Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 10, No.9, pp.865-874, 2011 jmmce.org Printed in the USA. All rights reserved 865 A Model for FeSiMg Alloy Production by Reduction Technique Saeed Nabil Saeed Ghali Steel Technology Department, Central Metallurgical Reseach & Development Institute (CMRDI), P.O Box 87 Helwan, 11722, Egypt. ABSTRACT Ferrosilicon magnesium is basic foundry alloys used for the production of ductile cast iron. Magnesium content plays an important role in the produced alloy grades from dolomite ore. The focus of the present work is to simulate mathematical model to predict magnesium content in the ferrosilicon magnesium, which produced by silicothermic reduction of calcined dolomite. The basic assumptions of this model are: constant low viscosity of molten charge, the reaction is irreversible of second order and the reaction is isothermal. The reaction is based on the following equation: 22 22 SiOSiMgSiMgO + → + The results of previous work was found to be in a good coincidence with the predicted values by the model ][])[( ]1][)[( ][ ]][)[( ]][)[( o SiMgOKt o SiMgOKt oo SieMgO eSiMgO Mg oo oo − − =− − where [Mg] is the concentration of magnesium metal in ferrosilicon magnesium alloy in mol/L. [Sio] and (MgOo) are the initial concentration of silicon and magnesium oxide in charge in mol/L, while t is time in second, K is the reaction rate constant ( 3.26588x10-7 L Sec-1 mol-1). The predicted values are greater than the experimental values; this may be attributed to the use of concentration instead of the activity. The predicted values of magnesium content in ferrosilicon magnesium alloy are in a good agreement with the experimental results obtained in previous work at low viscosity. Keywords: activity , model, ferrosilicon, magnesium, reduction, viscosity 1. INTRODUCTION Ferrosilicon magnesium is the one alloy of magnesium that is used to produce all types of ductile iron casting under all types of foundry conditions. Dolomite represents a source of both magnesium and calcium as it consists mainly of double carbonate of Ca and Mg. Calcinated dolomite seems to be a suitable cheap raw material for the production of  866 Saeed Nabil Saeed Ghali Vol.10, No.9 ferrosilicon magnesium alloy in EAF or in induction furnace [1-7]. The production of ferrosilicon magnesium from dolomite could be carried out either by metallothermic process using silicon and / or Al or by carbothermic process [8-13]. Magnesium content plays an important role in the produced alloy grades from dolomite ore. The silicothermic process of magnesium oxide is controlled by factors. The most important factors are physical properties of slag, the concentrations of reactants, reaction rate constant, reaction time and reaction temperature. At constant temperature, time and low viscosity the reaction rate mainly depends on the concentrations of reactants. In this paper, a mathematical model will be designed to predict magnesium content in ferrosilicon magnesium alloy which is