American Journal of Plant Sciences
Vol.08 No.09(2017), Article ID:78672,19 pages
10.4236/ajps.2017.89154
Evaluation of Cowpea Genotypes for Resistance to Fusarium redolens in Uganda
Roy Wanjala Namasaka1*, Geoffrey Tusiime1, Martin Orawu2, Paul Gibson1, Josiane Nyiramugisha1, Richard Edema1
1Department of Agricultural Production, Makerere University, Kampala, Uganda
2National Semi-Arid Resources Research Institute (NaSARRI), Soroti, Uganda

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 6, 2017; Accepted: August 21, 2017; Published: August 24, 2017
ABSTRACT
Fusarium redolens, a virulent fungus which causes damping off, leaf yellowing, wilting and root rots has recently been devastating cowpea fields in Uganda. This study aimed at identifying cowpea genotypes that are resistant to Fusarium redolens. Therefore, ninety cowpea genotypes were evaluated two times against a highly virulent Fusarium redolens (isolate from Zombo in Paidha district) in the screen house in 2016. Genotype effect was highly significant (P < 0.001) for root rot severity. Based on the Index of Susceptibility (IS), three genotypes (Asontem, Dan1 LA and IT89KD-88) remained resistant (IS < 3.5) over the two screening periods, 72 moderately resistant (3.5 ≤ IS < 6.5) and 11 susceptible (IS ≥ 6.5). Resistance was found to be enhanced by presence of lateral roots above or at the ground level. Further results suggested a difference in genetic control of resistance to root rots and seed rots caused by Fusarium redolens. All the released varieties tested (SECOW 1 T, SECOW 2 W, SECOW 3 B, SECOW 4 W and SECOW 5 T) had moderate resistance to Fusarium redolens. Correlation analysis revealed root rot severity was strongly correlated to disease incidence (+0.64, P < 0.001), to proportion of plants with lateral roots (−0.56, P < 0.001), to amount of leaf chlorophyll (−0.53, P < 0.001) and to proportion of plants that died prematurely due to Fusarium redolens infection (+0.45, P < 0.001). No significant correlation was detected between root rot severity and proportion of plants that germinated. The established resistance could be exploited for improvement of farmer preferred cowpea varieties towards Fusarium redolens resistance in Uganda.
Keywords:
Root Rot Severity, Lateral Roots, Seed Rots, Root Rots, Resistance, Index of Susceptibility

1. Introduction
Cowpea is the most important legume in the Eastern and Northern region of Uganda where both its leaves and grains are used as food [1] . Cowpea’s hardy nature against moisture stress and varying pH has enabled it to thrive in the drier regions of sub-Saharan Africa allowing resource poor farmers to reap both financial and nutritional benefits from it [2] [3] [4] . Its high protein content makes it an important source of protein in human and animal nutrition in the sub-Saharan region of Africa [4] . Moreover, cowpea’s nitrogen fixing ability is highly valuable when rotated or intercropped with other crops [5] .
Cultivation of cowpea in Uganda is constrained by many factors, among them cowpea root rots. These root rots are caused by a complex of pathogens including Fusarium redolens, F. cuneirostrum, F. oxysporum and Fusarium solani. Management of root rots is done following an integrated approach employing genetic resistance, cultural practices and chemical application [6] [7] [8] . While there are no known varieties resistant to cowpea root rots, fungicide application is associated with negative environmental, economic and health concerns that cannot be ignored [8] . Therefore, genetic resistance is the most sustainable strategy for managing root rots.
Root rot pathogens are known to be synergistic to one another [6] [9] [10] ; the more pathogens are involved in the complex, the more damage that is caused to the crop [9] . Genetic resistance to any one component pathogen in the complex reduces the damage caused to the crop. Therefore, breeding for resistance to any one or more fungal species in the complex causing cowpea root rot in Uganda will contribute greatly towards managing this disease. To successfully breed for host resistance, there is need to identify resistant materials to be used as parents. This study therefore aimed at screening cowpea genotypes against Fusarium redolens, a root rot causing pathogen that was found to be the most devastative to cowpea in greenhouse condition [11] . The resistant genotypes identified should be used by breeders to introgress resistance into other improved or preferred landraces that might be desirable but susceptible to root rots.
