Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 10, No.10, pp.888-903, 2011 jmmce.org Printed in the USA. All rights reserved 888 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel in Marine Environment 1 O.O. Daramola, 2 B.O. Adewuyi and 2 I.O. Oladele 1 Ajaokuta Steel Company Limited, Ajaokuta Steel City, Kogi State, Nigeria 2 Metallurgical and Materials Engineering Department, Federal University of Technology, Akure, Ondo State, Nigeria. 1 Corresponding Author: ojaythompsoms@yahoo.com Phone Number: 2348166814002 ABSTRACT Investigation were carried out to study the corrosion behaviour of heat treated rolled medium carbon steel and as-rolled medium carbon steel in sodium chloride medium. The as-rolled medium carbon steel was heated to a temperature of 830 o C to completely austenize it and water quenched; it was reheated to the ferrite-austenite dual phase region at a temperature of 745 o C below the effective Ac 3 point. The steel was then rapidly quenched in water and tempered at a temperature of 480 o C. The corrosion behaviour of the steel in marine medium (NaCl) was studied by weight loss measurement. The weight loss is between 0.02g-0.11g for the as-rolled steel and 0.01g – 0.013g for the heat treated steel. The results obtained showed that the as-rolled medium carbon steel is more susceptible to corrosion than the heat treated rolled medium carbon steel. Keywords: Corrosion, Medium carbon steel, sodium chloride. 1. INTRODUCTION Corrosion is the destruction of a metal by its reaction with the environment; this reaction is an electrochemical oxidation process that usually produces rust or other metal oxide [1]. Structural steel is used in most metal water front structures because it is strong, readily available, easily fabricated and not excessively costly. The steel is normally used for such accessories as bitts, bollards, cleats, and chocks [2]. There are many types of marine corrosion that can occur to steel water front structure (e.g. galvanic corrosion, stray current, differential environment,  Vol.10, No.10 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel 889 erosion corrosion, and biological corrosion) and many methods for corrosion control. Heat treatment of structural steel is one of the way of improving its resistance to corrosion. Heat treatment involves the application of heat to a material to obtain desired material properties (e.g. Mechanical, corrosion, electrical, magnetic