Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 10, No.15, pp.1379-1389, 2011 jmmce.org Printed in the USA. All rights reserved 1379 Growth, Structural, Optical, Mechanical and Dielectric Characterization of Diammonium Hydrogen Phosphate (DAHP) Single Crystals P. Selvarajan a* , N. Joseph John b and C.K. Mahadevan c a Department of Physics, Aditanar College of Arts and Science, Tiruchendur-628216, India. b Department of Physics, Malankara Catholic College, Mariagiri, Tamil Nadu- 629153, India c Physcis Research Centre, S.T. Hindu College, Nagercoil-629003, Tamil Nadu, India. *Corresponding Author: pselvarajanphy@yahoo.co.in ABSTRACT Solubility studies of Diammonium Hydrogen Phosphate(DAHP) in de-ionized water were performed at various temperatures in the range 30-50 o C. Single crystals of DAHP were grown from aqueous solutions by slow evaporation technique. Structural characterization of the grown crystals has been carried out by powder and single crystal X-ray diffraction (XRD) methods. DAHP crystals crystallize in monoclinic structure with the space group P2 1 /c. Second harmonic generation (SHG) for the material of this work was confirmed using Nd: YAG laser. The UV-Visible spectrum show that the grown crystals have wide optical transparency in the entire visible region. The dielectric measurements were made for grown DAHP crystal at the frequencies of 100 Hz and 1 KHz and in the temperature range 30-150 ° C. Vickers microhardness value of DAHP crystal is found to increase with increase in load. Value of density of the grown DAHP crystal has been measured and it is well matched with the data of XRD studies. MCCCLXXIX Keywords: Optical Materials; Solubility; Crystal Growth; XRD; hardness; dielectric constant; SHG; 1. INTRODUCTION The search for new conversion materials for various device applications has led to discovery of many organic, inorganic and semi-organic Nonlinear Optical (NLO) materials, which have potential applications in optoelectronics, Second Harmonic Generation (SHG), optical storage, optical communication, photonics, electro-optic modulation, optical parametric amplifiers, optical image processing, etc [1-4]. The salts of phosphate group such as  1380 P. Selvarajan , N. Joseph John Vol.10, No.15 Disodium Hydrogen Phosphate (DSHP), Trisodium Hydrogen Phosphate (TSHP) and Potassium Dihydrogen Phosphate (KDP) are reported to be NLO materials [5, 6]. Diammonium Hydrogen Phosphate (DAHP ) crystal belongs to the phosphate group and the hydrated nature of this crystal creates hydrogen bonding and this leads to have good optical properties. Solid DAHP loses ammonia readily on heating, for this reason it has been little studied at elevated temperatures. Many ammonium salts have characteristic transition temperatures for a wide temperature range [7]. X-ray studies of the title compound were reported by Smith et al and Khan et al [8, 9]. Watton et al had carried out NMR and thermal studies of DAHP sample and reported that the material undergoes characteristic phase transitions, resulting in dramatic changes in many physical properties as temperature is