2. Materials and Methods
2.1. Plant Materials Used in the Study
A total of ninety cowpea genotypes were evaluated for resistance to F. redolens. These included landraces (58), crosses (11), cultivars (5), breeding lines from IITA-Uganda (7) and exotic materials (9). The exotic genotypes were obtained from Ghana and Nigeria (Table 1).
2.2. Description of the Site
This study was conducted at Makerere University Agricultural Research Institute, Kabanyolo (MUARIK), located in Wakiso district 25 km north of Kampala (32˚37'E, 0˚28'N) and at 1200 m above sea level. Day length at MUARIK is 12
Table 1. Description of Cowpea genotypes used in the study.
IITA = International Institute of Tropical Agriculture; NE = North eastern Uganda; WC = West and Central Uganda and MU = Makerere University. 1Ghanaian material; 2Nigerian material.
hours throughout the year and the daily temperatures range from 17˚C to 33˚C. The region receives an average annual rainfall of 1200 mm. The screen house environment was modified by constructing a chamber inside the screen house with a transparent polyethene sheet which increased the humidity and the temperature to provide conducive environment for pathogen multiplication.
2.3. Multiplication of Inoculum in the Soil
Paidha 19, a Fusarium redolens isolate was used to screen materials in this study. Inoculum was multiplied at the International Centre for Tropical Agriculture (CIAT) laboratory at Kawanda, Uganda. A modification of the method descri- bed by [7] was used. Two flasks (500 ml capacity) of inoculated millet were added and mixed thoroughly with pre-sterilized soil (3:1-loam:sand) in each of the six trays (1.5 m long, 0.1 m wide and 0.13 m high). The trays were covered with dark polythene sheet for a week to raise the soil temperature and provide conducive environment for pathogen multiplication in the soil. Seeds of a susceptible line (WC 66) were surface sterilized and planted in each tray for 28 days after which they were uprooted. This was repeated three times to ensure the trays had adequate inoculum. During the crop cycles, the trays were watered four days per week as described by [12] . After each cycle, the soil was removed from the trays and mixed thoroughly then redistributed equally before the test genotypes were planted.
2.4. Screening of Cowpea Genotypes for Resistance to F. redolens Isolate
The seed samples of the genotypes (90 cowpea genotypes) were surface sterilized to remove surface contaminants and planted in the six wooden trays containing soil with mature F. redolens inoculum. Planting was done following an alpha lattice design of 6 blocks × 15 genotypes with two and three replications for first and second trial respectively. It was designed in such a way that there were two blocks per tray with fifteen rows of the test genotypes per block. Each row had 15 plants per genotype in the first trial and 7 plants per genotype in the second trial. The trays were placed on raised benches in the screen house and watered four days per week as described by [12] .
2.5. Data Collection
First reaction to disease was assessed by counting the number of germinated plants per genotype 6 days after planting (DAP) and recording it as a percentage of the total seeds planted per genotype. This was used as a measure of F. redolens induced seed rot in each genotype. This was then followed by assessment of chlorophyll content using Photosynq [Soil Plant Analysis Development 3 (SP- AD 3)] to determine the level of leaf chlorosis observed for each genotype [13] [14] 27 DAP. On the 28th DAP, dead plants were counted per genotype and recorded as percentage of the total plants that died. Finally, on the 29th DAP the plants were carefully uprooted and below-ground parts of the plant (roots and hypocotyls) washed under running tap water and examined visually for root rot symptoms. Disease incidence was then recorded by counting the symptomatic plants while root rot severity was scored according to the C1AT 1 - 9 scale [15] . Figure 1 represents the different classification of the root rots as observed. Percentage of plants that developed lateral roots at or above the ground level were also recorded.
2.6. Data Analysis
The data collected on the reaction of the cowpea genotypes to the pathogen were subjected to Restricted Maximum Likelihood (ReML) analysis in Genstat 12th edition, to detect any significant genotype effect on reaction to F. redolens. Linear mixed model was used in the analysis with genotypes as fixed factors while replication and blocks were random factors Equation (1). Genotype means were separated using Fisher’s Protected Least Significant Difference (LSD) at significance level tested at P ≤ 0.05 [16] .
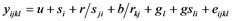 (1)
(1)
where 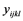 = observed value from each experimental unit, u = general mean,
= observed value from each experimental unit, u = general mean, 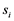 = effect of the ith trial,
= effect of the ith trial, 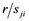 = Effect of jth replication within ith trial,
= Effect of jth replication within ith trial, 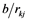
Figure 1. Photographic representation of the scale used to score root rot severity.
