Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 10, No.12, pp.1103-1110, 2011 jmmce.org Printed in the USA. All rights reserved Growth and Characterization of Non-Linear Optical Material G.R. Dillip a , C. Madhukar Reddy a , B. Deva Prasad Raju b,* a Department of Physics, Sri Venkateswara University, Tirupati - 517 502, India. b Department of Future Studies, Sri Venkateswara University, Tirupati - 517 502, India. * Corresponding Author: drdevaprasadraju@gmail.com ABSTRACT An organic nonlinear optical (NLO) single crystal has been synthesized by slow solvent evaporation technique from aqueous solutions of L-serine and sodium fluoride (NaF) at ambient temperature. The grown crystal was confirmed by single crystal X-ray diffraction analysis. The powder X-ray diffraction of the grown crystal was recorded and indexed. The functional groups of the grown crystals were determined by FTIR spectrum. The optical absorption study reveals that the transparency of the crystal in the entire visible region and the lower edge was found to be 258 nm. Relative powder second harmonic generation (SHG) efficiency of the grown crystal was tested by Kurtz and Perry powder technique using high intensity Nd:YAG laser operating at 1064 nm. Keywords: Nonlinear optical material; Solution growth; Optical absorption; SHG. 1. INTRODUCTION New nonlinear optical (NLO) materials, structures and devices with enhanced figure of merit has been developed over the past few decades as a major force to help drive nonlinear optics from the laboratory to real applications. They have wide range of applications that include optical storage communications and optical computing systems. The sprouting field of NLO research and applications still requires new materials for a large variety of processes. Now a days, the most widely used NLO crystal for SHG of Nd:YAG laser is KTP. However, many problems associated with the use of KTP crystals in terms of changes in the refractive, optical damage and