e-t-c) [3]. During the heat treatment process, the material usually undergoes phase microstructural and crystallographic changes and this has effect on the corrosion, mechanical and electrical properties of the steel [4]. Rolled medium carbon steel products are produced through a forming process called rolling. The process is carried out in a rolling mill which consist of a complex machine for deforming metal in rotary rolls and performing auxiliary operations such as transportation of stock to rolls, disposal after rolling, cutting, cooling, piling or coiling e-t-c [5]. To study the corrosion behaviour of heat treated rolled medium carbon steel and the as-rolled steel in marine medium (NaCl); 24 specimens were prepared from the as-rolled medium carbon steel sample; 12 out of the specimens were heat-treated. The corrosion susceptibility of the heat treated specimens and as-rolled specimens were investigated. The objective of this work is to investigate the effects of heat treatment on the corrosion susceptibility of rolled medium carbon steel. 2. MATERIALS AND METHODS The material used in this study was 12mm diameter rolled medium carbon steel. The spectrometric analysis of the steel was carried out; The Chemical compositions from the analysis are shown in Table 1. Twenty four specimens were prepared from this material using lathe machine; Twelve out of these specimens were heat treated. The corrosion test and metallographic examination of the as-rolled and heat treated specimens were carried out. 2.1.Determination of Operating Temperature The lower critical temperature (AC 1 ) and upper critical temperature (AC 3 ) were determined by Grange empirical formula [6] as presented here. AC 1 ( o C) = (1333 – 25Mn + 40Si +42Cr– 26 Ni)- (32) 5/9 ---------------1.1 AC 3 ( o C) = (1570 – 323C – 25Mn + 80Si – 3Cr – 32Ni) – (32) 5/9 –----1.2 Table 1. Chemical Composition of As – Rolled Medium Carbon Steel C Si Mn P S Cr Mo Ni Al Co Cu Nb Ti 0.353 0.290 0.987 0.050 0.057 0.071 0.005 0.11 0.025 0.015 0.185 0.005 0.0037 V W Pb Sn Zn Fe 0.0057 0.010 0.005 0.026 0.0076 97.805 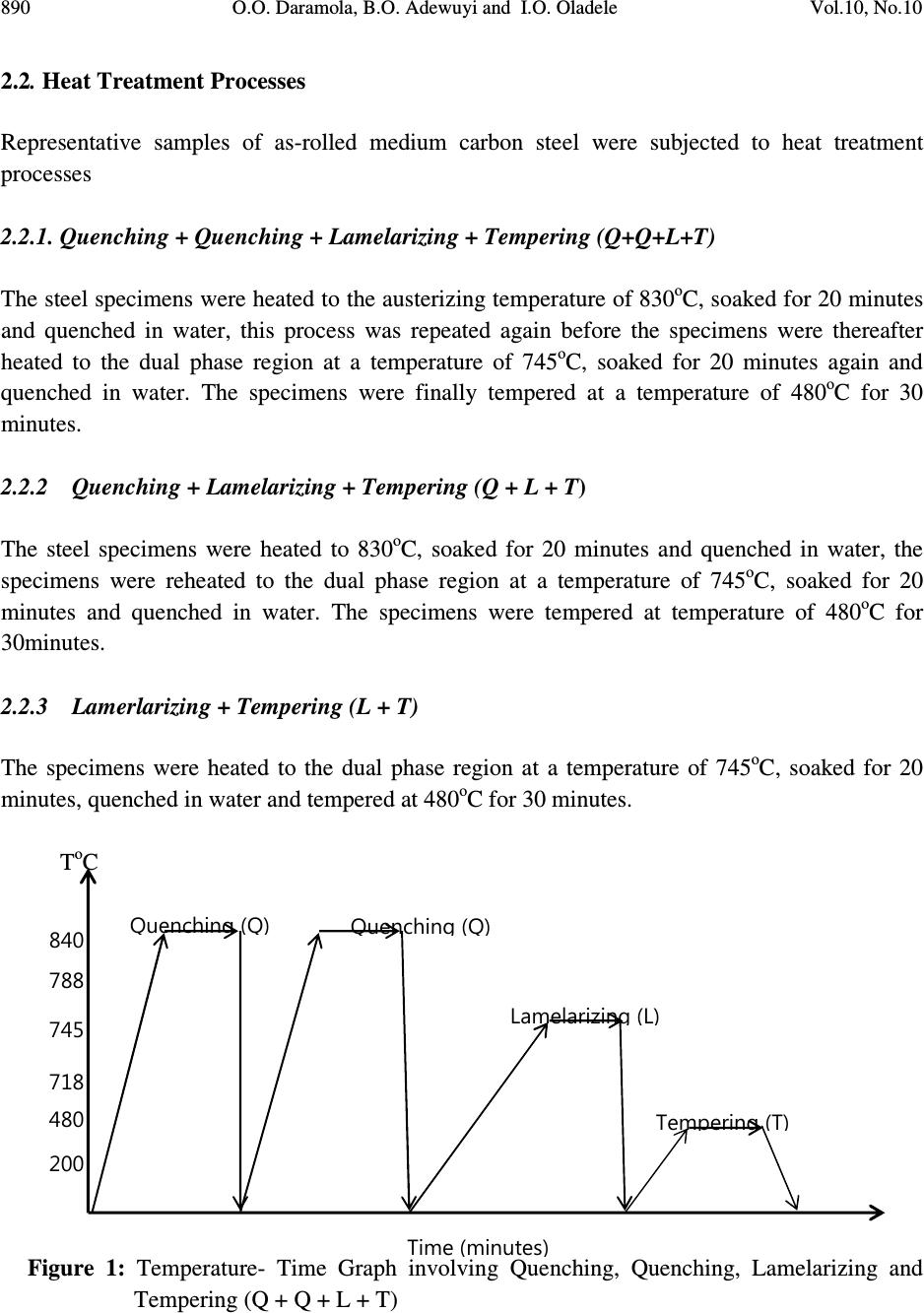 890 O.O. Daramola, B.O. Adewuyi and I.O. Oladele Vol.10, No.10 2.2. Heat Treatment Processes Representative samples of as-rolled medium carbon steel were subjected to heat treatment processes 2.2.1. Quenching + Quenching + Lamelarizing + Tempering (Q+Q+L+T) The steel specimens were heated to the austerizing temperature of 830 o C, soaked for 20 minutes and quenched in water, this process was repeated again before the specimens were thereafter heated to the dual phase region at a temperature of 745 o C, soaked for 20 minutes again and quenched in water. The specimens were finally tempered at a temperature of 480 o C for 30 minutes. 