= effect of the kth block confounded in the jth replication, 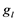 = effect of the lth genotype,
= effect of the lth genotype, 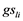 = effect of the lth genotype interacting with the ith trial and
= effect of the lth genotype interacting with the ith trial and 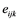 = the experimental error.
= the experimental error.
Correlation analysis of predicted means was performed in GENSTAT 12th edition between root rot severity, disease incidence, percentage of plants with lateral roots, percentage of plants that died, leaf chlorophyll level and percentage of plants that germinated to determine if there was a significant relationship between the traits at P ≤ 0.05.
The index of susceptibility (IS) was developed in Genstat 12th edition through multiple regression of five variables with root rot severity as the response variate and percentage lateral roots, disease incidence, amount of chlorophyll and percentage of dead plants as the explanatory variates Equation (2).
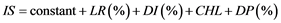 (2)
(2)
where, IS = index of selection, LR = lateral roots, DI = disease incidence, CHL = chlorophyll and DP = dead plants.
3. Results
3.1. Response of Ninety Cowpea Genotypes to F. redolens across Two Trials during 2016
Across trials results showed high significant differences in the mean performance of the genotypes when challenged with Fusarium redolens (Table 2). Genotype effects were significant (P ≤ 0.001) for percentage of plants that germinated, disease incidence, percentage lateral roots, amount of leaf chlorophyll and root rot severity. However, the genotype effect for percentage of plants that died as a result of the disease was non-significant at P = 0.05. The interactive effect of genotype by trial was highly significant (P ≤ 0.001) for percentage plant that germinated and level of leaf chlorophyll. Root rot severity, number of plants with lateral roots, disease incidence and percentage dead plants had no significant interaction with trial at P = 0.05. The resistant genotypes were observed to be distinctly different from the susceptible ones. These differences were mainly noticed in plant vigour, leaf chlorophyll and stunting (Figure 2).
Table 2. Mean of squares of different parameters of cowpea genotypes across first and second trials in 2016.
*, **, *** = significance at alpha 0.05, 0.01 and 0.001 respectively while ns = not significant.
Figure 2. Resistant vs susceptible genotypes to F. redolens.
3.2. Mean Performance of Cowpea Genotypes in Relation to Selected Parameters against F. redolens Infection during the First and Second Trials
Based on the Index of Susceptibility a clear difference was observed in the distribution of the various genotypes among the various resistant/susceptible clas- ses across the two trials (Table 3 and Figure 3). More genotypes were placed in resistant and intermediate classes compared to the susceptible classes in trial one when compared to the second trial. The genotypes were normally distributed across resistant/susceptible classes indicating genetic variability within the tested material though the class of moderately resistant genotypes was preponderant (Figure 3).
Table 3. Mean performance of selected cowpea genotypes in response to F. redolens infection across two trials.
R = resistant; I = intermediate and S = susceptible: First trial n = 90: second trial n = 86: trials 1, 2 and complete across trials predicted means table is available in Tables A1-A3 respectively; Resistant vs susceptible classification was based on index of susceptibility.
Moreover, a total of 3 genotypes (ASONTEM, Dan 1LA and IT89KD-288) were resistant (IS < 3.5) to F. redolens, 72 had intermediate resistance (3.5 < IS < 6.5) and 11 were susceptible (IS > 6.5) across the two trials (Figure 3). ASO- NTEM had the highest resistance with an index of susceptibility of 1.75 while NE 4 was the most susceptible (7.08). Released cultivars; SECOW 3 B (4.95), SECOW 5 T (5.36), SECOW 1 T (5.53) SECOW 2 W (5.62) and SECOW 4 W (6.00) had intermediate resistance to F. redolens (Table 3).
3.3. Correlation of Parameters Used to Evaluate the Genotypes against F. redolens Infection to Determine the Relationship between the Parameters
Correlations analysis of the variables (Table 4) displayed strong significant correlation of root rot severity to disease incidence (+0.64, P < 0.001), to percentage lateral roots (−0.56, P < 0.001), to amount of leaf chlorophyll (−0.53, P < 0.001) and to percentage dead plants (+45 < P < 0.001). However, percentage
Figure 3. Distribution of number of screened genotypes in the resistant/susceptible cla- sses in the two screening trials.
Table 4. Correlation of evaluated parameters across the two trials.