hygroscopic. Therefore, research for new NLO crystals is important [1-3]. In order to understand the microscopic origin of nonlinear behavior of organic NLO materials, considerable theoretical and experimental investigations have been done. NLO material capable of frequency conversion is generally composed of an electron donor (D), an acceptor (A) and a conjugated π - system as a bridge providing the electronic communication between the donor and acceptor [4, 5]. The advantages offered by organic over inorganic systems include high electronic susceptibility through high molecular polarizability (β), fast response time, facile modification through standard synthesis method 1103  1104 G.R. Dillip, C. Madhukar Reddy, B. Deva Prasad Raju Vol.10, No.12 and relative ease of device processing. Even though organic nonlinear optical materials with aromatic ring have been attracting much attention because of their high nonlinearity, fast response and high optical damage threshold, their practical applications are limited due to poor mechanical, thermal stabilities and the inability to produce and process large crystals [6]. Many donor-acceptor complexes exhibiting high second order nonlinearity have been reported [7-10]. Amino acids and their complexes belong to a family of organic materials that have been considered for photonic applications. Photonic crystals, which prohibit the propagation of light for frequencies within a band gap, have enabled exciting new ways to control and construct integrated optical devices. L-serine is an organic compound under amino acid category. It is one of the naturally occurring protenogenic amino acids [11, 12]. L-serine exists in zwitterionic form; the molecule can combine with anionic, cationic and overall neutral constituents. In this article, we report the growth, structural, vibrational, optical and second harmonic generation properties of the grown crystal. 