2.2.2 Quenching + Lamelarizing + Tempering (Q + L + T) The steel specimens were heated to 830 o C, soaked for 20 minutes and quenched in water, the specimens were reheated to the dual phase region at a temperature of 745 o C, soaked for 20 minutes and quenched in water. The specimens were tempered at temperature of 480 o C for 30minutes. 2.2.3 Lamerlarizing + Tempering (L + T) The specimens were heated to the dual phase region at a temperature of 745 o C, soaked for 20 minutes, quenched in water and tempered at 480 o C for 30 minutes. T o C Figure 1: Temperature- Time Graph involving Quenching, Quenching, Lamelarizing and Tempering (Q + Q + L + T) 200 480 718 745 788 840 Quenching (Q) Quenching (Q) Lamelarizing (L) Tempering (T) Time (minutes) 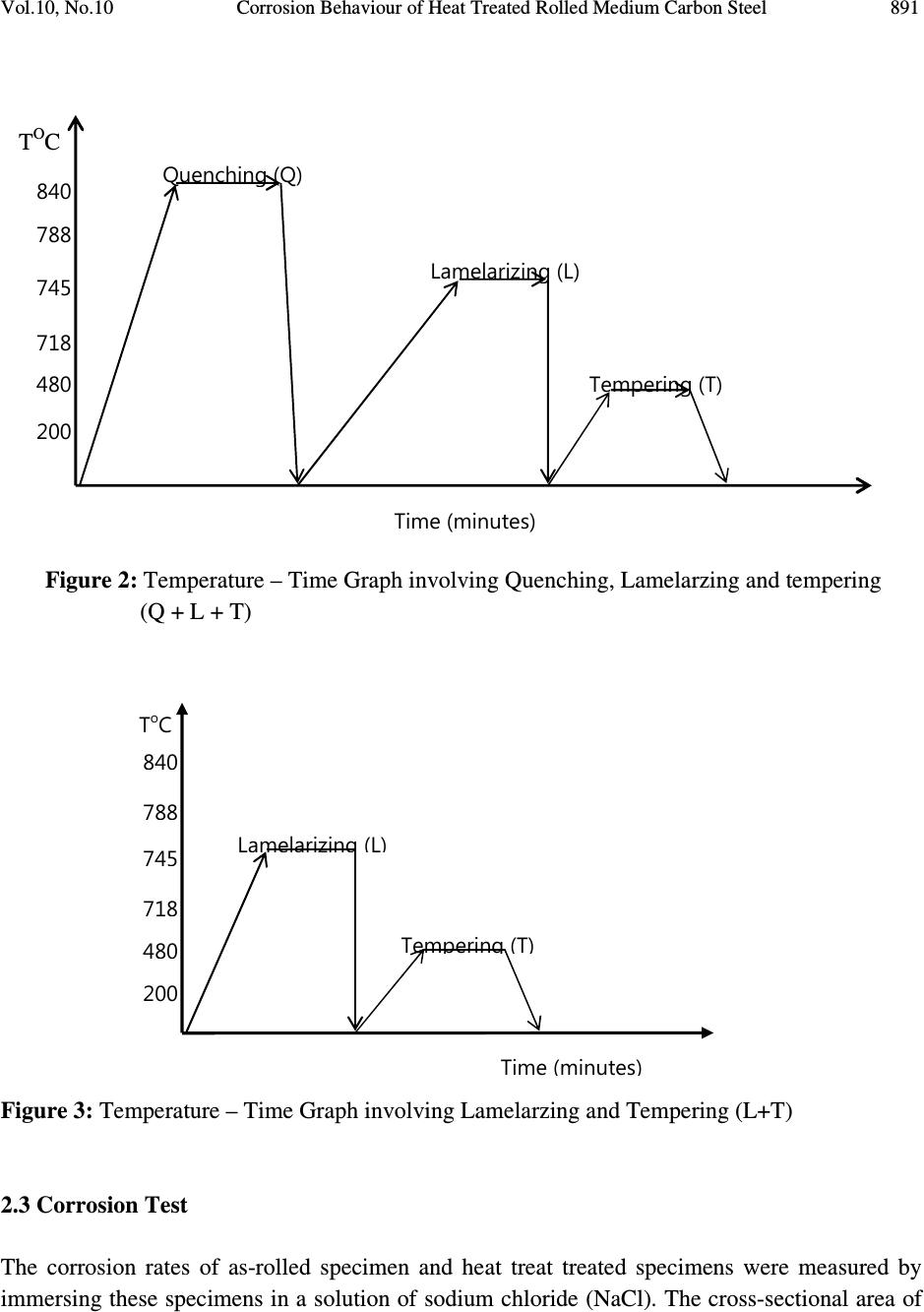 Vol.10, No.10 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel 891 T O C Figure 2: Temperature – Time Graph involving Quenching, Lamelarzing and tempering (Q + L + T) Figure 3: Temperature – Time Graph involving Lamelarzing and Tempering (L+T) 2.3 Corrosion Test The corrosion rates of as-rolled specimen and heat treat treated specimens were measured by immersing these specimens in a solution of sodium chloride (NaCl). The cross-sectional area of 200 480 718 745 788 840 Quenching (Q) Lamelarizing (L) Tempering (T) Time (minutes ) 200 480 718 745 788 840 T o C Lamelarizing (L) Tempering (T) Time (minutes)  892 O.O. Daramola, B.O. Adewuyi and I.O. Oladele Vol.10, No.10 each of the specimens was calculated; each of the specimens was also weighed on a chemical balance and the weight recorded. The PH and the electrode potential of the corrosive medium were measured and recorded. After every 3 days interval (72 hours); specimens were retrieved, washed properly in water, dried and weighed on a weighing balance to determine the weight loss during exposure. The laboratory simulation experiments were carried out in NaCl (O.5M and 1.OM) medium.. Finally, the corrosion rate was calculated using mpy = 3.45 x 106W ATD where W = weight loss in g D = density of specimen