*, **, *** = significance at alpha 0.05, 0.01 and 0.001 respectively while ns = not significant.
germination had a negative but non-significant correlation to root rot severity and disease incidence but strongly significant correlation (−0.30, P < 0.001) to percentage dead plants. The percentage lateral roots had significant but weak correlation (+0.16, P < 0.05) to leaf chlorophyll, strongly significant correlation (−0.47, P < 0.001) to percentage dead plants and strongly significant correlation to percentage germination (+0.34, P < 0.001). In addition, amount of chlorophyll in the leaf had a negative and non-significant correlation to percentage dead plants and a strongly significant correlation (−0.27, P < 0.001) to percentage germination. On the other hand, percentage germination showed strong and significant correlation to percentage dead plants (−0.30, P < 0.001).
4. Discussion
In the two screening trials of cowpea genotypes to identify resistance to Fusarium redolens root rot, varying levels of symptoms were developed within the first two weeks after planting. These symptoms consisted of seed rot, damping off, leaf yellowing, root rots and premature senescence. The root rot caused by Fusarium redolens gradually increased from 14 days after planting.
In this study, the results showed highly significant effect (P ≤ 0.001) of cowpea genotypes reaction to F. redolens root rots across the two trials. A factor attributed to the presence of a considerable genetic diversity among the genotypes used. Genotypes by trial interaction was non-significant for percentage of dead plants, percentage lateral roots, disease incidence and root rot severity which implied that the genotypes performance in relation to these parameters was consistent from one trial to the next. Therefore, the expression of these traits were more independent of environmental influence and performance of the genotypes in one trial could be used to predict their performance in the other trials. Contrastingly, genotype by trial interaction for percentage germination and amount of chlorophyll were highly significant indicating the performance of the genotypes for these parameters were influenced by the environmental conditions. The expression of resistance to diseases results from the plant genetic makeup and the influence of the environment in which the plant is grown [17] . Plants that have been stressed by poor unfavourable growth conditions tend to be infected easily by Fusarium spp. [18] . In this study, modification of the environment as outlined by [6] ensured fast multiplication of the pathogen in the soil hence high initial inoculum which was essential for the high infection observed. The final levels of root rot severity observed was dependent on susceptibility/resistance of the genotypes, the environmental condition and the virulence levels of F. redolens isolate. The artificial environment created implies the genotypes that did not succumb to the infection had were resistant. Low soil fertility due to poor land management practices is a common factor that creates stress conditions to crops thus weakening them and increasing their susceptibility [6] . More so, infected soil debris are usually ploughed back into the soil therefore ensuring the inoculum is maintained. Both conditions were achieved in this experiment by using sterile soil without adding any nutrient for all the subsequent planting and ploughing the crop debris back in the soil.
Evaluation of resistance/susceptibility to Fusarium root rot has majorly been done by targeting a single trait (root rot severity). However, in this study, five susceptibility traits were cumulatively used to determine the resistance of the various genotypes to F. redolens. This is because resistance to root rot is complex response by the plant to the pathogen and which does not just depend on the root alone but other traits that come into play. Indeed [19] [20] documented the impact of more lateral roots and chlorophyll respectively in enhancing plant resistance to diseases. Thus emphasising the need to include such traits in the overall evaluation of resistance/susceptibility of the plant. Results from Index of Susceptibility (IS) across the two trials showed that 3 genotypes (ASONTEM, Dan 1LA and IT89KD-288) were resistant (IS < 3.5) to root rots, 72 genotypes moderately resistant (3.5 < IS < 6.5) and 11 were susceptible (IS ≥ 6.5). This outcome suggested that sources of resistance exist and can therefore be introgressed into susceptible genotypes especially in farmer preferred varieties. However, most of the resistance was observed from exotic genotypes. All Ugandan released varieties were moderately resistant to the pathogen. Noticeably, WC 67 A and WC 27 (Appendix) performed well compared to other local germplasm implying presence of some resistance to F. redolens in the landrace cowpea of Uganda. Despite the resistance of some of the genotypes to F. redolens, none of the genotypes was immune to the pathogens as indicated by the recorded incidence.