changed [10]. Rotational Echo Double Resonance (REDOR) measurements on polycrystalline sample of diammonium hydrogen phosphate were reported by Gil Goobes et al [11]. Since few properties of DAHP crystals have been reported in the literature, it is planned to carry out a systematic study on various properties of the material in this work. Hence, the aim of this paper is to report the results of growth, solubility studies, structural, microhardness, UV-Visible-NIR transmittance studies of DAHP crystals. Also measurements of density, melting point, dielectric constant, dielectric loss of the grown crystals of DAHP are reported and discussed. 2.EXPERIMENTAL PROCEDURE 2.1 Experimental for Solubility and Growth The commercially available Analar Reagent (AR) grade sample of Diammonium Hydrogen Phosphate (DAHP) was purchased from Merck India and it was further purified by repeated re-crystallization process for three times using de-ionzed water as the solvent. In order to obtain crystals of high quality, purification of starting material was found to be an important step and hence the re-crystallized salt was used for the growth of crystals of DAHP. Before the growth of single crystals of DAHP, solubility study was carried out using a hot- plate magnetic stirrer and a digital thermometer. A voltage regulator was attached with hot- plate magnetic stirrer in order to maintain the temperature constant (here accuracy is + 0.1 o C ). Initially, the temperature was maintained at 30 o C. The re-crystallized salt of DAHP was added step by step to 50 ml of de-ionized water in an air-tight container kept on the hot-plate magnetic stirrer and stirring was continued till a small precipitate was formed. This gave confirmation of supersaturated condition of the solution. Then, 25 ml of the solution was pipetted out and taken in a petri dish and it was warmed up till the solvent was evaporated out. By measuring the amount of salt present in the petri dish, the solubility ( in g/100 ml) of DAHP in de-ionized water was determined gravimetrically [12]. The same procedure was followed to find solubility of DAHP sample at other temperatures such as 35, 40 , 45 and 50 o C. Single crystals of DAHP were grown by solution method with slow solvent evaporation technique at room temperature (30 o C). In accordance with the solubility data, the saturated solution of the re-crystallized salt of DAHP was prepared and it was constantly stirred for 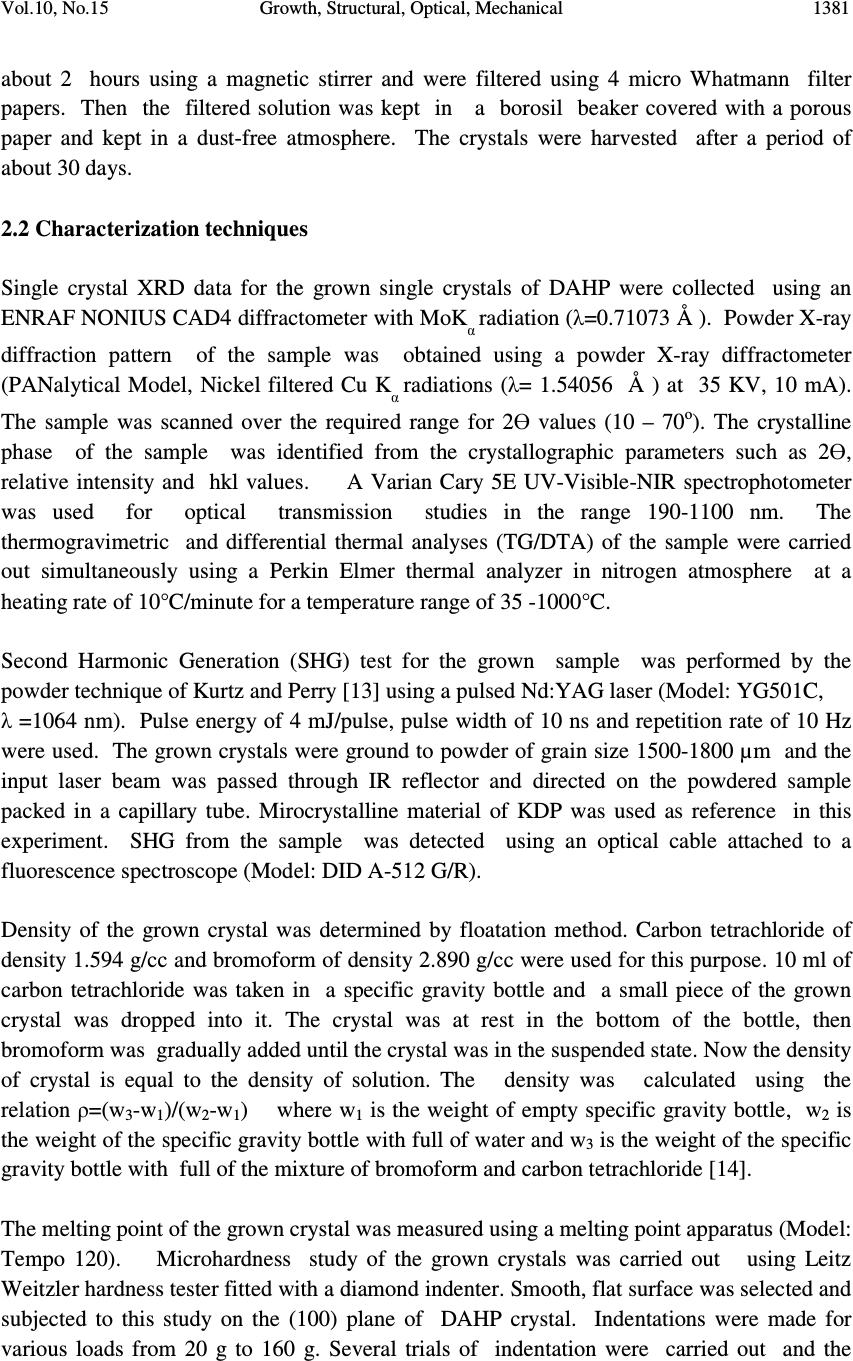 Vol.10, No.15 Growth, Structural, Optical, Mechanical 1381 about 2 hours using a magnetic stirrer and were filtered using 4 micro Whatmann filter papers. Then the filtered solution was kept in a borosil beaker covered with a porous paper and kept in a dust-free atmosphere. The crystals were harvested after a period of about 30 days. 2.2 Characterization techniques Single crystal XRD data for the grown single crystals of DAHP were collected using an ENRAF NONIUS CAD4 diffractometer with MoK α radiation (λ=0.71073 Å ). Powder X-ray diffraction pattern of the sample was obtained using a powder X-ray diffractometer (PANalytical