in g/cm 3 A = Area in cm 2 T = Exposure Time in hour 2.4 Metallographic Examination Samples of as-rolled and heat – treated specimen were mounted in hot phenolic powder and were ground on a water lubricated hand grinding set-up of abrasive papers, progressing through from the coarsest to the finest grit sizes. The 240, 320, 400 and 600 grades were used in that order. Polishing was carried out on a rotating disc of a synthetic velvet polishing cloth impregnated with micron alumna paste. Final polishing was carried out with diamond paste. The specimens were then etched with the standard 2% Nital so as to reveal the ferrite grain boundaries. The optical microscopic examinations were carried out on a metallurgical microscope at a magnification of 400X. The specimens were illuminated with 100 kilowatts detachable quartz iodine lamp. 3. RESULTS AND DISCUSSION 3.1 Corrosion Properties The results of the corrosion rate of the heat treated specimens and as-rolled specimen is shown in Tables 2–8. The corrosion rates of the heat treated specimens in marine medium (NaCl) is low when compared to the as-rolled steel, as shown in Figures 4-9, this is because the as-rolled steel consist of mainly pearlite in which each crystal consist of alternate layers of ferrite and cementite, it was understood that ferrite is anodic to cementite and this corrode with moisture as the electrolyte [7]. This was confirmed from the microstructure of the as-rolled steel shown in Plates 1–7. In the NaCl environment, the corrosion rate of the as-rolled steel was very high within the first 2 days and after this, the corrosion decreases with increase in exposure time; this is because the FeCl 2 formed during the process is insoluble and forms a protective film on the corroding surface of the steel which effectively prevent corrosive medium from coming into 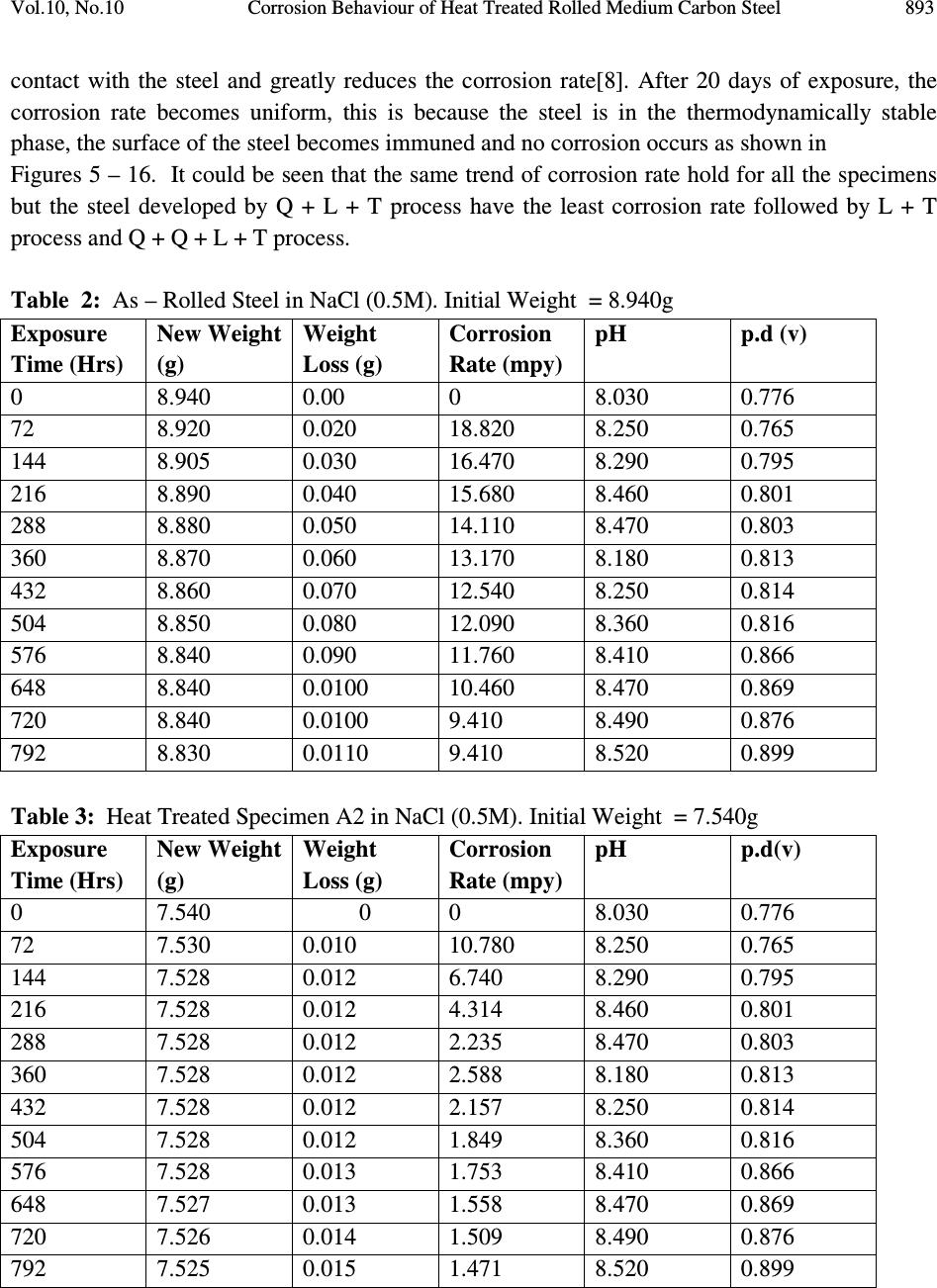 Vol.10, No.10 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel 893 contact with the steel and greatly reduces the corrosion rate[8]. After 20 days of exposure, the corrosion rate becomes uniform, this is because the steel is in the thermodynamically stable phase, the surface of the steel becomes immuned and no corrosion occurs as shown in Figures 5 – 16. It could be seen that the same trend of corrosion rate hold for all the specimens but the steel developed by Q + L + T process have the least corrosion rate followed by L + T