Paper Menu >>
Journal Menu >>
 Journal of Mi nerals & Materials Characteriza tion & Engineering, V ol. 10, No.9, pp.817-825, 2011 jmmce.org Printed in the USA. All rights reserved 817 Laboratory Studies on Phosphorus Removal from Nigeria’s Agbaja Iron Ore by Bacillus Subtilis C. N. Anyakwo1 and O.W. Obot2 1Department of Metallurgical and Materials Engineering, Federal University of Technology, Owerri, Nigeria. Email: charlesanyakwo@yahoo.com 2Department of Mechanical Engineering, Faculty of Engineering, University of Uyo, Nigeria. Email: obotowo2009@gmail.com ABSTRACT The investigation into whether or not Bacillus subtilis can remove phosphorus from the Nigerian Agbaja iron ore was carried out with careful monitoring of the population of the removing agent as well as pH of the system. 1.00mm/0.50mm, 0.50mm/0.25mm, and 0.25mm/0.125mm ore fractions obtained from sieving of the crushed iron ore in Shital Test kits, were used in sub-merged cultur e of nutrien t broth (NB) medium for 10 weeks. B. s ubtili s which was part of the rich microflora found on the ore surface was cultivated in nutr ient-rich media and later inoculated in sterilized 100ml of NB in 250ml conical flask and 1g of each of the equally sterilized ore fractions was added. At weekly intervals, a set of samples was removed, treated through series of chemical reactions to obtain ammonium phospho- molybdate precipitate which was back-titrated with 0.1 N-HCl to determine the amount of phosphorus left in samples and consequently, the amount removed. The laboratory investigations found out that B. subtilis has the capability to remove phosphorus from the Nigerian Agbaja iron ore, recording an impressive average of 65.73% P . Also found out was the systematic reduction in bacterial cells count in colony forming unit per mililitre, the initial load 3.4x105 cfu/ml increased to 4.8x107 cfu/ml from where it declined to 1.3x106 cfu/ml, which justified the pH trend observed during the process of cumulative phosphorus removal. The reduction in microbial activity may be attributed to antimicrobial components of the ore, pyrite, and other heavy metals which may have affected the phosphorus uptake from ore. 1. INTRODUCTION In 2007 the world iron ore production rose by 11% to 1.640 billion tons. It was expected that in 2008 and 2009 the trend was likely to continue. According to the latest report released by 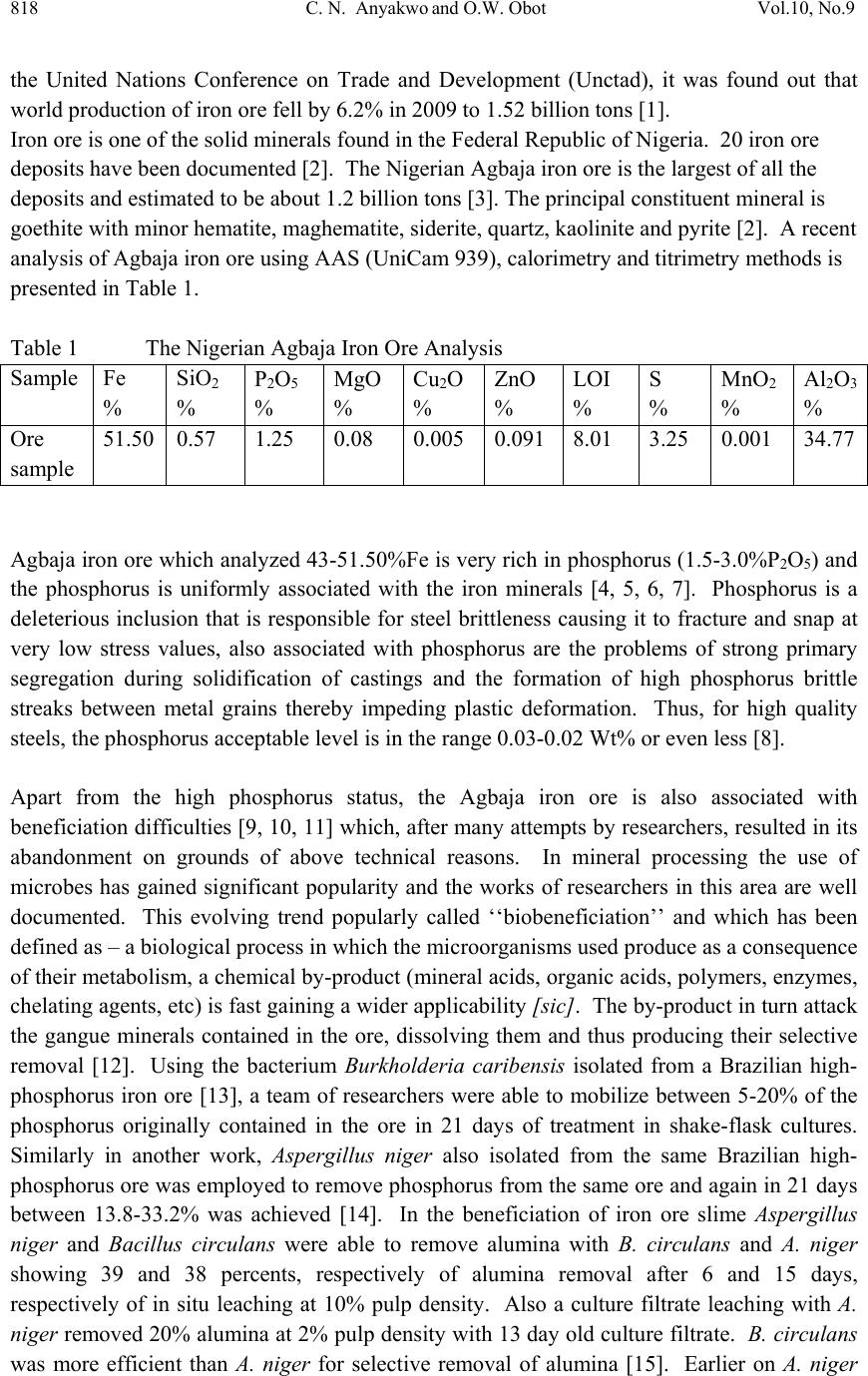 818 C. N. Anyakwo and O.W. Obot Vol.10, No.9 the United Nations Conference on Trade and Development (Unctad), it was found out that world production of iron ore fell by 6.2% in 2009 to 1.52 billion tons [1]. Iron ore is one of the solid minerals found in the Federal Republic of Nigeria. 20 iron ore deposits have been documented [2]. The Nigerian Agbaja iron ore is the largest of all the deposits and estimated to be about 1.2 billion tons [3]. The principal constituent mineral is goethite with minor hematite, maghematite, siderite, quartz, kaolinite and pyrite [2]. A recent analysis of Agbaja iron ore using AAS (UniCam 939), calorimetry and titrimetry methods is presented in Table 1. Table 1 The Nigerian Agbaja Iron Ore Analysis Sample Fe % SiO2 % P2O5 % MgO % Cu2O % ZnO % LOI % S % MnO2 % Al2O3 % Ore sample 51.50 0.57 1.25 0.08 0.005 0.091 8.01 3.25 0.001 34.77 Agbaja iron ore which analyzed 43-51.50%F e is very rich in phosphorus (1.5-3.0%P2O5) and the phosphorus is uniformly associated with the iron minerals [4, 5, 6, 7]. Phosphorus is a deleterious inclusion that is responsible for steel brittleness causing it to fracture and snap at very low stress values, also associated with phosphorus are the problems of strong primary segregation during solidification of castings and the