Model, Nickel filtered Cu K α radiations (λ= 1.54056 Å ) at 35 KV, 10 mA). The sample was scanned over the required range for 2Ө values (10 – 70 o ). The crystalline phase of the sample was identified from the crystallographic parameters such as 2Ө, relative intensity and hkl values. A Varian Cary 5E UV-Visible-NIR spectrophotometer was used for optical transmission studies in the range 190-1100 nm. The thermogravimetric and differential thermal analyses (TG/DTA) of the sample were carried out simultaneously using a Perkin Elmer thermal analyzer in nitrogen atmosphere at a heating rate of 10°C/minute for a temperature range of 35 -1000°C. Second Harmonic Generation (SHG) test for the grown sample was performed by the powder technique of Kurtz and Perry [13] using a pulsed Nd:YAG laser (Model: YG501C, λ =1064 nm). Pulse energy of 4 mJ/pulse, pulse width of 10 ns and repetition rate of 10 Hz were used. The grown crystals were ground to powder of grain size 1500-1800 µm and the input laser beam was passed through IR reflector and directed on the powdered sample packed in a capillary tube. Mirocrystalline material of KDP was used as reference in this experiment. SHG from the sample was detected using an optical cable attached to a fluorescence spectroscope (Model: DID A-512 G/R). Density of the grown crystal was determined by floatation method. Carbon tetrachloride of density 1.594 g/cc and bromoform of density 2.890 g/cc were used for this purpose. 10 ml of carbon tetrachloride was taken in a specific gravity bottle and a small piece of the grown crystal was dropped into it. The crystal was at rest in the bottom of the bottle, then bromoform was gradually added until the crystal was in the suspended state. Now the density of crystal is equal to the density of solution. The density was calculated using the relation ρ=(w 3 -w 1 )/(w 2 -w 1 ) where w 1 is the weight of empty specific gravity bottle, w 2 is the weight of the specific gravity bottle with full of water and w 3 is the weight of the specific gravity bottle with full of the mixture of bromoform and carbon tetrachloride [14]. The melting point of the grown crystal was measured using a melting point apparatus (Model: Tempo 120). Microhardness study of the grown crystals was carried out using Leitz Weitzler hardness tester fitted with a diamond indenter. Smooth, flat surface was selected and subjected to this study on the (100) plane of DAHP crystal. Indentations were made for various loads from 20 g to 160 g. Several trials of indentation were carried out and the  1382 P. Selvarajan , N. Joseph John Vol.10, No.15 average diagonal lengths were measured for an indentation time of 10 seconds. The Vickers micro hardness number was calculated using the relation H v = 1.8544 P/d kg/mm 2 where P is the applied load and d is the diagonal length of the indentation impression [15]. A sample crystal having a thickness about 4 mm was polished for dielectric measurements. Opposite faces of the sample crystal was coated with good quality silver paste to obtain a good ohmic contact with the electrodes. The capacitance and