process and Q + Q + L + T process. Table 2: As – Rolled Steel in NaCl (0.5M). Initial Weight = 8.940g Exposure Time (Hrs) New Weight (g) Weight Loss (g) Corrosion Rate (mpy) pH p.d (v) 0 8.940 0.00 0 8.030 0.776 72 8.920 0.020 18.820 8.250 0.765 144 8.905 0.030 16.470 8.290 0.795 216 8.890 0.040 15.680 8.460 0.801 288 8.880 0.050 14.110 8.470 0.803 360 8.870 0.060 13.170 8.180 0.813 432 8.860 0.070 12.540 8.250 0.814 504 8.850 0.080 12.090 8.360 0.816 576 8.840 0.090 11.760 8.410 0.866 648 8.840 0.0100 10.460 8.470 0.869 720 8.840 0.0100 9.410 8.490 0.876 792 8.830 0.0110 9.410 8.520 0.899 Table 3: Heat Treated Specimen A2 in NaCl (0.5M). Initial Weight = 7.540g Exposure Time (Hrs) New Weight (g) Weight Loss (g) Corrosion Rate (mpy) pH p.d(v) 0 7.540 0 0 8.030 0.776 72 7.530 0.010 10.780 8.250 0.765 144 7.528 0.012 6.740 8.290 0.795 216 7.528 0.012 4.314 8.460 0.801 288 7.528 0.012 2.235 8.470 0.803 360 7.528 0.012 2.588 8.180 0.813 432 7.528 0.012 2.157 8.250 0.814 504 7.528 0.012 1.849 8.360 0.816 576 7.528 0.013 1.753 8.410 0.866 648 7.527 0.013 1.558 8.470 0.869 720 7.526 0.014 1.509 8.490 0.876 792 7.525 0.015 1.471 8.520 0.899 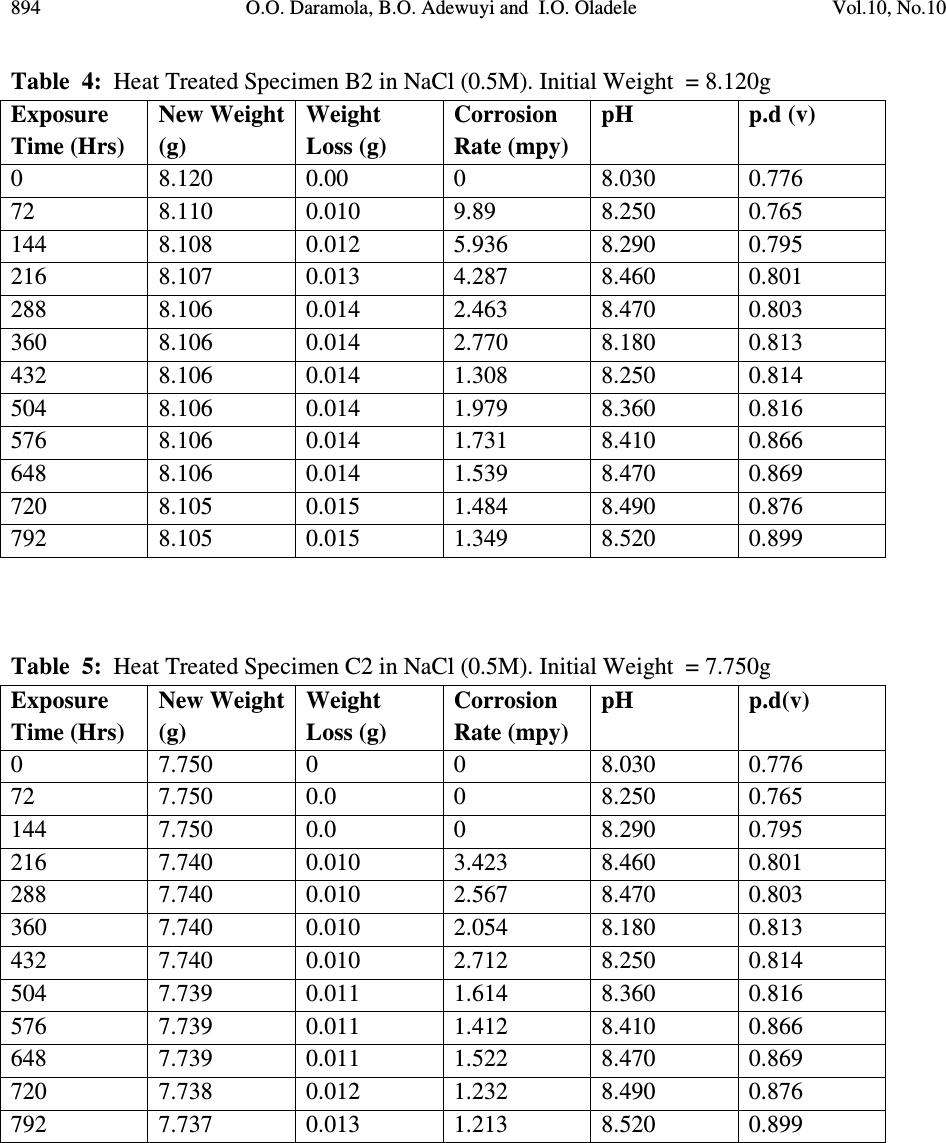 894 O.O. Daramola, B.O. Adewuyi and I.O. Oladele Vol.10, No.10 Table 4: Heat Treated Specimen B2 in NaCl (0.5M). Initial Weight = 8.120g Exposure Time (Hrs) New Weight (g) Weight Loss (g) Corrosion Rate (mpy) pH p.d (v) 0 8.120 0.00 0 8.030 0.776 72 8.110 0.010 9.89 8.250 0.765 144 8.108 0.012 5.936 8.290 0.795 216 8.107 0.013 4.287 8.460 0.801 288 8.106 0.014 2.463 8.470 0.803 360 8.106 0.014 2.770 8.180 0.813 432 8.106 0.014 1.308 8.250 0.814 504 8.106 0.014 1.979 8.360 0.816 576 8.106 0.014 1.731 8.410 0.866 648 8.106 0.014 1.539 8.470 0.869 720 8.105 0.015 1.484 8.490 0.876 792 8.105 0.015 1.349 8.520 0.899 Table 5: Heat Treated Specimen C2 in NaCl (0.5M). Initial Weight = 7.750g Exposure Time (Hrs) New Weight (g) Weight Loss (g) Corrosion Rate (mpy) pH p.d(v) 0 7.750 0 0 8.030 0.776 72 7.750 0.0 0 8.250 0.765 144 7.750 0.0 0 8.290 0.795 216 7.740 0.010 3.423 8.460 0.801 288 7.740 0.010 2.567 8.470 0.803 360 7.740 0.010 2.054 8.180 0.813 432 7.740 0.010 2.712 8.250 0.814 504 7.739 0.011 1.614 8.360 0.816 576 7.739 0.011 1.412 8.410 0.866 648 7.739 0.011 1.522 8.470 0.869 720 7.738 0.012 1.232 8.490 0.876 792 7.737 0.013 1.213 8.520 0.899 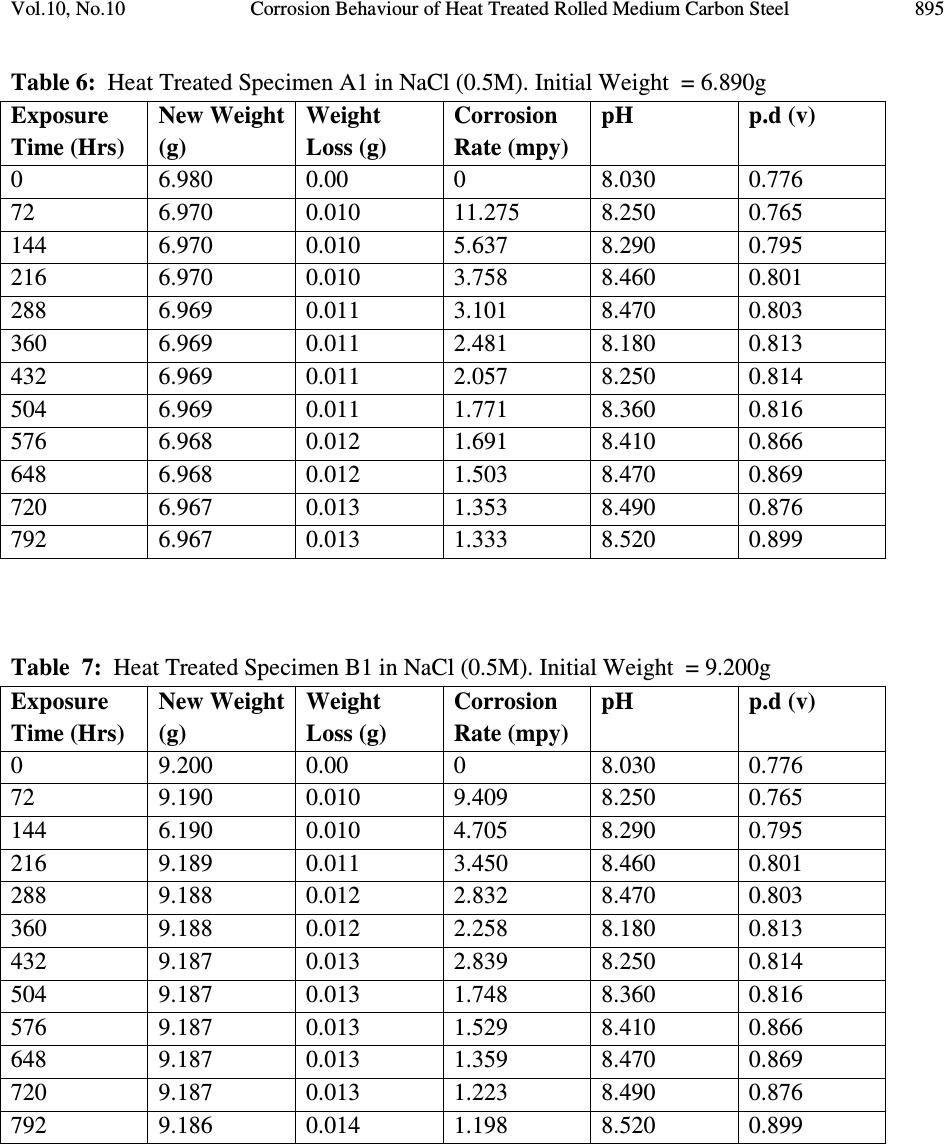 Vol.10, No.10 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel 