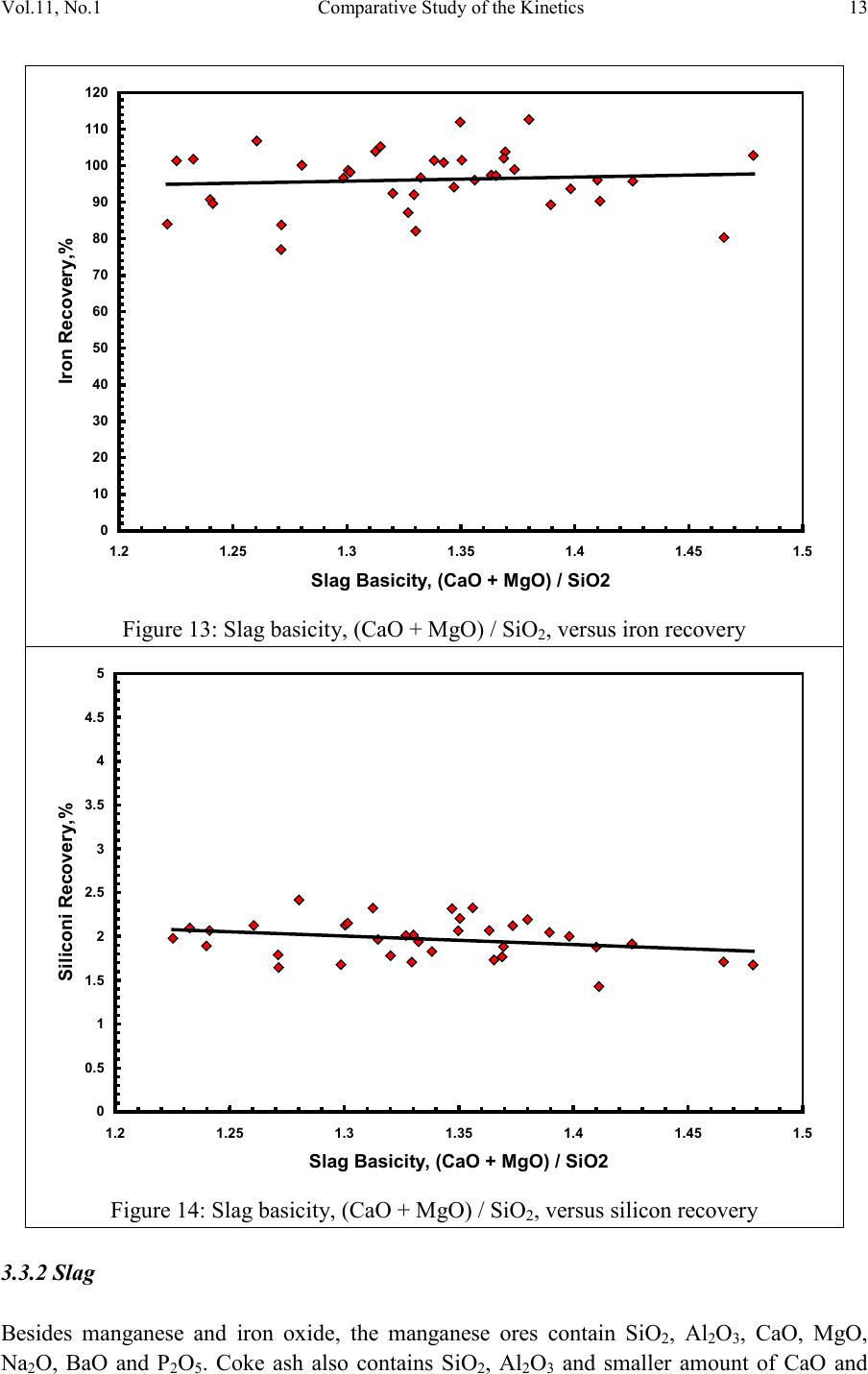 Vol.11, No.1 Comparative Study of the Kinetics 13 0 10 20 30 40 50 60 70 80 90 100 110 120 1.2 1.25 1.3 1.35 1.4 1.45 1.5 Slag Basicity, (CaO + MgO) / SiO2 Figure 13: Slag basicity, (CaO + MgO) / SiO2, versus iron recovery 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 1.2 1.25 1.3 1.351.4 1.45 1.5 Slag Basicity, (CaO + MgO) / SiO2 Figure 14: Slag basicity, (CaO + MgO) / SiO2, versus silicon recovery 3.3.2 Slag Besides manganese and iron oxide, the manganese ores contain SiO2, Al2O3, CaO, MgO, Na2O, BaO and P2O5. Coke ash also contains SiO2, Al2O3 and smaller amount of CaO and 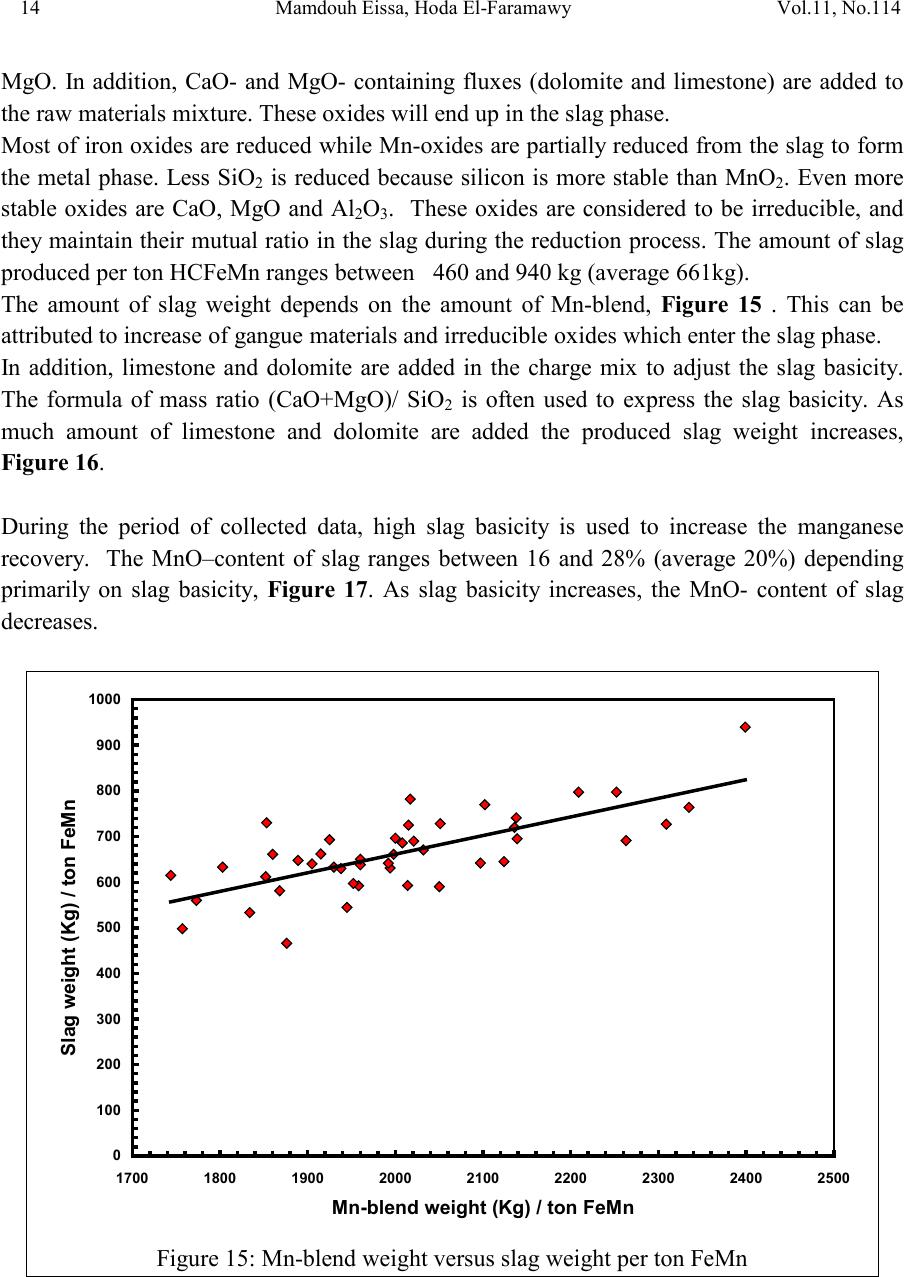 14 14 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 MgO. In addition, CaO- and MgO- containing fluxes (dolomite and limestone) are added to the raw materials mixture. These oxides will end up in the slag phase. Most of iron oxi des are reduced while Mn-oxides are partially reduced from the slag to form the metal phase. Less SiO2 is reduced because silicon is more stable than MnO2. Even more stable oxides are CaO, MgO and Al2O3. These oxides are considered to be irreducible, and they maintain their mutual ratio in the slag during the reduction process. The amount of slag produced per ton HCFeMn ranges between 460 and 940 kg (average 661kg). The amount of slag weight depends on the amount of Mn-blend, Figu re 15 . This can be attributed to increase of gangue materials and irreducible oxides which enter the slag phase. In addition, limestone and dolomite are added in the charge mix to adjust the slag basicity. The formula of mass ratio (CaO+MgO)/ SiO2 is often used to express the slag basicity. As much amount of limestone and dolomite are added the produced slag weight increases, Figure 16. During the period of collected data, high slag basicity is used to increase the manganese recovery. The MnO–content of slag ranges between 16 and 28% (average 20%) depending primarily on slag basicity, Figure 17. As slag basicity increases, the MnO- content of slag decreases. 0 100 200 300 400 500 600 700 800 900 1000 1700 1800 1900 2000 2100 2200 2300 2400 2500 Mn-blend weight (Kg) / ton FeMn Slag weight (Kg) / ton FeMn Figure 15: Mn-blend weight versus slag weight per ton FeMn  Vol.11, No.1 Comparative Study of the Kinetics 15 0 100 200 300 400 500 600 700 800 900 1000 100 150 200250 300 350 400450 500 Flux weight (Kg) / ton FeMn alloy Slag weightt (Kg) / ton FeMn alloy Figure 16: Flux weight versus slag weight per ton FeMn 0 5 10 15 20 25 30 1.2 1.25 1.3 1.351.4 1.45 1.5 Slag basicity, (CaO + MgO) / SiO2 Figure 17: Slag basicity, (CaO + MgO) / SiO2, versus MnO % in