Several symptoms have been reported to be associated with Fusarium root rot of cowpea. From farmer field observations [11] and earlier studies [21] , F. redolens was observed causing seed rot leading to low germination, leaf yellowing, root rots and plant senescence in grain legumes. Similar symptoms were confirmed during screen house evaluation in this study thus corroborating the findings of [21] . To understand this relationship, this study investigated a number of symptoms in relation to infection by F. redolens. These symptoms included seed rot, leaf chlorosis, lateral root formation, and plant death. A clear understanding of the reaction of germplasm after infection with F. redolens is important in providing an insight of the host pathogen relationship to facilitate effort for breeding for resistance.
Pathogenic Fusarium spp. have been known to cause reduction in seed germination potential [22] . In this study, a highly significant variation (P ≤ 0.001) in the genotypes percentage germination was observed across the two trials. This revealed that individual genotypes had varying germination potential when challenged with the pathogen. In addition, there was high significant genotype by trial interaction in percentage germination which underlines that the environmental variations do influence the seed germination. For instance, the cold period experience in the first week of the second trial delayed germination thus extending the exposure time of the seed to the pathogen. Consequently, four genotypes failed to germinate. Similarly, [23] observed that seed-coat traits whi- ch restrict entry of fungi are influence by environmental changes. They further stated that prolonged exposure of the seeds to the pathogen due to cold induced dormancy increases the chance of infection. Furthermore, [24] , study on cowpea seed-coat revealed that seeds with rough texture have thin seed-coat compared to smooth walled seeds. This factor could have influenced susceptibility of Dan 1LA to seed rot despite its resistance to root rot. Contrary to the expectation, there seemed to be a very low negative and non-significant correlation between root rot severity and percentage of plants that germinated. Thus, some of the resistant lines (e.g. Dan 1LA-50.64%) had high seed rot while susceptible lines (e.g. WC 66% - 89.23%) had very low seed rot. More so, WC 44 recorded the highest resistance to seed rot (100% germination) across the two trials despite its intermediate resistance to root rot (4.96). In the second trial, the cross WC 32 × SECOW 5 failed to germinate due to seed rot despite its high resistance to root rot in the first trial. This suggested that resistance to seed rots due to F. redolens are controlled differently from resistance to root rots. Previous studies have indeed established that Fusarium related seed rots are highly influenced by the structure of the natural openings in the seed-coat which restrict or allow entry of the pathogen [23] . Applicability of this to cowpea is however yet to be established.
Leaf yellowing (chlorosis) is one of the main symptoms of F. redolens [21] . In this study, resistant plants remained relatively green thus retaining high chlorophyll in their leaves and stalks while susceptible plants turned yellow. There was a highly significant difference (P ≤ 0.001) among the genotypes for chlorophyll content across the two trials. However, the genotypes were not consistent in chlorosis observed as a response to the pathogen across the two trials as shown by the high significant (P ≤ 0.001) genotype by trial interaction. This could be due to infestation of mites in the second trial that might have influence the observed chlorosis. Correlation analysis of root rot severity with leaf chlorophyll content showed a highly significant (P ≤ 0.001) negative correlation thus confirming that chlorosis can be used to infer to the presence of the pathogen [21] .
Results from this study revealed that the genotypes had significantly different (P ≤ 0.001) ability to produce lateral roots. Besides, there was a highly significant (P ≤ 0.001) negative correlation between root rot severity and percentage of plants that produced lateral roots per genotype suggesting that genotypes with ability to produce lateral roots showed more resistance to F. redolens. The same genotypes with lateral roots producing ability seemed to have better survival mechanism even when infected. Previous studies have suggested that lateral roots are produced as a survival mechanism response to Fusarium infection [19] . Similar results were observed in this experiment when the base of the stem was completely cut by the root rot and the genotypes could still produce lateral roots which sustained them. In fact, some genotypes developed single lateral roots that were almost as strong as the tap root and would produce more lateral roots from them to sustain the plants. Indeed, [19] in their study confirmed that production of lateral roots do compensate for the function of the other infected roots.
According to [25] , Fusarium root rots have the ability to cause plant mortality and this was also observed in the present study in susceptible plants. Indeed, a positive and significant correlation was observed between root rot severity and dead plants. This substantiated that the deaths observed resulted from the pathogen infection. Though there was no statistical significant difference in the variation of percentage of plants that died across the genotypes, plants were observed to die prematurely as from 14 days after planting especially in the susceptible lines.