dielectric loss factor (tan δ) were measured using the conventional two probe technique at frequencies of 100 Hz and 1 KHz and in the temperature range 30-150 o C using an LCR meter (Model APLAB) The dielectric constant of the crystal was calculated using the relation (as the crystal area was smaller than the plate area of the cell). where C crys is the capacitance with crystal (including air), C air is the capacitance of air, A crys is the area of the crystal touching the electrode and A air is the area of the electrode[16, 17] 3. RESULTS AND DISCUSSION 3.1 Solubility and Growth Finding solubility of a material in a solvent is a useful analysis to grow bulk single crystals from solution. The size of a crystal depends on the amount of material available in the solution which in turn is decided by the solubility of the material in that solvent. To grow bulk crystals from solution growth technique, selection of solvent is an important step. To select a suitable solvent, the solubility of DAHP in different solvents such as water, ethanol and methanol were determined. Solubility is very low in ethanol and methanol and it is high in de-ionized water. Hence de-ionized water was used as solvent in this work. Solubility curve for DAHP sample at various temperatures ranging from 30 to 50 o C is shown in Fig. 1. Here solubility is defined as the amount of solute in grams present in 100 ml of saturated solution at a particular temperature and it corresponds to saturation between a solid and its solution at given temperature and pressure. It is observed from the results that the solubility increases with temperature for the sample. Since solubility increases with temperature, the sample has positive temperature coefficient of solubility. Hence DAHP crystals can be grown by slow evaporation method. The harvested crystals of DAHP are displayed in Fig. 2. The grown crystals are found stable, non-hygroscopic at ambient temperature, colourless, transparent and have well defined appearance. Since the temperature was not completely kept constant during the growth of the crystals in the present work, there are slight morphological changes among the grown crystals. The dimensions of the biggest DAHP crystal harvested within a period of 30 days is about 15 x 12 x 6 mm 3 . C crys – C air ( 1 – A crys /A air ) C air ε r = (A air ) (A crys )  Vol.10, No.15 Growth, Structural, Optical, Mechanical 1383 3.2 Single Crystal and Powder XRD Studies Single crystal XRD studies for grown DAHP crystal were carried out using single crystal X-ray diffractometer with graphite monochormated MoK α radiation and the structure was solved by single crystal XRD analysis by direct method and refined by the full matrix-least- square technique using SHELXL program. The obtained data from single crystal XRD studies are presented in the table 1. From the data, it is observed that the grown crystals crystallize in monoclinic system with the space group P2 1 /c . The number of molecules per unit cell (Z) for DAHP crystal is found to be 4. The unit cell parameters of DAHP crystals obtained in this work are very close