slag  16 16 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 3.3.3 Gases The amount and analysis of the gases leaving the furnace is not available at SMC due to unavailability of measuring devices. Assumptions have been made in order to calculate the amount and composition of the gases. It is assumed that the temperature of the gases at the furnace top, which is in excess of 200oC, being high enough for evaporation of moisture from charge as soon as the materials fall into the furnace. Evaporation of moisture is very rapid. Thus, all H2O (l) enters the furn ace leav es as H2O (g) with the off-gas. The other constituents of gas phase are CO and CO2. By determining the amount of carbon and oxygen in gases, the amount of CO and CO2 can be calculated. The carbon amount in CO and CO2 mixture in off-gas can be calculated by subtraction the carbon contained in the produced HCFeMn from the sum of carbon contained in the added coke, fluxing materials and consumed electrodes, C {CO+CO2} = C ( C oke) + C (electrodes) + C (flu x) – C (alloy) (1) The oxygen amount in CO and CO2 mixture in off-gas can be calculated by subtraction the oxygen contained in the produced slag from the oxygen contained in the raw materials (local Mn-Ores, Mn-Sinter, limestone, dolomite and coke ash), O {CO+CO2} = O (Raw materials) – O (slag) (2) The sum of carbon and oxygen in (CO +CO2) mixture equals the sum of CO and CO2 in off- gas: C {CO+CO2} + O {CO+CO2} = {CO +CO2} in gases (3) Consequently, the total weight of gases can be calculated: Gases weight = CO + CO2 + H2O (4) Carbon / oxygen ratio is calculated and correlated with CO2/ (CO+CO2) as shown in Fig (18). The following equation is fitting this relation: CO2 / (CO+CO2) wt ratio = -266.67 (C/O) + 200 (5) The amount of gases per one ton HCFeMn ranges between 855 and 1316 kg (average 1016 kg). The CO2 / (CO+CO2) wt ratio ranges between 0.05 and 0.45 (average 0.25) Figures (19 -21) reveal increasing the amount of gases per one ton HCFeMn by increasing both Mn-blend and coke and sum of Mn-blend and coke, respectively. This can be attributed to the higher oxygen and carbon in the charge mix.  Vol.11, No.1 Comparative Study of the Kinetics 17 -10 0 10 20 30 40 50 60 70 80 90 100 110 0.3 0.4 0.5 0.60.7 0.8 Carbon:oxygen wt ratio in CO-CO2 mixture Figure18: Carbon: oxygen ratio versus CO2 % in CO – CO2 mixture 0 200 400 600 800 1000 1200 1400 1700 1800 1900 2000 2100 2200 2300 2400 2500 Mn-blend weight (Kg) / ton FeMn Gases weight (Kg) / ton FeM n Figure 19: Mn-blend wt versus gases wt per ton FeMn  18 18 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 0 200 400 600 800 1000 1200 1400 350 