2. MATERIAL SYNTHESIS The titled compound was grown by slow solvent evaporation solution growth technique. Commercially available analytical grade (AR) L-serine (99%) and sodium fluoride (99%) supplied by MERCK, India were taken in 1:1 stoichiometric ratio as starting materials without further purification to synthesize the title compound. The measured amounts of these materials were dissolved in the deionized water of resistivity 18.2 M Ω cm -1 and the solution was filtered by Whatman filter paper of pore size 11µm. Then the filtered solution is kept at a dustless environment and optimally closed for controlled evaporation. The purity of the synthesized salt was further improved by successive recrystallization process. Transparent single crystals of size 9×8×7 mm 3 are harvested in a period of 25 days. The grown single crystals are shown in Fig. 1. Fig. 1: The grown NLO crystal 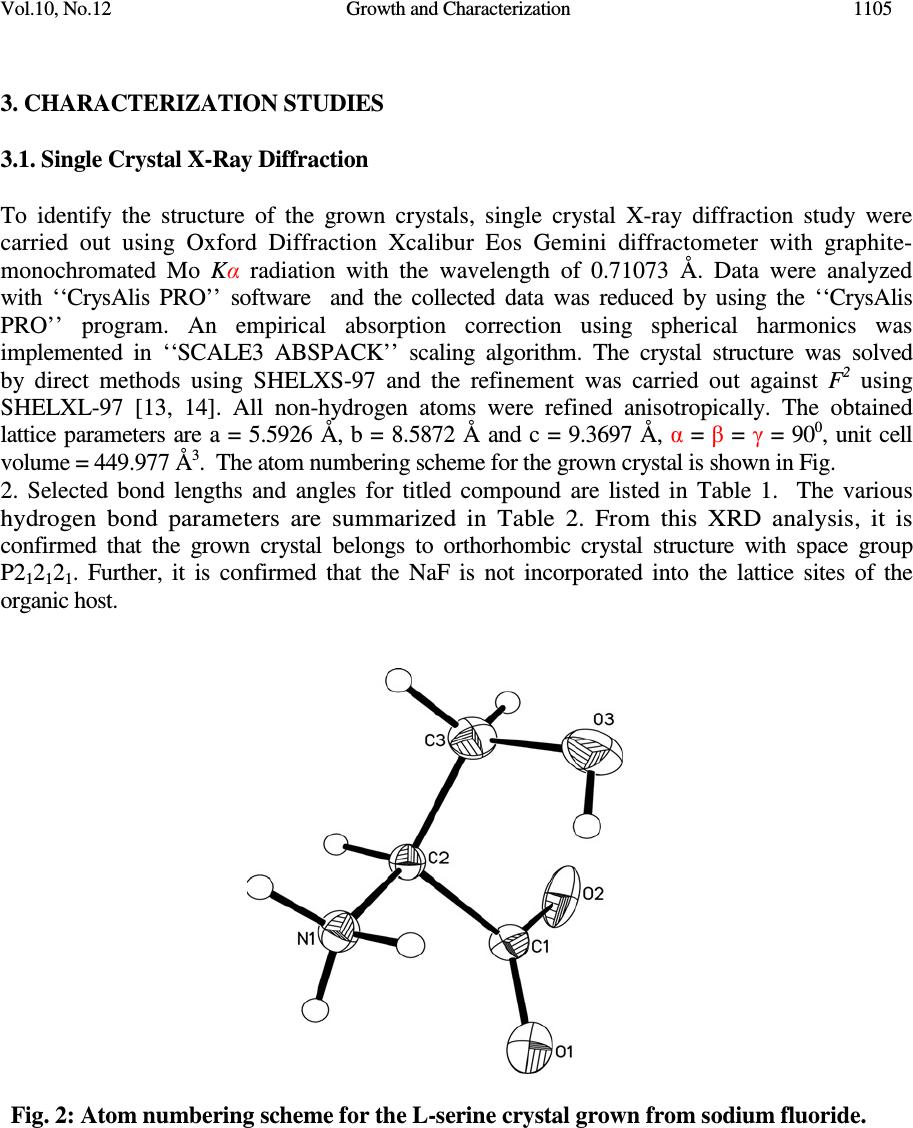 Vol.10, No.12 Growth and Characterization 1105 3. CHARACTERIZATION STUDIES 3.1. Single Crystal X-Ray Diffraction To identify the structure of the grown crystals, single crystal X-ray diffraction study were carried out using Oxford Diffraction Xcalibur Eos Gemini diffractometer with graphite- monochromated Mo K α radiation with the wavelength of 0.71073 Å. Data were analyzed with ‘‘CrysAlis PRO’’ software and the collected data was reduced by using the ‘‘CrysAlis PRO’’ program. An empirical absorption correction using spherical harmonics was implemented in ‘‘SCALE3 ABSPACK’’ scaling algorithm. The crystal structure was solved by direct methods using SHELXS-97 and the refinement was carried out against F 2 using SHELXL-97 [13, 14]. All non-hydrogen atoms were refined anisotropically. The obtained lattice parameters are a = 5.5926 Å, b = 8.5872 Å and c = 9.3697 Å, α = β = γ = 90 0 , unit cell volume = 449.977 Å 3 . The atom numbering scheme for the grown crystal is shown in Fig. 2. Selected bond lengths and angles for titled compound are listed in Table 1. The various hydrogen bond parameters are summarized in Table 2. From this XRD