895 Table 6: Heat Treated Specimen A1 in NaCl (0.5M). Initial Weight = 6.890g Exposure Time (Hrs) New Weight (g) Weight Loss (g) Corrosion Rate (mpy) pH p.d (v) 0 6.980 0.00 0 8.030 0.776 72 6.970 0.010 11.275 8.250 0.765 144 6.970 0.010 5.637 8.290 0.795 216 6.970 0.010 3.758 8.460 0.801 288 6.969 0.011 3.101 8.470 0.803 360 6.969 0.011 2.481 8.180 0.813 432 6.969 0.011 2.057 8.250 0.814 504 6.969 0.011 1.771 8.360 0.816 576 6.968 0.012 1.691 8.410 0.866 648 6.968 0.012 1.503 8.470 0.869 720 6.967 0.013 1.353 8.490 0.876 792 6.967 0.013 1.333 8.520 0.899 Table 7: Heat Treated Specimen B1 in NaCl (0.5M). Initial Weight = 9.200g Exposure Time (Hrs) New Weight (g) Weight Loss (g) Corrosion Rate (mpy) pH p.d (v) 0 9.200 0.00 0 8.030 0.776 72 9.190 0.010 9.409 8.250 0.765 144 6.190 0.010 4.705 8.290 0.795 216 9.189 0.011 3.450 8.460 0.801 288 9.188 0.012 2.832 8.470 0.803 360 9.188 0.012 2.258 8.180 0.813 432 9.187 0.013 2.839 8.250 0.814 504 9.187 0.013 1.748 8.360 0.816 576 9.187 0.013 1.529 8.410 0.866 648 9.187 0.013 1.359 8.470 0.869 720 9.187 0.013 1.223 8.490 0.876 792 9.186 0.014 1.198 8.520 0.899 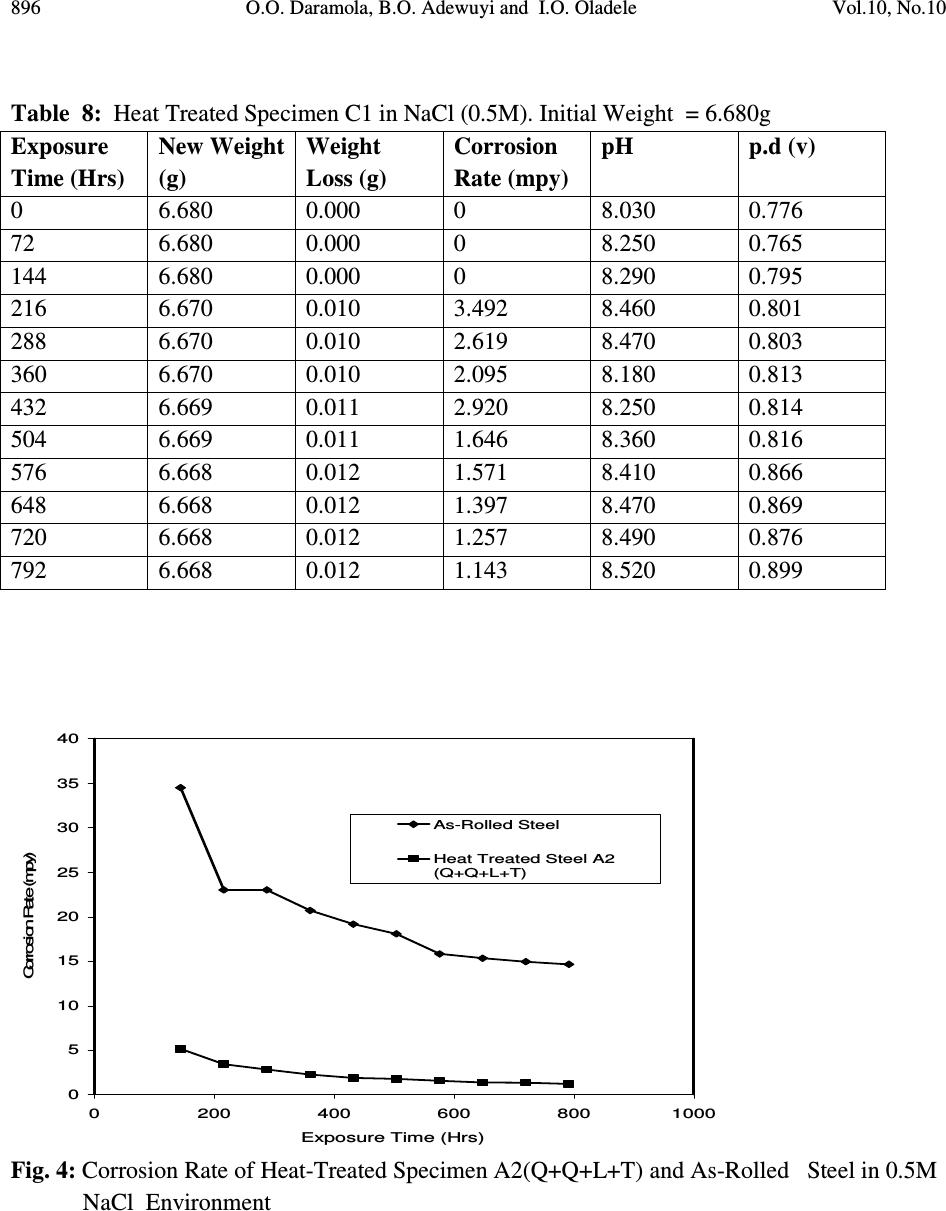 896 O.O. Daramola, B.O. Adewuyi and I.O. Oladele Vol.10, No.10 Table 8: Heat Treated Specimen C1 in NaCl (0.5M). Initial Weight = 6.680g Exposure Time (Hrs) New Weight (g) Weight Loss (g) Corrosion Rate (mpy) pH p.d (v) 0 6.680 0.000 0 8.030 0.776 72 6.680 0.000 0 8.250 0.765 144 6.680 0.000 0 8.290 0.795 216 6.670 0.010 3.492 8.460 0.801 288 6.670 0.010 2.619 8.470 0.803 360 6.670 0.010 2.095 8.180 0.813 432 6.669 0.011 2.920 8.250 0.814 504 6.669 0.011 1.646 8.360 0.816 576 6.668 0.012 1.571 8.410 0.866 648 6.668 0.012 1.397 8.470 0.869 720 6.668 0.012 1.257 8.490 0.876 792 6.668 0.012 1.143 8.520 0.899 0 5 10 15 20 25 30 35 40 0200 400 600 8001000 Exposure Time (Hrs) Corrosion Rate (mpy) As-Rolled Steel Heat Treated Steel A2 (Q+Q+L+T) Fig. 4: Corrosion Rate of Heat-Treated Specimen A2(Q+Q+L+T) and As-Rolled Steel in 0.5M NaCl Environment 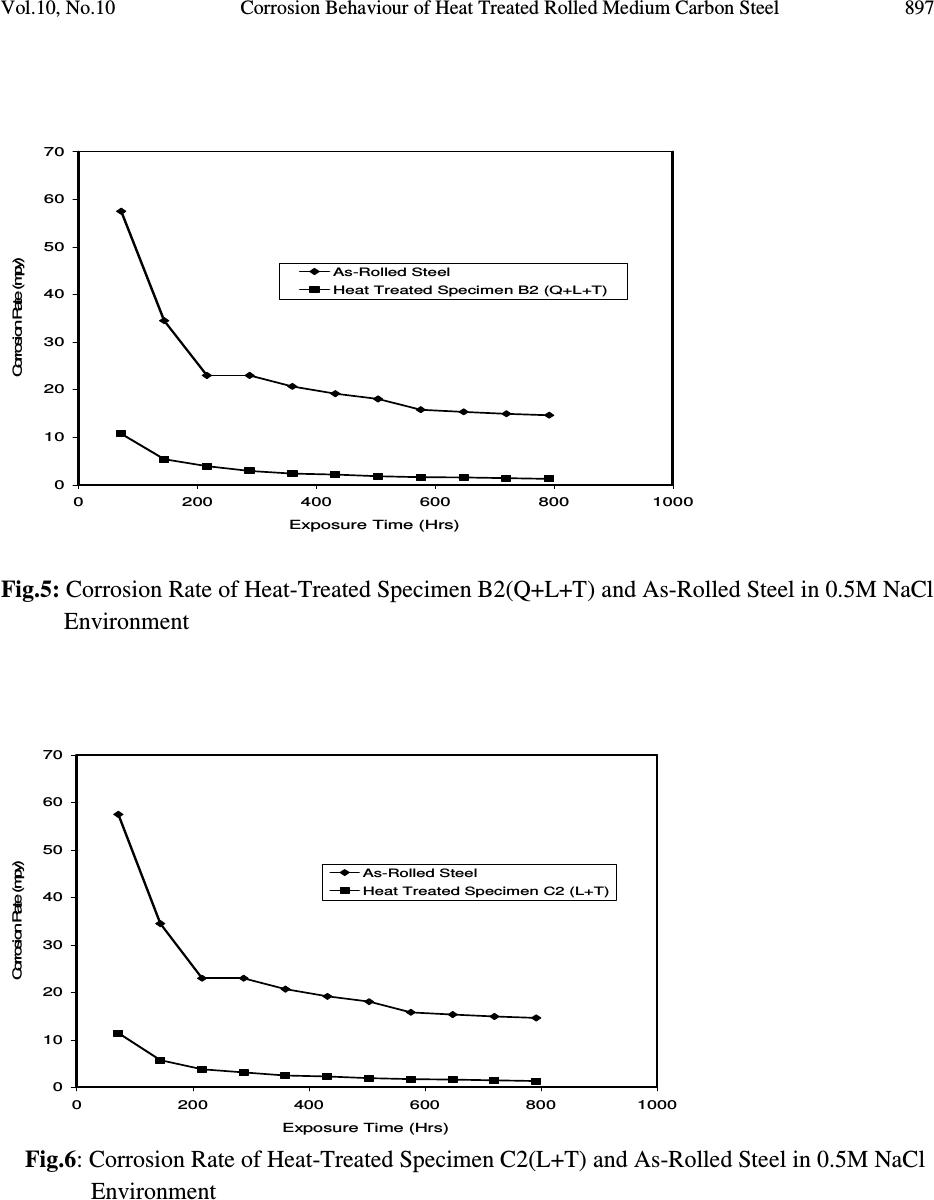 Vol.10, No.10 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel 897 0 10 20 30 40 50 60 70 0200 400 600 8001000 Exposure Time (Hrs) Corrosion Rate (mpy) As-Rolled Steel Heat Treated Specimen B2 (Q+L+T) Fig.5: Corrosion Rate of Heat-Treated Specimen B2(Q+L+T) and As-Rolled Steel in 0.5M NaCl Environment 0 10 20 30 40 50 60 70 0200 400 600 8001000 