produced by silicothermic reaction of calcinated dolomite at low viscosity of slag. 2. MATHEMATICAL MODEL The focus of the present work is to create a mathematical model to predict magnesium content in ferrosilicon magnesium alloy, which is produced by silicothermic reduction of calcinated dolomite. The basic assumption of this model is the low and almost unchangeable viscosity of slag during the reaction. Ferrosilicon magnesium alloy is produced by reduction of calcinated dolomite ore with ferrosilicon containing silicon 75 mass content in %. The reaction is based on the equation: )(][][2)(2 22 SiOSiMgSiMgO K +→+ (1) This system is controlled by chemical and kinetic roles. The limitations of this model are the following: • The reaction takes place at low slag viscosity and nearly unchanged • The above reaction is isothermal and irreversible • The reaction is second order • Concentration of reducing agent (in molar) is greater than the magnesium oxide ( [Si] > (MgO)) • There is a mass balance in the above reaction The rate of change of magnesium content in ferrosilicon magnesium alloy is directly proportional to the concentration of magnesium oxide and silicon metal. There is a mass balance in this system. )( 2 1 ][ 2 1 ][)( 22 SiOSiMgSiMgO K +→+ (2) [Mg 2 Si] go to molten metal and (SiO 2 ) go to slag. Every one mole from [Mg 2 Si] has two moles from Mg, i.e. the rate of change of Mg with time depends on the initial concentrations of the reactants.  Vol.10, No.9 A Model for FeSiMg Alloy Production 867 The reaction in equation (2) means that one mole from magnesium oxide reduced by one mole of silicon metal to give one mole of magnesium. This can be writing as: ][][)( MgSiMgO K →+ (3) The above reaction can be rewrite as: XBA K →+ (4) XXBXA K oo →−+− )()( (5) Where at zero time: (MgO) = initial concentration of magnesium oxide = (MgO o ) = A o (6) [Si] = initial concentration of silicon metal = [Si o ] = B o (7) [Mg] = initial concentration of magnesium metal = X = Zero (8) At any time: (MgO) = A = A o – X (9) [Si] = B = B o – X (10) [Mg] = X (11) From equation (3), the rate of change in magnesium content is directly proportional with the concentrations of reactants. ])[( ][ SiMgOK dt Mgd= (12) From equations 9, 10, 11 & 12, we obtain ))(( XBXAK dt dx oo −−= (13) Rearrange and integration of two sides of equation (13) ∫ ∫ = −− dtK XBXA dx oo ))(( (14) ∫ += −− tconsKt XBXA dx oo tan ))(( (15) The left side of equation (15) )()())(( 121 XB C XA C XBXA oooo − + − = −− (16) ))(( )()( ))(( 1 21 XBXA XACXBC XBXA oo oo oo −− −+− = −− (17)  868 Saeed Nabil Saeed Ghali Vol.10, No.9 From the parameters of X 21 0CC − − = (18) From the parameters of X 0 o ACBC 2 0 1 1+= (19) From equations (18) and (19) oo A B C − =1 1 (20) oo A B C − =1 2 (21) From equations (15), (16), (20) and (21), the left side of equation (15) ∫∫ ∫ −− + −− = −− )( 1 )( 1 ))((X o B dx o B o AX o A dx o A o BX o BX o A dx (22) Take y = A 0 – x (23) and hence dy = -dx (24) z =B 0 – x (25) and hence dz= -dx (26) From equations (22-26) ∫∫ ∫ +− − = −− ][ 1 ))((z dz y dy ABXBXA dx oooo (27) ∫∫ ∫ − − = −− ][ 1 ))((z dz y dy BAXBXA dx oooo (28) ∫ − − = −− ]ln[ln 1 ))((zy BAXBXA dx oooo (29) ∫ − = −− ][ln 1 ))(( z y BAXBXA dx oooo (30) From equations (23), (25) and (30) ∫ − − − = −− ] )( )( [ln 1 ))((XB XA BAXBXA dx o o oooo (31) From equations (15) and (31) .] )( )( [ln 1constKt XB XA BA o o oo += − − − (32) From boundary conditions, at zero time, from equation (8), X = 0 .][ln 1const B A B A o o oo = − (33) From equations (32) and (33) ][ln 1 ] )( )( [ln 1 o o ooo o oo B A BA Kt XB XA BA − += − − − (34) Kt B A BAXB XA BA o o ooo o oo = − − − − −][ln 1 ] )( )( [ln 1 (35)  Vol.10, No.9 A Model for FeSiMg Alloy Production 869 Kt XBA XAB BA oo oo oo = − − −] )( )( [ln 1 (36) )(] )( )( [ln oo oo oo BAKt XBA XAB −= − − (37) )( )( )( oo BAKt oo oo e XBA XAB − = − − (38) )( )()( oo BAKto o o o eXB B A XA − −=− (39) )( )()( oo BAKt o o oo eX B A AXA − −=− (40) oBAKtoBAKt o o AeAXeX B A oooo −=− −− )()( )*( (41) ]1[]1[ )()( −=− −− oooo BAKtoBAKt o o eAe B A X (42) ]1[ ]1[ )( )( − − = − − oo oo BAKt o o BAKto e B A eA X (43) ][ ]1[ )( )( oBAKto BAKtoo BeA eBA X oo oo − − = − − (44) ][])[( ]1][)[( ][ ]][)[( ]][)[( o SiMgOKt o SiMgOKt oo SieMgO eSiMgO Mg oo oo − − = − − (45) At initial time, t = 0 1 ][])[( ]1][)[( ][ 0 0 = − − = oo oo SieMgO eSiMgO Mg (46) This means that the model verifies the boundary conditions In case of K is very large, and [Si] > (MgO), From equation (45) ][])[( ]1][)[( ][ ]][)[(* ]][)[(* o SiMgOt o SiMgOt oo SieMgO eSiMgO Mg oo oo − − = −∞ −∞ (47) ][])[( ]1][)[( ][ oo oo SieMgO eSiMgO Mg − − = ∞− −∞ (48) )( ][ ])[( ][ o o oo MgO Si SiMgO Mg = − − = (49) This means that all magnesium oxide will be reduced by silicon In case of at infinity time and [Si]> (MgO) )( ][])[( ]1][)[( ][ ]][)[(** ]][)[(** o o SiMgOK o SiMgOK oo MgO SieMgO eSiMgO Mg oo oo = − − = −∞ −∞ (50)  870 Saeed Nabil Saeed Ghali Vol.10, No.9 It is clear that the model verify both the boundary conditions and logical limits. From equation (1), it can be calculate the rate of reaction using Gibbs free energies of constituents )(][][2)(2 22 SiOSiMgSiMgO K +→+ (1) RP GGG ∆ − ∆ = ∆ (51) 22 SiOSiMgP GGG ∆+∆=∆ (52) SiMgOR GGG ∆ + ∆ = ∆ 22 (53) ./8.26796./104.6 3 2 mol JmolCalxG SiMg ==∆ [14] (54) STHG ∆ − ∆ = ∆ [15] (55) mol J mol Cal G SiO / 16 . 528342 / 33 . 126186 )79.48)(1873(217570 2 −=− = − − − = ∆ (56) . / 37 . 733848 / 3 . 175275 )9.41)(1873(253754[22 mol J mol Cal G MgO −=−= − − − = ∆ (57) From equations (51) & (54-57) ./01.232303]037.733848[)]16.528342(8.26796[ molJG = + − − − + = ∆ 117486.6 3.2 **1026588.31010 −−−− ∆− ===SecmolLxK RT G (58) The model is applied for the experimental results of Hoda et al [16]. Tables (1-2) show the constituents and chemical compositions of charges respectively. Table 1: The charge of experimental Input Output No . Dolomite FeSi Fluorspar Limestone Al Quartzite Bauxite CaSO 4 Metal mass Mg mass content in % Mg mass content in % Predicted 1 1250 750 100 50 25 725 2.25 