analysis, it is confirmed that the grown crystal belongs to orthorhombic crystal structure with space group P2 1 2 1 2 1 . Further, it is confirmed that the NaF is not incorporated into the lattice sites of the organic host. Fig. 2: Atom numbering scheme for the L-serine crystal grown from sodium fluoride. 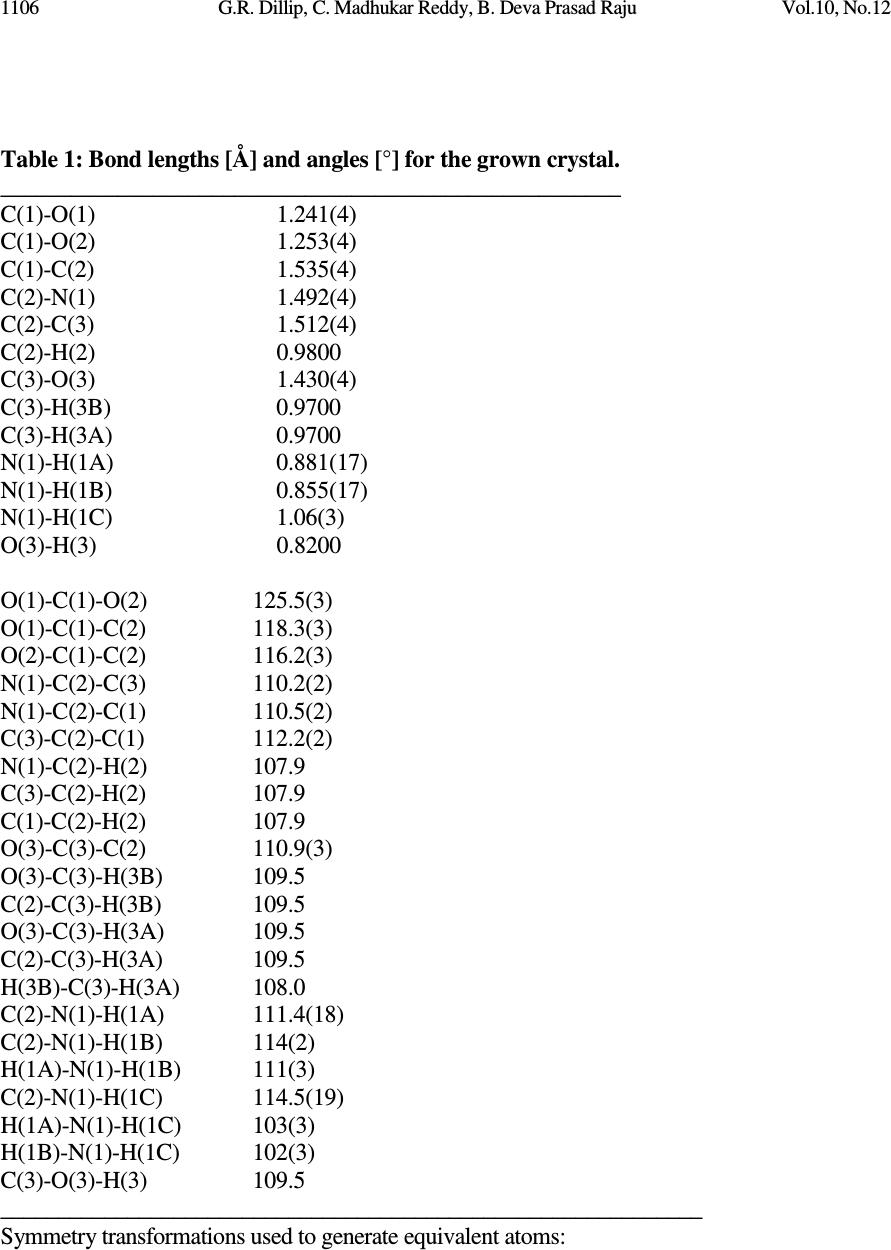 1106 G.R. Dillip, C. Madhukar Reddy, B. Deva Prasad Raju Vol.10, No.12 Table 1: Bond lengths [Å] and angles [°] for the grown crystal. _____________________________________________________ C(1)-O(1) 1.241(4) C(1)-O(2) 1.253(4) C(1)-C(2) 1.535(4) C(2)-N(1) 1.492(4) C(2)-C(3) 1.512(4) C(2)-H(2) 0.9800 C(3)-O(3) 1.430(4) C(3)-H(3B) 0.9700 C(3)-H(3A) 0.9700 N(1)-H(1A) 0.881(17) N(1)-H(1B) 0.855(17) N(1)-H(1C) 1.06(3) O(3)-H(3) 0.8200 O(1)-C(1)-O(2) 125.5(3) O(1)-C(1)-C(2) 118.3(3) O(2)-C(1)-C(2) 116.2(3) N(1)-C(2)-C(3) 110.2(2) N(1)-C(2)-C(1) 110.5(2) C(3)-C(2)-C(1) 112.2(2) N(1)-C(2)-H(2) 107.9 C(3)-C(2)-H(2) 107.9 C(1)-C(2)-H(2) 107.9 O(3)-C(3)-C(2) 110.9(3) O(3)-C(3)-H(3B) 109.5 C(2)-C(3)-H(3B) 109.5 O(3)-C(3)-H(3A) 109.5 C(2)-C(3)-H(3A) 109.5 H(3B)-C(3)-H(3A) 108.0 C(2)-N(1)-H(1A) 111.4(18) C(2)-N(1)-H(1B) 114(2) H(1A)-N(1)-H(1B) 111(3) C(2)-N(1)-H(1C) 114.5(19) H(1A)-N(1)-H(1C) 103(3) H(1B)-N(1)-H(1C) 102(3) C(3)-O(3)-H(3) 109.5 _____________________________________________________________ Symmetry transformations used to generate equivalent atoms:  Vol.10, No.12 Growth and Characterization 1107 Table 2: Hydrogen coordinates ( × 10 4 ) and isotropic displacement parameters (Å 2 ×10 3 ) for the grown crystal. __________________________________________________________________________ x y z U(eq) __________________________________________________________________________ H(2) 6620 5190 7936 27 H(3B) 5332 4991 5576 41 H(3A) 7997 4469 5705 41 H(1A) 9290(30) 3240(40) 8040(30) 41 H(1B) 7550(50) 2780(40) 9070(20) 41 H(1C) 7670(60) 1860(40) 7710(40) 48(11) H(3) 5841 2053 5981 68 __________________________________________________________________________ 