agreement with reported work[8]. Powder XRD pattern of DAHP crystal is shown in Fig. 3. The well-defined peaks at specific 2Ө values show high crystallinity of the grown crystals. All the reflections of powder XRD pattern of the sample were indexed using the TREOR software package following the procedure of Lipson and Steeple [18]. The (hkl) planes satisfy the general reflection conditions of space group observed from the structure determination of the crystal. The obtained lattice parameters from powder XRD studies using UNITCELL software package are observed to be in close agreement with data obtained from single crystal XRD studies. Fig.1: Variation of solubility with temperature for DAHP Fig.2: Harvested DAHP crystals 25 30 35 40 45 50 50 55 60 65 70 Solubility in grams ( 100 ml ) Temperature( o C)  1384 P. Selvarajan , N. Joseph John Vol.10, No.15 Fig.3: Powder XRD pattern of DAHP crystals 3.3 Measurement of Density and Melting Point The value of density of DAHP crystal was measured by floatation method and it was found to be 1.6183 g/cc. This value is well matched with the reported value [19] . The density was also calculated from the crystallographic XRD data using the relation ρ=(MZ)/(NV) where M is the molecular weight of DAHP crystal, Z is the number of molecules per unit cell, N is Avogadro’s number and V is volume of the unit cell and it was found to be 1.620 g/cc. Using the melting point apparatus, the value of melting point of DAHP crystal was found to be 202 °C . The values of density is in good agreement with the data obtained from XRD studies. Table 1 : Single crystal XRD data for DAHP crystal Chemical formula ( NH4)2 H PO4 Molecular weight 132.05 Crystal system Monoclinic Space group P21/c a ( Ǻ) 10.923(4) b ( Ǻ) 6.645(2) c (Ǻ) 8.041(2) α 90 ο β 112.06 ο γ 90 ο V (Ǻ)3 540.91(3) Z 4 Density 1.620 g/cc  Vol.10, No.15 Growth, Structural, Optical, Mechanical 1385 3.5 UV-Visible Transmittance Studies Usually an NLO material must have a wide transparency window and this could be checked by recording an absorption or transmittance spectrum of the sample in UV-visible- IR region. The optical transmittance spectrum of DAHP crystal in the wavelength range 190-1100 nm is recorded and it is shown in Fig. 4. The study of the absorption edge is essential in connection with the theory of electronic structure, which leads to the prediction of whether the band structure is affected near the band extreme. From the transmittance spectra, it is noticed that DAHP crystal has high transmittance in the entire visible-NIR region of the spectrum. The high transmission in the entire visible region and short cut off wavelength facilitates the grown crystal to be a potential nonlinear optical material for second harmonic and third harmonic of Nd:YAG laser [20]. A sharp fall in the transmittance is observed at 196 nm for the grown DAHP crystal and this corresponds to the fundamental absorption. Absorption in the near ultraviolet region arises from electronic transitions associated within the sample. Using the formula E g = 1240 / λ (nm), the band gap of the sample is calculated to