5. Conclusions and Recommendation
The study showed that there were resistant genotypes against F. redolens in Uganda. Three genotypes (ASONTEM, Dan 1LA and IT89KD-288) were resistant to F. redolens). The released cultivars evaluated (SECOW 2 W, SECOW 3 B, SECOW 4 W, SECOW 5 T and SECOW 1 T) were all moderately resistant to Fusarium redolens. Resistance in these varieties can be improved through bre- eding (both forward and back crosses) in order to ensure that qualities for which they were selected are retained. The study also showed that cowpea genotypes that produced lateral roots above or at the ground level were more resistant to F. redolens. In addition, the study has shown that resistance to Fusarium root rots and Fusarium seed rots may be conferred by separate genetic responses in cowpea. This makes seed from some F. redolens root rot resistant lines to succumb to rot.
Field evaluation studies are recommended as it is essential to evaluate the stability of this resistance in varying environments especially under severe stresses to avoid disease escapes. The landraces WC 67 A and WC 27 had relatively low IS thus they can easily be accepted for release if tested in for stability and adaptability to other agronomic traits.
Acknowledgements
We acknowledge the Alliance for a Green Revolution in Africa (AGRA) for the full financial support of the study and Makerere University Agricultural Research Institute, Kabanyolo (MUARIK) administration, for allowing the research to be conducted in their laboratory and screen house.
Cite this paper
Namasaka, R.W., Tusiime, G., Orawu, M., Gibson, P., Nyiramugisha, J. and Edema, R. (2017) Eva- luation of Cowpea Genotypes for Resistance to Fusarium redolens in Uganda. Ame- rican Journal of Plant Sciences, 8, 2296- 2314. https://doi.org/10.4236/ajps.2017.89154
References
- 1. Ronner, E. and Giller, K.E. (2012) Background Information on Agronomy, Farming Systems and Ongoing Projects on Grain Legumes in Uganda. N2 Africa Milestones. Accessed, 34.
http://www.N2Africa.org - 2. Bisikwa, J., Ekere, W., Kawooya, R., Biruma, M. and Okello, D. (2014) Farmers Guide to Sustainable Cowpea Production in Uganda.
- 3. Mwale, S.E., Ochwo-Ssemakula, M., Sadik, K., Achola, E., Okul, V., Gibson, P., Edema, R. and Rubaihayo, P. (2017) Response of Cowpea Genotypes to Drought Stress in Uganda. American Journal of Plant Sciences, 8, 720-733.
https://doi.org/10.4236/ajps.2017.84050 - 4. Badiane, F.A., Gowda, B.S., Cissé, N., Diouf, D., Sadio, O. and Timko, M.P. (2012) Genetic Relationship of Cowpea (Vigna unguiculata) Varieties from Senegal Based on SSR Markers. Genetics and Molecular Research, 11, 292-304.
https://doi.org/10.4238/2012.February.8.4 - 5. Timko, M.P., Ehlers, J.D. and Roberts, P.A. (2007) Cowpea. Pulses, Sugar and Tuber Crops, 49-67.
- 6. Agrios, G.N. (2005) Plant Pathology. 5th Edition. Elsevier Academic Press, Cambridge, Massachusetts, 8.
- 7. Mukankusi, C.M., Melis, R.J., Derera, J., Buruchara, R.A. and Mark, D. (2011) A Screening Technique for Resistance to Fusarium Root Rot of Common Bean. African Journal of Plant Science, 5, 152-161.
- 8. Pottorff, M., Wanamaker, S., Ma, Y.Q., Ehlers, J.D., Roberts, P.A. and Close, T.J. (2012) Genetic and Physical Mapping of Candidate Genes for Resistance to Fusarium oxysporum f. sp. Tracheiphilum Race 3 in Cowpea. PLoS One, 7, e41600.
https://doi.org/10.1371/journal.pone.0041600 - 9. de Jensen, E.C. (2000) Etiology and Control of Dry Bean Root Rot in Minnesota. INIAP Archivo Historico.
- 10. Tusiime, G. (2003) Variation and Detection of Fusarium solani f. sp. Phaseoli and Quantification of Soil Inoculum in Common Bean Fields. Ph.D. Thesis, Makerere University, Kampala.
- 11. Namasaka, R.W. (2017) Inheritance of Resistance to Fusarium Root Rot (Fusarium redolens) Disease of Cowpea in Uganda. Master’s Thesis, Makerere University, Kampala.
- 12. Mugisha, C.M. (2008) Improving Resistance to Fusarium Root Rot [Fusarium solani (Mart.) Sacc. f. sp. phaseoli (Burkholder) WC Snyder & HN Hans.] in Common Bean (Phaseolus vulgaris L.). Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg.