400450 500 550600 650 Coke consumption (Kg) / ton FeMn Gases weight (Kg) / ton FeMn Figure 20: Coke consumption versus gases wt per ton FeM n Figure 21: Sum of Mn-blend and coke wt versus gases wt per ton FeMn 0 200 400 600 800 1000 1200 1400 2100 22002300 2400 2500 2600 2700 2800 2900 3000 (Mn-blend+Coke) wt (Kg) / ton FeMn Gases wt (Kg) / ton F eM n  Vol.11, No.1 Comparative Study of the Kinetics 19 3.3.4 Losses From material balance calculations, weight losses up to 350 kg per one ton produced HCFeMn has been detected (average 162 kg). The average weight losses represent about 5.5% of the charge mix. These weight losses could be attributed to materials losses in handling and charging process, dust leaving the furnace with the off-gas, weight errors and metal lost in slag and in crushing to the suitable sizes. 4. CONCLUSIO NS From the results of analysis of industrial data for producing HC-FeMn in closed submerged arc furnace, the following conclusions can be deduced: • As Mn/Fe ratio of local Mn-ores increases, t he M n-sint er weight per one ton produced HCFeMn decr eases , and Mn-sinter% in the Mn-blend decreases. • The coke consumption increases as the Mn-blend weight increases and Mn/Fe of the Mn-blend decreases. • As the silica amount or percent in Mn-blend increase, the flux addition increases. On the other hand, as the basicity of Mn-blend increases, the flux addition decreases. • The electrodes consumption increases as the coke consumption per ton HCFeMn increases . • Most of the phosphorus in the ore goes to the finished product. The recovery of phosphorus is high of about 98% and slightly decreases with increasing the slag basicity. For producing standard HCFeMn containing < 0.2 % P, charging of Mn- blend with low phosphorus content is required. • The recovery of Mn ranges between 70 and 80 %. Much higher basic slag has slight effect on Mn- recovery. The negative higher viscosity of much higher basic slag hinders the positive effect of increasing the activity of manganese oxide in the slag melt due to existing of higher content of basic oxides CaO and MgO. • The manganese cont ent of produced HCFeMn depends mainly on Mn/Fe ratio of Mn- blend. For obtaining HCFeMn alloy containing minimum 75%Mn, it is necessary to use Mn-blend with Mn/Fe ratio of higher than 6. • The recovery of iron is higher than the recovery of manganese. Its average is about 96%. In the range of basic slag used, slag basicity has insignificant effect on iron recovery. • The silicon content in the produced alloy is very low (average 0.18 %) as a result of high slag basicity and low operating temperature, leading to low Si recovery of only 2 %. • The amount of produced slag depends on the amounts of Mn-blend and fluxing materials. • The MnO–content of slag depends primarily on slag basicity, as slag basicity increases , t he Mn O- con ten t of sl ag decreases .  