be 6.326 eV. Fig.4: UV-Visible-NIR transmittance spectrum for DAHP crystal 3.6 Microhardness Studies Microhardness studies for the grown DAHP crystal were performed at room temperature (30 o C) to determine microhardness number and hence the mechanical strength and this study plays an important role in the fabrication of opto-electronic devices. The hardness of a material is a measure of its resistance to plastic deformation. In an ideal crystal, the hardness value should be independent of applied load. But in a real crystal, the load dependence is observed. This is due to normal indentation size effect (ISE) [21]. The variation of microhardness number (H V ) with load applied to the plane (100) of the sample is displayed in Fig. 5 and it is noticed that Vickers hardness number (H v ) increases with  1386 P. Selvarajan , N. Joseph John Vol.10, No.15 the applied load satisfying the indentation size effect. The maximum value of microhardness of DAHP crystal is observed to be 68.55 kg/ mm 3 at 160 g , beyond this load significant cracking occurs which may be due to the release of internal stresses produced within the indentation [22]. Fig.5: Variation of microhardness number with load for DAHP crystal 3.7 Dielectric Characterization The dielectric parameters like dielectric constant (ε r ) and dielectric loss ( (tan δ) ) are the basic electrical properties of solids. The measurement of dielectric constant and loss as a function of frequency and temperature gives the ideas of electrical processes that are taking place in material and these parameters were measured on (100) face of the sample. Variations of dielectric constant and dielectric loss of DAHP crystal at frequencies 100 Hz and 1 KHz and in the temperature range 30- 150 o C are displayed in Figs. 6 and 7. The results suggest that the dielectric constant and loss strongly depend on the frequency of a.c. signal and the temperature of the sample. It is observed from the figures that both dielectric constant and loss are found to be more at 100 Hz than those at 1 KHz and observed to be increased when temperature of DAHP crystal is increased. The high values of dielectric constant and loss at 100 Hz are ascribed to space charge polarization. The dielectric constant of a material is known to consist of contributions from electronic, ionic, dipolar and space change polarizations, each dominating in a particular frequency range. It is well established that the space charge polarization is very predominant at lower frequencies. This polarization is known to arise from charged defects present and also due to the creation and distribution of dipoles either within the bulk or at the surface of the crystal. The dipolar orientational effect can sometimes be seen in some materials up to 10 10 Hz. The ionic and electronic polarizations always exist below 10 13 Hz. The nature of decrease of ε r and (tan δ) with frequency suggests that the grown DAHP crystal seem to contain dipoles of continuously 020406080100120 140 160 180 40 45 50 55 60 65 70 Hardness number (kg/mm 2 ) Load (g) 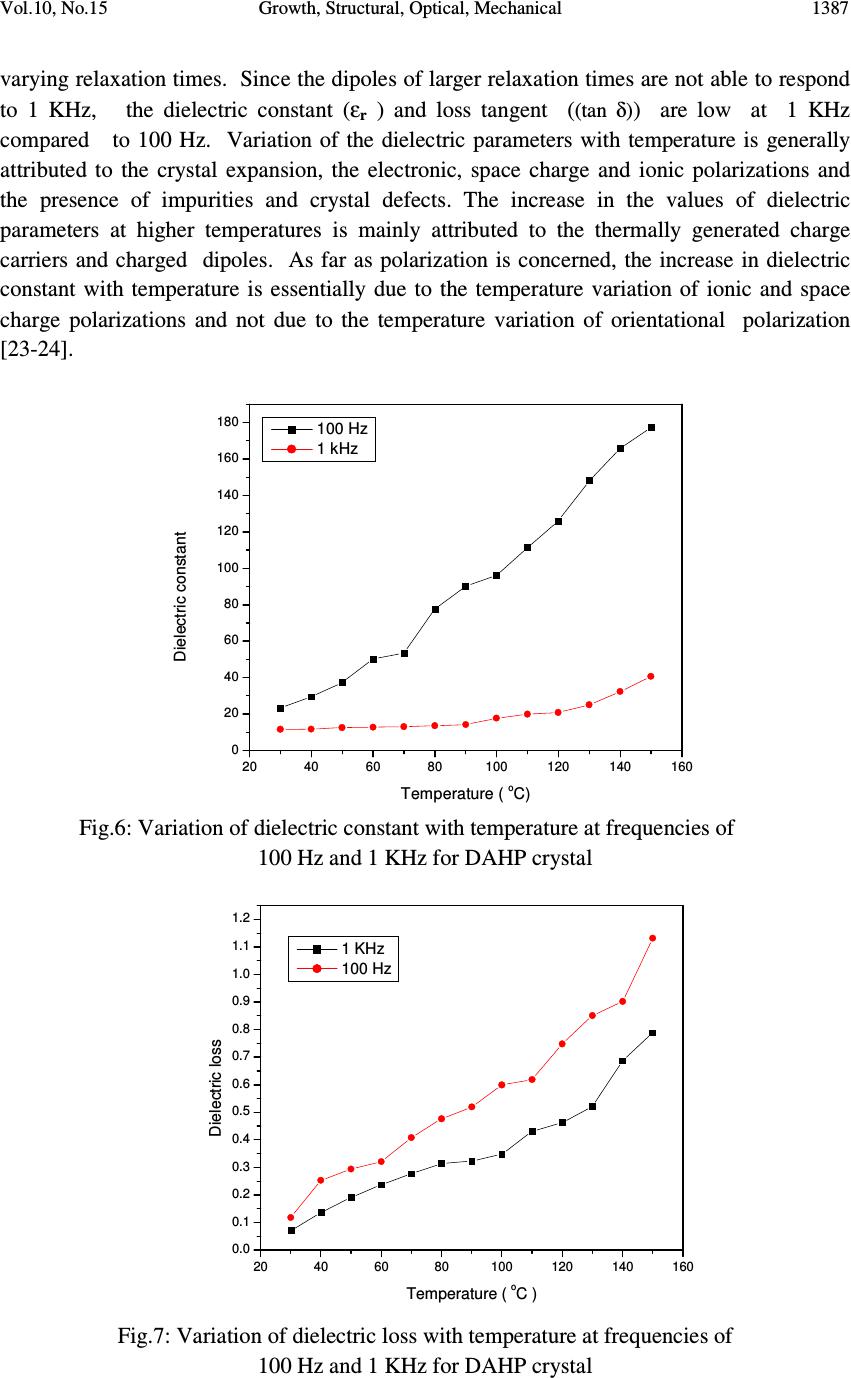 Vol.10, No.15 