4.38 2 1250 750 100 70 25 590 2.86 5.30 3 1250 750 100 100 25 550 4.1 5.54 4 1250 750 100 130 25 430 2.0 6.90 5 1250 750 100 150 25 441.5 1.3 6.61 6 1250 600 160 50 50 25 480 1.7 5.48 7 1250 600 160 50 75 25 558 1.8 4.60 8 1250 600 160 50 100 25 622 3.2 4.04 9 1250 600 160 50 150 25 813 1.6 2.96 10 1250 600 40 50 25 750 1.76 4.11 11 1250 600 80 50 25 489 3.5 6.06 12 1250 600 120 50 25 550 3.5 5.19 13 1250 600 160 50 25 575 4.24 4.78 14 1250 600 200 50 25 595 3.25 4.45 15 1250 600 400 50 25 550 3.25 4.05 16 1250 950 160 50 25 983 2.45 3.19 17 1250 950 160 100 25 828 2.84 3.67 18 1250 950 160 150 25 731 3.05 3.99 19 1250 950 160 200 25 805 2.52 3.50 20 1250 950 160 250 25 750 2.63 3.62  Vol.10, No.9 A Model for FeSiMg Alloy Production 871 Table 2: Chemical composition of charge Chemical composition, mass content in % Constituents Calcinated dolomite Fluorspar Rare earth metals Limestone Quartzite Bauxite FeSi Al SiO 2 1.4 12.6 3.88 95 6.43 Fe 2 O 3 1.45 0.35 0.5 0.2 CaO 62.4 1 51.78 MgO 33.6 1 0.6 0.3 L.O.I. at 1000 o C 0.43 41.3 Al 2 O 3 1 2.4 0.8 2.5 85 Na 2 O 1 1.64 K 2 O 0.35 CaF 2 82 CaCO 3 2.2 P 2 O 3 0.01 CeO 2 /ReO 45 Fe 0.005 0.34 0.14 23.3 Pb 0.001 P 2 O 5 0.001 SO 3 0.03 FeO 1.8 C 0.09 S 0.003 P 0.031 Al 1.41 99. Ca 0.31 Si 74.8 Figs (1-4) show the actual and predicted magnesium content at different contents of bauxite, limestone, fluorspar and quartzite respectively, the time of reaction is two hours. Fig. 1 shows that the effect of bauxite (alumina content) as given in Tables (1-2) on the magnesium content and the difference between predicted and actual magnesium content. It is clear that the difference between the magnesium mass content in % pred. and magnesium mass content in % actual increase as the alumina increase. This behaviour can be attributed to the negative effect of Al 2 O 3 on the activity of magnesium oxide due to the formation of calcium aluminates [8; 17-18]. Fig.2 shows the difference between the predicted magnesium content and the actual magnesium cont at different limestone. It is noted that – as clear from Fig.2 - the difference between the predicted and the actual magnesium content decreases as the limestone increases (from heats 1 to 3) then the difference, by further limestone increase (heats 4 & 5) , the difference sharply increase. This can be explained by two significant opposite effect. The first one is the positive effect of increasing CaO content – due to increase limestone- in SiO 2 rich slag leading to the formation of 2CaO.SiO 2 , 3CaO.SiO 2 and CaO.SiO 2 [19-25]. these compounds are formed first and are very stable leading to free MgO for reduction, this mean that the activity of magnesium oxide increase by increasing limestone to some extent. The second factor, is the negative effect of increasing the content of these high molten compounds CaO.SiO 2 , 2CaO.SiO 2 and 3CaO.SiO 2 with melting temperatures of 1564 o C, 2130 o C and 2070 o C, respectively, resulting in higher viscous slag and hence the activity of magnesium