- 13. Lombard, K., O’Neill, M., Mexal, J., Ulery, A., Onken, B., Bettmann, G. and Heyduck, R. (2010) Can Soil Plant Analysis Development Values Predict Chlorophyll and Total Fe in hybrid Poplar? Agroforestry Systems, 78, 1-11.
https://doi.org/10.1007/s10457-009-9214-1 - 14. Gholizadeh, A., Soom, M., Amin, M., Abdul Rahim, A. and Wayayok, A. (2011) Using Soil Plant Analysis Development Chlorophyll Meter for Two Growth Stages to Assess Grain Yield of Malaysian Rice (Oryzasativa). American Journal of Agricultural and Biological Sciences, 6, 209-213.
https://doi.org/10.3844/ajabssp.2011.209.213 - 15. Abawi, G.S. and Corrales, M.A.P. (1990) Root Rots of Beans in Latin America and Africa: Diagnosis, Research Methodologies, and Management Strategies (No. 35) CIAT.
- 16. Popoola, A.R., Aluko, C.T., Afolabi, C.G. and Uzochukwu, S.V.A. (2013) Rapid Screening of In-Vitro Regenerated Plantlets of Four Nigerian Cowpea Varieties to Artificially-Inoculated Fungal Wilt Pathogen. Nigerian Journal of Biotechnology, 25, 12-17.
- 17. Fehr, W.R. (1987) Principles of Cultivar Development, Volume 1, Theory and Technique. Macmillan Publishing Company, London.
- 18. Armstrong, G.M. and Armstrong, J.K. (1980) Cowpea wilt Fusarium oxysporum f. sp. Tracheiphilum Race I from Nigeria. Plant Disease, 64, 954-955.
https://doi.org/10.1094/PD-64-954 - 19. Snapp, S., Kirk, W., Román-Avilés, B. and Kelly, J. (2003) Root Traits Play a Role in Integrated Management of Fusarium Root Rot in Snap Beans. Hort Science, 38, 187-191.
- 20. Thomas, H. and Ougham, H. (2014) The Stay-Green Trait. Journal of Experimental Botany, 65, 3889-3900.
https://doi.org/10.1093/jxb/eru037 - 21. Jiménez-Fernández, D., Navas-Cortés, J.A., Montes-Borrego, M., Jiménez-Díaz, R.M. and Landa, B.B. (2011) Molecular and Pathogenic Characterization of Fusarium redolens, a New Causal Agent of Fusarium Yellows in Chickpea. Plant Disease, 95, 860-870.
https://doi.org/10.1094/PDIS-12-10-0946 - 22. Aigbe, S.O. and Fawole, B. (2009) First Report of a Cowpea Seed Rot Caused by Fusarium Compactum in Nigeria. American-Eurasian Journal of Sustainable Agriculture, 3, 388-392.
- 23. Souza, F.H. and Marcos-Filho, J.ú.L.I.O. (2001) The Seed Coat as a Modulator of Seed-Environment Relationships in Fabaceae. Brazilian Journal of Botany, 24, 365-375.
https://doi.org/10.1590/S0100-84042001000400002 - 24. Lush, W.M. and Evans, L.T. (1980) The Seed Coats of Cowpeas and Other Grain Legumes: Structure in Relation to Function. Field Crops Research, 3, 267-286.
https://doi.org/10.1016/0378-4290(80)90034-9 - 25. Rodrigues, A.A.C. and Menezes, M. (2005) Identification and Pathogenic Characterization of Endophytic Fusarium Species from Cowpea Seeds. Mycopathologia, 159, 79-85.
https://doi.org/10.1007/s11046-004-7138-x
Appendix
Table A1. First trial means of 90 genotypes for the six susceptibility traits to F. redolens.
Table A2. Means of 86 genotypes for the six traits evaluated during second trial.
R = resistant; I = intermediate and S = susceptible: IS = Index of susceptibility; NB: Four genotypes failed to germinate and were therefore excluded from the overall analysis.
Table A3. Means of 86 genotypes for the six traits across two trials.
R = resistant; I= intermediate and S = susceptible; First trial n = 90: second trial n = 86: IS = Index of Susceptibility; NB: Four genotypes failed to germinate in the second trial and were therefore excluded from the overall analysis.