Growth, Structural, Optical, Mechanical 1387 varying relaxation times. Since the dipoles of larger relaxation times are not able to respond to 1 KHz, the dielectric constant (ε r ) and loss tangent ( (tan δ) ) are low at 1 KHz compared to 100 Hz. Variation of the dielectric parameters with temperature is generally attributed to the crystal expansion, the electronic, space charge and ionic polarizations and the presence of impurities and crystal defects. The increase in the values of dielectric parameters at higher temperatures is mainly attributed to the thermally generated charge carriers and charged dipoles. As far as polarization is concerned, the increase in dielectric constant with temperature is essentially due to the temperature variation of ionic and space charge polarizations and not due to the temperature variation of orientational polarization [23-24]. Fig.6: Variation of dielectric constant with temperature at frequencies of 100 Hz and 1 KHz for DAHP crystal Fig.7: Variation of dielectric loss with temperature at frequencies of 100 Hz and 1 KHz for DAHP crystal 20406080100 120 140 160 0 20 40 60 80 100 120 140 160 180 Dielectric constant Temperature ( o C) 100 Hz 1 kHz 20406080100 120 140 160 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 Dielectric loss Temperature ( o C ) 1 KHz 100 Hz  1388 P. Selvarajan , N. Joseph John Vol.10, No.15 3.8 Second Harmonic Generation (SHG) Test To confirm the nonlinear optical property of sample, powder form of DAHP crystals was subjected to NLO test obeying the Kurtz and Perry powder method [13] and Second Harmonic Generation (SHG) was confirmed by emission of green light (λ =532 nm). From the study, it was observed that the SHG relative efficiency of DAHP sample was found to be about 0.9 times as that of KDP. The good optical transmittance in the visible–NIR region and SHG conversion efficiency makes the grown crystal a potential material for device applications in optoelectronics. 4. CONCLUSIONS Solubility of Diammonium Hydrogen Phosphate (DAHP) in de-ionized water was determined at various temperatures and optical quality single crystals of DAHP were grown by solution growth method with slow solvent evaporation technique. The grown crystals are transparent and with well defined external appearance. Single crystal and powder X-ray diffraction (XRD) methods reveal that the structure of the grown DAHP crystal belongs to monoclinic. The values of density and melting point of DAHP crystal are measured to be 1.618 g/cc and 202 o C respectively. It is observed that the measured value of density of DAHP crystal by floatation method is well coincided with the density calculated from XRD data. UV-visible-NIR transmittance spectral studies for DAHP crystal show that the material has good optical transmittance in the entire visible region and low cut-off wavelength and hence it may be suitable for device fabrication in optoelectronics. Dielectric constant and dielectric loss tangent (tan δ) measurements were carried out for the grown DAHP crystal at frequencies of 100 Hz and 1 KHz and in the temperature range 30 - 150 