oxide decrease. Furthermore, the presence of CO 2 gas, resulting from the decomposition of limestone leads to more oxidation of magnesium. Fig. 3 shows the variation between the 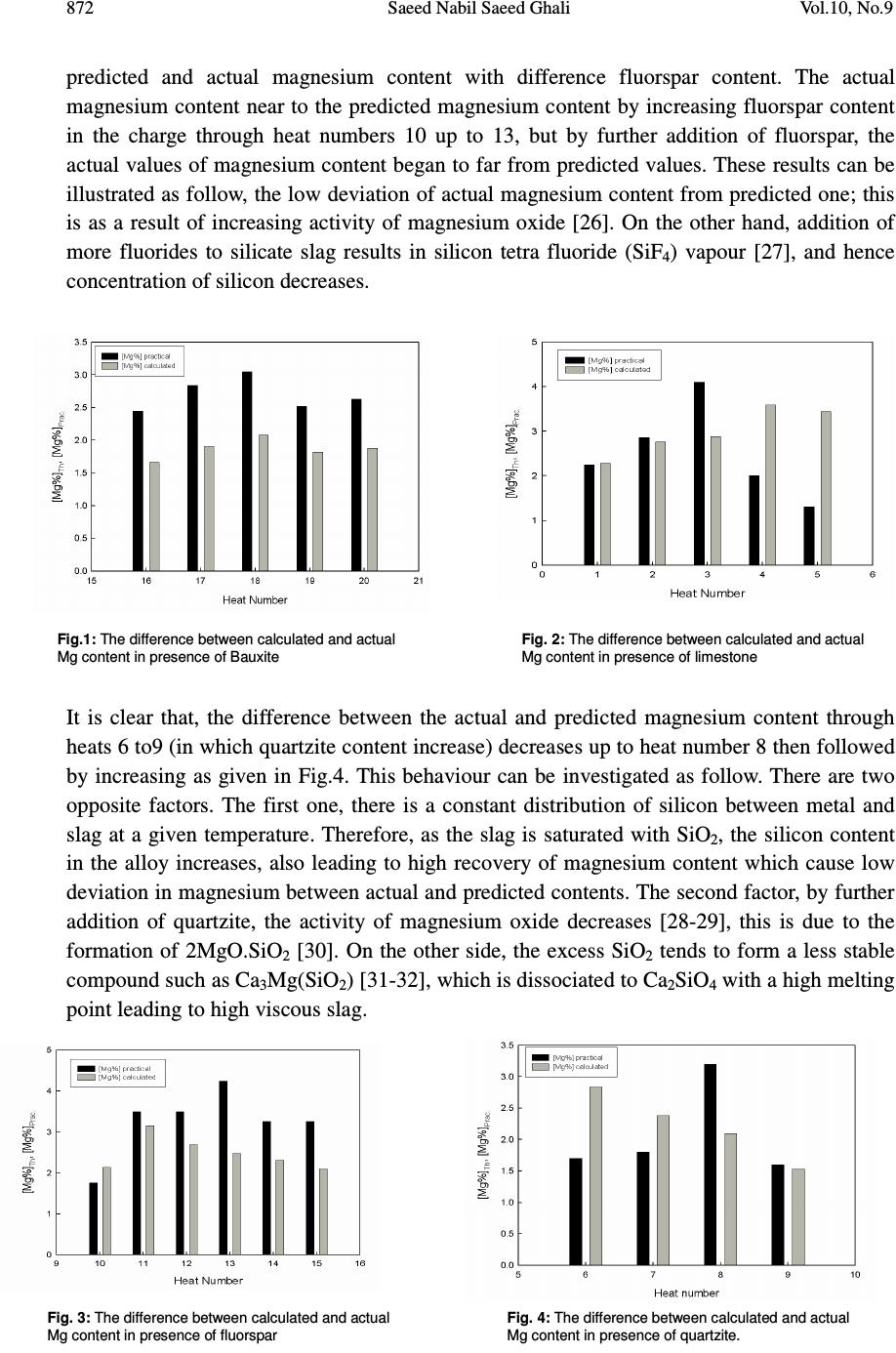 872 Saeed Nabil Saeed Ghali Vol.10, No.9 predicted and actual magnesium content with difference fluorspar content. The actual magnesium content near to the predicted magnesium content by increasing fluorspar content in the charge through heat numbers 10 up to 13, but by further addition of fluorspar, the actual values of magnesium content began to far from predicted values. These results can be illustrated as follow, the low deviation of actual magnesium content from predicted one; this is as a result of increasing activity of magnesium oxide [26]. On the other hand, addition of more fluorides to silicate slag results in silicon tetra fluoride (SiF 4 ) vapour [27], and hence concentration of silicon decreases. It is clear that, the difference between the actual and predicted magnesium content through heats 6 to9 (in which quartzite content increase) decreases up to heat number 8 then followed by increasing as given in Fig.4. This behaviour can be investigated as follow. There are two opposite factors. The first one, there is a constant distribution of silicon between metal and slag at a given temperature. Therefore, as the slag is saturated with SiO 2 , the silicon content in the alloy increases, also leading to high recovery of magnesium content which cause low deviation in magnesium between actual and predicted contents. The second factor, by further addition of quartzite, the activity of magnesium oxide decreases [28-29], this is due to the formation of 2MgO.SiO 2 [30]. On the other side, the excess SiO 2 tends to form a less stable compound such as Ca 3 Mg(SiO 2 ) [31-32], which is dissociated to Ca 2 SiO 4 with a high melting point leading to high viscous slag. Fi g.1: The difference between calculated and actual Mg content in presence of Bauxite Fig. 2: The difference between calculated and actual Mg content in presence of limestone Fig. 3: The difference between calculated and actual Mg content in presence of fluorspar Fig. 4: The difference between calculated and actual Mg content in presence of quartzite.  Vol.10, No.9 A Model for FeSiMg Alloy Production 873 3. CONCLUSIONS The predicted magnesium contents are greater than the experimental values. The most important reason is attributed to the use of concentrations instead of activities. • Based on the assumptions, low viscosity, and the reduction of magnesium oxide by silicon metal, the reaction is controlled by rate of reaction and concentration of reactants (MgO) and [Si]. • The equation have been derived is function in initial concentration of reactants, time, reaction rate constant as shown, ][])[( ]1][)[( ][ ]][)[( ]][)[( o SiMgOKt o SiMgOKt oo SieMgO eSiMgO Mg oo oo − − = − − • The reaction rate constant of the reaction : )(][][2)(2 22 SiOSiMgSiMgO K +→+ is 117 **1026588.3 −−− =SecmolLxK • Volatilization of magnesium metal during the reduction of magnesium oxide process has a great negative significant effect on the gab between the predicted and actual values of magnesium content. • Finally the difference between the actual and predicted mainly depends on effect of additions on the activities of magnesium oxide and reducing agent, and viscosity of the reaction medium. REFERENCES 1. 