3.2. Powder X-Ray Diffraction Analysis A sample in powder form was subjected to X-ray diffraction study by employing a SIEFERT 3003 TT diffractometer with a characteristic Cu K α ( λ =1.540598Å) radiation from 20° to 60° at a scan rate of 2°/min. Powder XRD profile of the grown crystal is shown in Fig. 3. The appearance of sharp and strong peaks confirmed the good crystallinity nature of the grown sample. The estimated lattice parameters of the grown crystal are agreed well with the data available in JCPDS file no: 27-1989. The slight shift in the sharp peak position at 22.87° in lower angle side may be due to the addition of sodium fluoride in the crystal and it is also confirmed by the slight variation observed in lattice parameters of the grown crystal. Fig. 3: Powder XRD profile of the grown crystal 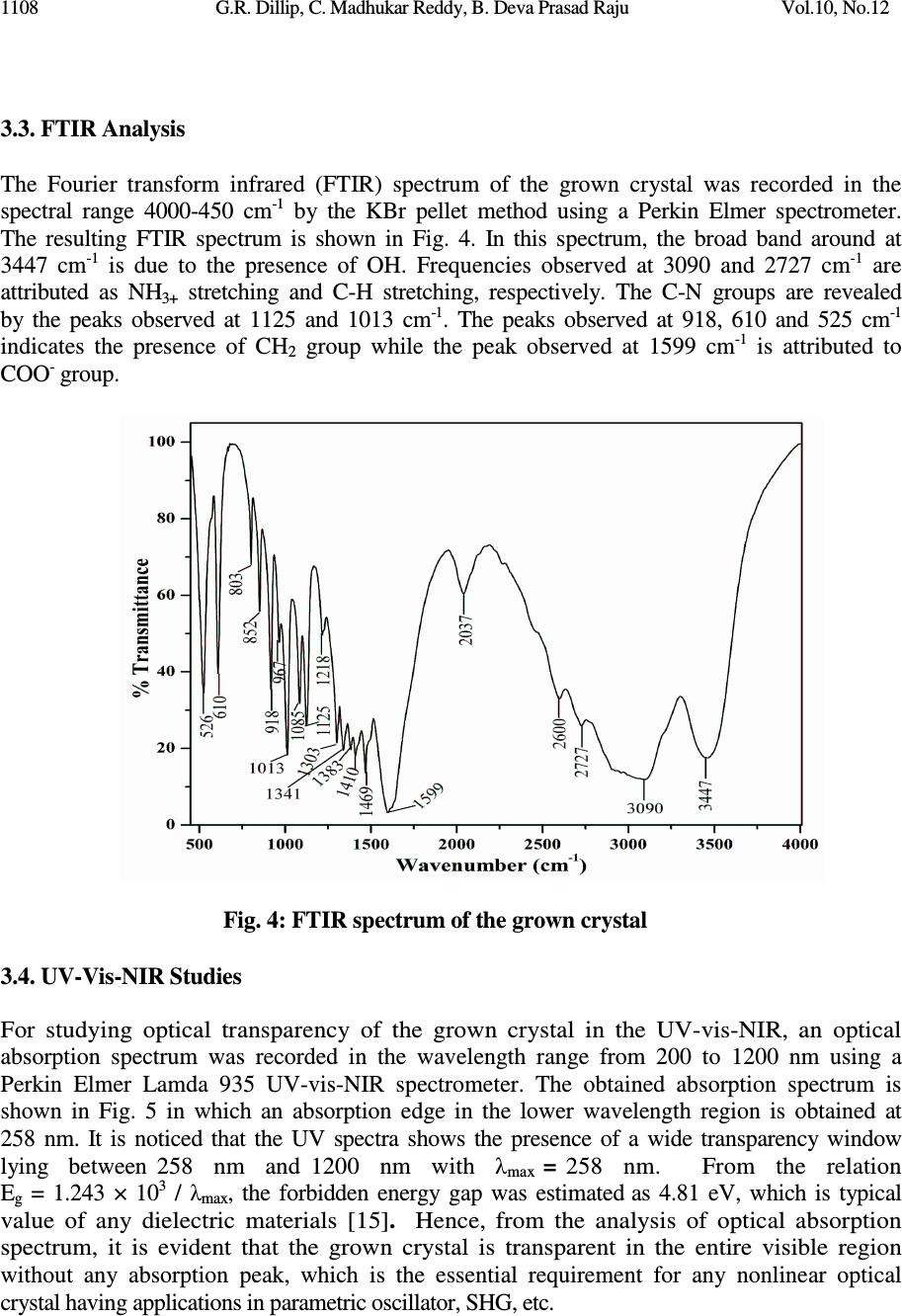 1108 G.R. Dillip, C. Madhukar Reddy, B. Deva Prasad Raju Vol.10, No.12 3.3. FTIR Analysis The Fourier transform infrared (FTIR) spectrum of the grown crystal was recorded in the spectral range 4000-450 cm -1 by the KBr pellet method using a Perkin Elmer spectrometer. The resulting FTIR spectrum is shown in Fig. 4. In this spectrum, the broad band around at 3447 cm -1 is due to the presence of OH. Frequencies observed at 3090 and 2727 cm -1 are attributed as NH 3+ stretching and C-H stretching, respectively. The C-N groups are revealed by the peaks observed at 1125 and 1013 cm -1 . The peaks observed at 918, 610 and 525 cm -1 indicates the presence of CH 2 group while the peak observed at 1599 cm -1 is attributed to COO - group. Fig. 4: FTIR spectrum of the grown crystal 3.4. UV-Vis-NIR Studies For studying optical transparency of the grown crystal in the UV-vis-NIR, an optical absorption spectrum was recorded in the wavelength range from 200 to 1200 nm using a Perkin Elmer Lamda 935 UV-vis-NIR spectrometer. The obtained absorption spectrum is shown in Fig. 5 in which an absorption edge in the lower