o C and these values show the normal behaviour of insulating-type materials like DAHP. Vickers microhardness study shows that the DAHP crystal has high hardness values, revealing the reasonable mechanical strength of the material. The relative SHG efficiency of the grown sample was found to be about 0.9 times as that of KDP. ACKNOWLEDGEMENTS The authors are thankful to the staff members of IIT, Chennai, M.K.University, Madurai, Regional Research Laboratory, Trivananthapuram, Crescent Engineering College, Chennai for helping us to carry out various studies of the grown crystals of DAHP. Also the authors like to thank the management authorities of Aditanar College of Arts and Science, Tiruchendur and S.T. Hidnu College, Nagercoil for the constant encouragement to carry out the research in crystal growth. REFERENCE [1] S.B. Monaco, L.E. Davis, S.P. Velsko, F.T. Wang, D. Eimerl, A.J. Zalkin J. Crystal Growth 85 (1987) 252.  Vol.10, No.15 Growth, Structural, Optical, Mechanical 1389 [2] K. Meera, R. Muralidharan, R. Dhanasekaran, Prapun Manyum, P. Ramasamy J. Crystal Growth 263 (2004) 510. [3] C. Justin Raj, S. Dinakaran, S. Krishnan, B. Milton Boaz, R. Robert, S. Jerome Das Optics Commun. 281 (2008) 2285. [4] K. Ambujam , K. Rajarajan , S. Selvakumar , J. Madhavan, Gulam Mohamed , P. Sagayaraj Optical Mater. 29 (2007) 657. [5] M.Gunasekaran, N.Vijayan, R.Ramesh Babu, R.Gopalakrishnan, P.Ramasamy J. Crystal Growth 244(2002) 194. [6] N.Joseph John, P.Selvarajan, S. Sylvia, C.K.Mahadevan Mat.Manufact.Process. 22 (2007) 379. [7] R.Navil Kumar and C.P.G. Vallaban J.Phys.Condens. Matter 1(1989) 6095. [8] J.P.Smith, J.R.Lehr, W.E.Brown Acta Crystallogr. 10 (1957) 50. [9] A.A.Khan, J.P.Roux, W.J.James Acta Crystallogr. B28(1978) 2065. [10] A.Watton, E.C.Reynhardt, H.S. Sandhu, H.E.Petch J.Chem. Phys. 67(1977)887. [11] G.Goobes , Vinodhkumar Raghunathanm Elizabeth A.Louie, James M.Gibson, Gregory L. Olsen, Gary P.Drobny Sold State Nucl. Mag. Resonance 29(2006) 242. [12] P. Selvarajan, A. Siva dhas , T.H. Freeda, C.K. Mahadevan, Physica B 403(2008)4205. [13] S.K. Kurtz , T.T. Perry J.Appl. Phys.39 (1968) 3798. [14] C. Krishnan, P. Selvarajan, T.H. Freeda J. Crystal Growth 311 (2008)141. [15] K.Kishan Rao, V.Surender and B.Saritha Rani Bull.Mater.Sci. 25(2002)641. [16] C. Krishnan , P. Selvarajan , T.H. Freeda , C.K. Mahadevan Physica B 404 (2009) 289. [17] S. Krishnan, C. Justin Raj, S. M. Navis Priya, R. Robert, S. Dinakaran, and S. Jerome Das Cryst. Res. Technol. 43, No. 8 (2008) 845. [18] H. Lipson, H. Steeple, Interpretation of X-ray powder Diffraction Patterns, Fifth Ed, Macmillan, NewYork (1970) , p.165. [19] J.A.Dean, Lange’s Hand book of Chemistry, 12 th edn., McGraw Hill , New York (1979), p.4-16. [20] T. Balakrishnan, K. Ramamurthi Spectrochimica Acta Part A 72 (2009) 269. [21] J.B. Charles, F.D. Gnanam J. Mater. Sci. Lett. 12 (1993) 1395. [22] N. Theresita Shanthi, P. Selvarajan, C.K. Mahadevan, Curr. Appl. Phys. 9(2009)1155. [23] K. V. Rao and A. Smakula J. Appl. Phys. 36(1965) 2031. [24] P. Selvarajan, B.N. Das, H.B. Gon and K.V. Rao, J. Mater. Sci. 29 (1994) 4061. |