461964 (U.S.S.R.), 8(1975), M. A. Kekelidze et al., Auth. Cert., Bynll. Izobr., 2. M. A. Ryss et al.: Stal, April, (1973), p. 303. 3. D. I. Saginadze et al.: Stal, 2 (1977), p.140. 4. D. I. Saginadze et al.: Steel in the USSR, 16 (1986), p. 533 5. F. N. Tavadze et al.: Nauka Proizvodstva, C51, (1983), p. 243. 6. 727704 (U.S.S.R.), 14(1980), F. N. Tavadze et al., Auth. Cert., Bynll. Izobr. 7. V. Varbanov et al.: Works of the Iron and Steel Inst., VIII (1977). 8. D. J. G. Ives: Principles of the extraction of metals, London, (1969). 9. H. J. T. Ellingham: I. Soc. Chem. Ind., Lond., 1944 & C.W. Dannatt and H.J.T. Ellingham: Disc Faraday Soc., 4 (1948), p. 126 10. N. P. Lyakiskev et al.: Alyuminotermiya "Aluminothermy", Moscow, (1978), p. 424 11. M. A. Merritt and E. V. Marcossi: Electric Furnace Conf. Proc., New York, 29 (1972), p. 184. 12. V. Ryachikov, V. G.. Mizin., and A. S. Dubrovin: Steel In U.S.S.R., 12 (1982), p. 264. 13. P. Sokolov and N. L. Ponomarov: Introduction to Metallothermic, Moscow, (1990), p. 134. 14. W. Klemm and H. Wwstlinning: Z.anorg. Chem., 245, (1941), p. 365. 15. R.G. Ward, M.A., Ph. D: An introduction to the physical chemistry of iron & steel making, Edward Arnold (Publishers) LTD, (1962), p. 212/213. 16. H. El-Faramawy et al.: Ferrosilicon Magnesium Production, Scandinavian Journal of Metallurgy, 32 (2003) p. 37/46. 17. M. Alper, et al: J. Am. Ceram. Soc., 45 (1962) No. 6, p. 263/8  874 Saeed Nabil Saeed Ghali Vol.10, No.9 18. R. A. Howald & N. B. Roy: CALPHAD 15(1991) No. 2, p. 159/72 19. B. Ellingstaeter & T. Rosenquist: Journal of Metals, 6 (1956), p. 111. 20. L M. Pidgeon: Trans Met Soc AIME, 226 (1963) p. 821 21. L. M. Pidgeon & J. K. King: Trans Faraday Soc, 40 (1948) p. 197. 22. V. Ryabchikov & A. S. Mikulinskii: Chemical Abstract, 35 (1962) p. 62. 23. J. M. Taguri & L M. Pidgeon: Can J Chem, 39 (1961) p. 540 24. K. Tomasek, I. Imsis & O. Sesveiec: Light Metals, Proc. Conf., Montreal, Quebec, 25- 29 August (1996) p. 213 25. J. R. Wynnyckj, L. M. Pidgeon: Met Trans 2 (1971) p. 979 26. C. Chen & W. Zhao: Acta Met. Sinica, 19 (1983) No. 1 , A1/A8 27. K. C. Mills & B. J. Keene: Int Metal Review, 1 (1981) p. 21/69 28. J. F. Elliott, M. Gleiser & V. Ramakrishna: Thermochemistry for Steelmaking. Vol. II, Reading. Palo Alto/London (1963). 29. H. R. Rein, J. Chipman: Trans. Metallurg. Soc. AIME, 233 (1965), p. 415/425 30. S. Kambayashi, E. Katao: J. Chem. Thermodyn. 15 (1983) No. 8, p. 701/707 31. W. T. Hughes, C. E. Ransky & E. F. Emle: Advan Extr Met Proc Symp, London (1968) 429 32. R. B.Winkler: Vacuum metallurgy, Elsevier, London, (1971)193 |