Exposure Time (Hrs) Corrosion Rate (mpy) As-Rolled Steel Heat Treated Specimen C2 (L+T) Fig.6: Corrosion Rate of Heat-Treated Specimen C2(L+T) and As-Rolled Steel in 0.5M NaCl Environment 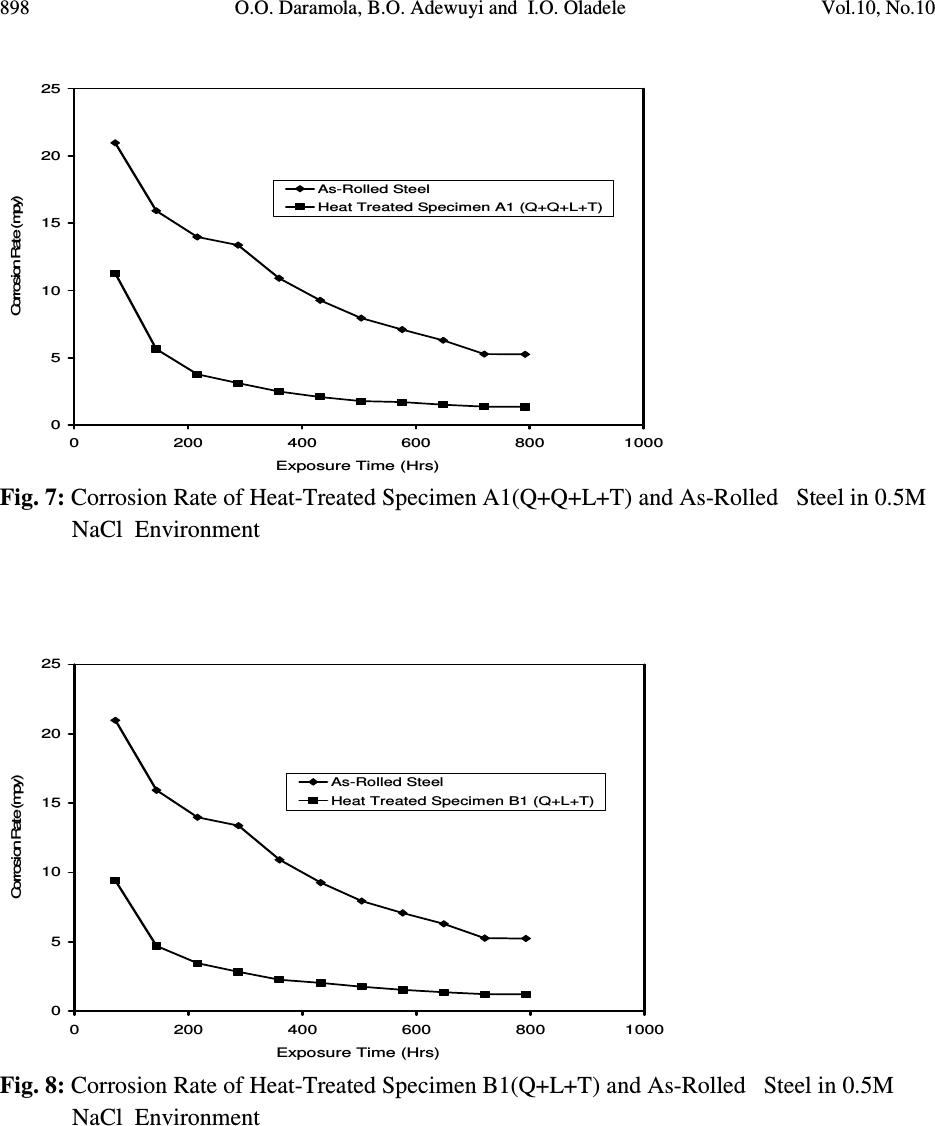 898 O.O. Daramola, B.O. Adewuyi and I.O. Oladele Vol.10, No.10 0 5 10 15 20 25 0200 400 600 8001000 Exposure Time (Hrs) Corrosion Rate (mpy) As-Rolled Steel Heat Treated Specimen A1 (Q+Q+L+T) Fig. 7: Corrosion Rate of Heat-Treated Specimen A1(Q+Q+L+T) and As-Rolled Steel in 0.5M NaCl Environment 0 5 10 15 20 25 0200400600800 1000 Exposure Time (Hrs) Corrosion Rate (mpy) As-Rolled Steel Heat Treated Specimen B1 (Q+L+T) Fig. 8: Corrosion Rate of Heat-Treated Specimen B1(Q+L+T) and As-Rolled Steel in 0.5M NaCl Environment  Vol.10, No.10 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel 899 Fig. 9: Corrosion Rate of Heat-Treated Specimen C1(L+T) and As- Rolled Steel in 0.5M NaCl Environment 3.2 Microstructure The microstructures obtained are shown in Plates 1-7. The microstructure produced by the as- rolled steel consist of pearlite while the microstructure produced by Q + Q + L + T, Q + L + T and L + T processes consist of a duplex ferrite martensite but may contain bainite and retained austenite [9]. The corrosion rates of the heat treated specimens in marine medium (NaCl) is low when compared to that of as-rolled steel. This is because the as-rolled steel which consist of mainly alternate layers of ferrite and cementite, ferrite is anodic to cementite and this corrode with moisture as electrolyte [9-10]. 0 2 4 6 8 10 12 14 16 0 200 400 600 800 1000 Exposure Time (Hrs) Corrosion Rate ( mpy ) As- Rolled Steell Heat Treated Specimen C1(L+T) 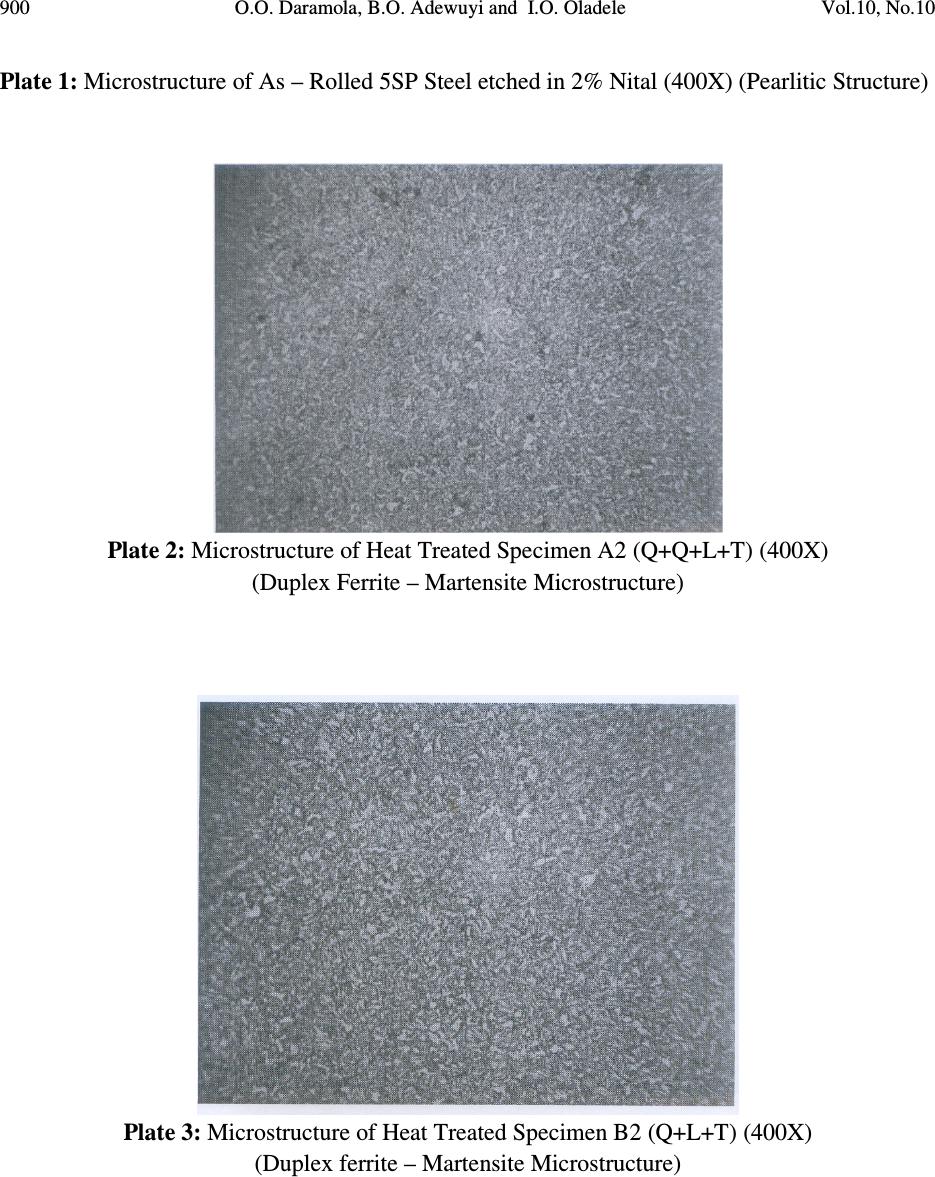 900 O.O. Daramola, B.O. Adewuyi and I.O. Oladele Vol.10, No.10 Plate 1: Microstructure of As – Rolled 5SP Steel etched in 2% Nital (400X) (Pearlitic Structure) Plate 2: Microstructure of Heat Treated Specimen A2 (Q+Q+L+T) (400X) (Duplex Ferrite – Martensite Microstructure) Plate 3: Microstructure of Heat Treated Specimen B2 (Q+L+T) (400X) (Duplex ferrite – Martensite Microstructure)  Vol.10, No.10 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel 901 Plate 4: Microstructure of Heat Treated Specimen C2 (L+T) (400X) (Duplex ferrite – Martensite Microstructure) Plate 5: Microstructure of Heat Treated Specimen A1 (Q+Q+L+T) (400X) (Duplex ferrite – Martensite Microstructure)  902 