wavelength region is obtained at 258 nm. It is noticed that the UV spectra shows the presence of a wide transparency window lying between 258 nm and 1200 nm with λ max = 258 nm. From the relation E g = 1.243 × 10 3 / λ max , the forbidden energy gap was estimated as 4.81 eV, which is typical value of any dielectric materials [15] . Hence, from the analysis of optical absorption spectrum, it is evident that the grown crystal is transparent in the entire visible region without any absorption peak, which is the essential requirement for any nonlinear optical crystal having applications in parametric oscillator, SHG, etc. 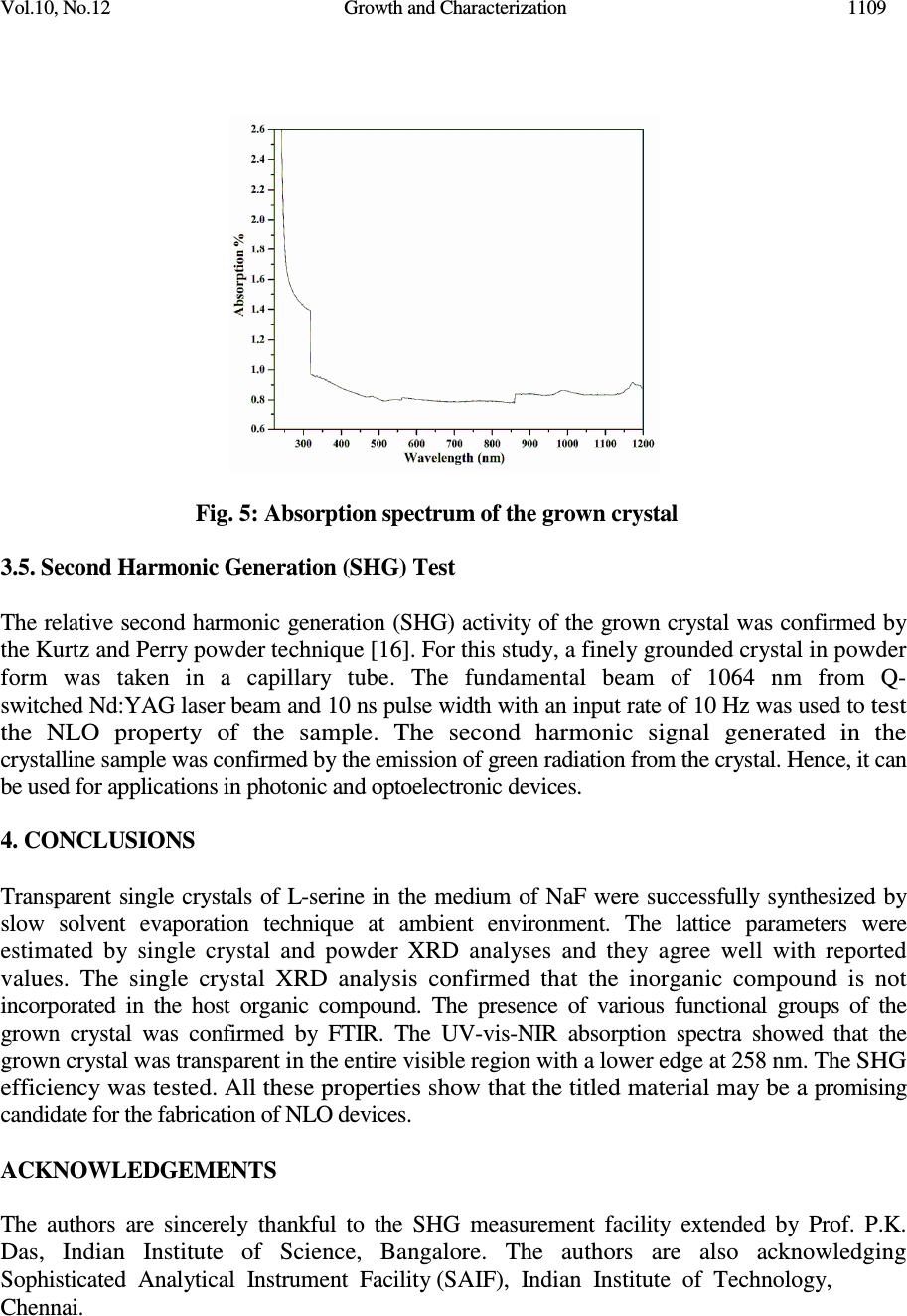 Vol.10, No.12 Growth and Characterization 1109 Fig. 5: Absorption spectrum of the grown crystal 3.5. Second Harmonic Generation (SHG) Test The relative second harmonic generation (SHG) activity of the grown crystal was confirmed by the Kurtz and Perry powder technique [16]. For this study, a finely grounded crystal in powder form was taken in a capillary tube. The fundamental beam of 1064 nm from Q- switched Nd:YAG laser beam and 10 ns pulse width with an input rate of 10 Hz was used to test the NLO property of the sample. The second harmonic signal generated in the crystalline sample was confirmed by the emission of green radiation from the crystal. Hence, it can be used for applications in photonic and optoelectronic devices. 4. CONCLUSIONS Transparent single crystals of L-serine in the medium of NaF were successfully synthesized by slow solvent evaporation technique at ambient environment. The lattice parameters were estimated by single crystal and powder XRD analyses and they agree well with reported values. The single crystal XRD analysis confirmed that the inorganic compound is not incorporated in the host organic compound. The presence of various functional groups of the grown crystal was confirmed by FTIR. The UV-vis-NIR absorption spectra showed that the grown crystal was transparent in the entire visible