20 20 Mamdouh Eissa, Hoda El-Faramawy Vol.11, No.1 • A model for determination of the amount and composition of off-gases has been derived based on the chemical composition and material balance of the input raw materials and the produced alloy and slag. • The amount of off-gases increases b y increas ing bot h Mn-blend and coke amounts. • In the production process, about 5.5% of the charge mix weight is lost due to materials losses in handling and charging process, dust leaving the furnace with the off-gases , weight errors and metal lost in slag and in crushing to the suitable sizes. ACKNOWLEDGEMENT This work is a part of complex study carried out through a project financed by the Science and Technological Development Fund (STDF), Egypt. The authors would like to acknowledge STDF due to their financial support and all facilities they offered to perform this work. Cordial thanks and deep appreciation are due to Eng. Mohamed Abdel Samie Chairman & Managing Director of Sinai Manganese Company (SMC), for his encouragement, sound support and providing technical data. The counterparts of SMC offered all facilities and required data for performing this study. Special thanks and gratitude are due to all members in Steel and Ferroalloys Department, CMRDI and technical staff of SMC Company. REFERENCES [1] Ol sen S. E., Tangstad M. and Lindstad T., 2007, Production of Manganese Ferroalloys, P. 247, SINTEF and Tapir Academic Press, Trondheim [2] Tan gstad M. , 1996, The Hi gh Carb on Ferroman ganese Process-Coke Bed Relations, PhD Thesis, 49, Department of Metallurgy, The Norwegian Institute of Technology, Trondheim [3] V.I. Nikoleav, 1974, Steel in the USSR, pp. 299-302 [4] N. Chernyatin, V. G. Mizin, I.A. Kopyrin and E. A. Simonova, 1981, Stal, 9, pp. 38-44 [5] Y. Kamel, T. Miyazaki, H. Yamaoka, 1993, ISIJ International, 33 , No.2, pp. 259-266 [6] X. Bi et al, 1993, Ironmaking and Steelmaking, 20, No. 6, pp.476-481 [7] P.A.Kravchenko et al, Steel in Translation, 38(2008), No. 9, pp. 764-760 [8] V.P. Vorob,ev, A. D. Godunov and V. Ignat,v, 2009, Steel in Translation, 39, No.3, pp.243-247 [9] R. Kononov, O. Ostrovski and Ganguly, 2009, ISIJ International, 49, No.8, pp. 1099- 1106 [10] V.Tathavadkar, V.Singh, P.K.Mishra, P. Mallick and B. D. Nanda, 2010, Ironmaking and steelmaking, 37, No. 2, pp.103-111. [11] Slag Atlas, 1981, Ed. Verein Deutscher Eisenhuttenleute, Verlag Staheisen GmbH, Dusseldorf, p. 282.
|