O.O. Daramola, B.O. Adewuyi and I.O. Oladele Vol.10, No.10 Plate 6: Microstructure of Heat Treated Specimen B1 (Q+L+T) (400X) (Duplex ferrite – Martensite Microstructure) Plate 7: Microstructure of Heat Treated Specimen C1 (L+T) (400X) (Duplex ferrite – Martensite Microstructure) 4. CONCLUSION From the findings, the heat treated medium carbon steel have better corrosion properties than the as-rolled medium carbon steel. The steel developed by Quenching + Lamelarizing + Tempering  Vol.10, No.10 Corrosion Behaviour of Heat Treated Rolled Medium Carbon Steel 903 (Q+L+T) process has the best corrosion properties followed by Lamelarizing + Tempering (L+T) process and Quenching + Quenching + Lamelarzing + Tempering (Q+Q+L+T) process. REFERENCES 1. Scully, J.C. (1990). The Fundamental of Corrosion. Maxwell Macmillan Perganman Publishing Corporation, Oxford. 2. Mamoru, O. Yukito, T; Hitoshi, K. and Yuji, F. (1990). Development of New Steel Plates for building Structural use, Nippon Steel Technical Report, No44 pp. 8 – 15. 3. Rajan, T.V; Sharma, C.P. and Sharma, A. (1989). Heat Treatment Principles and Techniques, Prentice Hall of India Private Limited, New Delhi. pp. 36 – 58. 4. O.O. Daramola, B.O. Adewuyi and I.O. Oladele (2010), Effects of Heat Treatment on the Mechanical Properties of Rolled Medium Carbon Steel, Journal of Minerals and Materials Characterization and Engineering; Vol. 9, No. 8, pp. 693 – 708. 5. Thomas, G., Ahn, J.H. and Kin, N.J. (1986) Controlled Rolling Process for Dual-Phase Steel and Shapes. The Metal Society, London, Book 285, pp. 121 – 124. 6. Gorni, A.A. (2004). Steel Forming and Heat-Treating Hand Book; Vol. 2, Saova Center, Brazil, p. 4. 7. Faleke, E.O. (1987). Metallurgical Investigation of a Corroded Peugeot Car Body. Unpublished Thesis, Federal University of Technology, Akure 8. Baboian and Turcotte, (1985). Corrosion Sceince. Elservier Science Limited, Great Britain. Volume 25, No 13, pp. 958 – 1009. 9 Davies, D.J. and Harold, W. (1971). Practical Microcopical Metallography, Chapman and Hall Limited, London. 10. Davis, D.J. and Oelman, L.A. (1983). The Structure, Properties, and Heat Treatment of Metals, London, Pitman Books, pp. 44 – 52. 11. Smith, W.F. and Hashemi, J. (2006). Foundations of Materials Science and Engineering, 4 th Edition; Mcgraws – Hill Book. pp. 28 – 36. 12. Dieter, G.E (2000). Mechanical Metallurgy, 5 th Edition. Singapore, Mcgraw – Hill Book, pp. 186 - 195 |