region with a lower edge at 258 nm. The SHG efficiency was tested. All these properties show that the titled material may be a promising candidate for the fabrication of NLO devices. ACKNOWLEDGEMENTS The authors are sincerely thankful to the SHG measurement facility extended by Prof. P.K. Das, Indian Institute of Science, Bangalore. The authors are also acknowledging Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology, Chennai.  1110 G.R. Dillip, C. Madhukar Reddy, B. Deva Prasad Raju Vol.10, No.12 REFERENCES 1. D.S. Chemla, J. Zyss, Nonlinear optical properties of organic molecules and crystals, Academic Press, Wiley-Interscience, New York, 1987. 2. P.N. Prasad, D.J. Williams, Introduction to nonlinear optical effects in molecules and polymers, Wiley-Interscience, New York, 1991. 3. C.H. Bosshard, M. Bosch, I. Liakatas, M. Jager, P. Gunter, Springer series in Optical Science, Berlin, Heideberg, New York, 2000. 4. L. Li, Z. Wang, X. Song, S. Sun, Spectrochim. Acta Part A 72 (2009) 816. 5. K. Naseema, Vijayalakshmi Rao, K.V. Sujith, Balakrishna Kalluraya, Curr. App. Physics 10 (2010) 1236. 6. S. Singh, B. Lal, J. Cryst. Growth 312 (210) 301. 7. M.N. Bhat, S.M. Dharmaprakash, J. Cryst. Growth 242 (2002) 245. 8. T. Pal, T. Karand, B. Gabriel, L. Rigi, Cryst. Growth Des. 4 (2004) 743. 9. T. Raghavalu, G.R. Kumar, S.G. Raj, V. Mathivanan, R. Mohan, J. Cryst. Growth 307 (2007) 112. 10. M.R.S. Kumar, H.J. Ravindra, A. Jayarama, S.M. Dharmaprakash, J. Cryst. Growth 286 (2006) 451. 11. S. Moitra, T. Kar, J. Cryst. Growth 310 (2008) 4539. 12. T.U. Deva, N. Lawrence, R.R. Babu, K. Ramamurthi, J. Cryst. Growth 310 (2008) 116. 13. Oxford Diffraction, CrysAlis PRO, Oxford Diffraction Ltd, Yarnton, England, 2009. 14. G.M. Sheldrick Acta Cryst. A 64 (2008) 112. 15. G.R. Dillip, P. Raghavaiah, K. Mallikarjuna, C.M. Reddy, G. Bhagavannarayana, V.R. Kumar, B.D.P. Raju, Spectrochim. Acta Part A 79 (2011) 1123. 16. S.K. Kurtz, T.T. Perry, J. App. Physics 39 ( 1968) 3798. |