formation of high phosphorus brittle streaks between metal grains thereby impeding plastic deformation. Thus, for high quality steels, the phosphorus acceptable level is in the range 0.03-0.02 Wt% or even less [8]. Apart from the high phosphorus status, the Agbaja iron ore is also associated with beneficiation difficulties [9, 10, 11] w hich, after many attempts by research ers, resulted in it s abandonment on grounds of above technical reasons. In mineral processing the use of microbes has gained significant popularity and the works of researchers in this area are well documented. This evolving trend popularly called ‘‘biobeneficiation’’ and which has been defined as – a biological process in which the microorganisms used produce as a consequence of their metabolism, a ch emical by -produ ct (mineral acids, or ganic acid s, poly mers, enzy mes, chelating agents, etc) is fast gaining a wider applicab ility [sic]. The by-product in turn attack the gangue minerals contained in the ore, dissolving them and thus producing their selective removal [12]. Using the bacterium Burkholderia caribensis isolated from a Brazilian high- phosphorus iron ore [13], a team of researchers were able to mobilize between 5-20% of the phosphorus originally contained in the ore in 21 days of treatment in shake-flask cultures. Similarly in another work, Aspergillus niger also isolated from the same Brazilian high- phosphorus ore was employed to remove phosphorus from the same ore and again in 21 days between 13.8-33.2% was achieved [14]. In the beneficiation of iron ore slime Aspergillus niger and Bacillus circulans were able to remove alumina with B. circulans and A. niger showing 39 and 38 percents, respectively of alumina removal after 6 and 15 days, respectively of in situ leaching at 10% pulp density. Also a culture filtrate leaching with A. niger removed 20% alumina at 2% pulp density with 13 day old culture filtrate. B. circulans was more efficient than A. niger for selective removal of alumina [15]. Earlier on A. niger  Vol.10, No.9 Labortory Studies on Phosphorus Removal 819 isolated from Nigeria’s Agbaja iron ore was used to mobilize phosphorus from the same ore and in 49 days of leaching, 81%, 63% and 68% efficiencies for Mesh 5, Mesh 100 and Mesh 250 grain sizes, respectively, were achieved [16]. The objective of the present study therefore, is aimed at using B. subtilis, a bacterium, to biobeneficiate the Nigerian Agbaja iron ore to a metallurgical acceptable level. Biobeneficiation is an alternative, ecologically friendlier approach, to the traditional physical and chemical methods of dephosphorisation. 2. MATERIALS AND METHODS The iron ore, Plate 2.1, was collected from Agbaja, an indigenous community near Lokoja, Kogi State of Nigeria. Standard glass wares, heavy equipment, chemicals and disposables used in the phosphorus removal process were sourced locally. Plate 2.1 Agbaja iron ore 1kg of the iron ore was manually crushed in the laboratory using hammer and anvil to generate tiny particles which were screened using Shital Test sieves into 1.00/0.50mm, 0.50/0.25mm and 0.25/0.125mm particle size fractions, Plates 2.2. (a) (b) (c) Plate 2.2 Ore Sample Particle Size Fractions (a) 1.00/0.50mm, (b) 0.50/0.25mm, (c) 0.25/0.125mm 10 g of each of the iron ore particle size fractions was weighed using Adventurer-AR3130 (with readability 001g) and placed in 90 ml of sterile water in 250 ml conical flask and then serially diluted to 10-6. Using a pipette 1 ml from each final dilution was taken to seed 10 sterile Petri dishes and about 20 ml of mineral oil medium, the recipe of which is given in Table 2.1., at 45 OC was added to each seeded Pet ri dish, swirled and allowed to stand for 14 days. 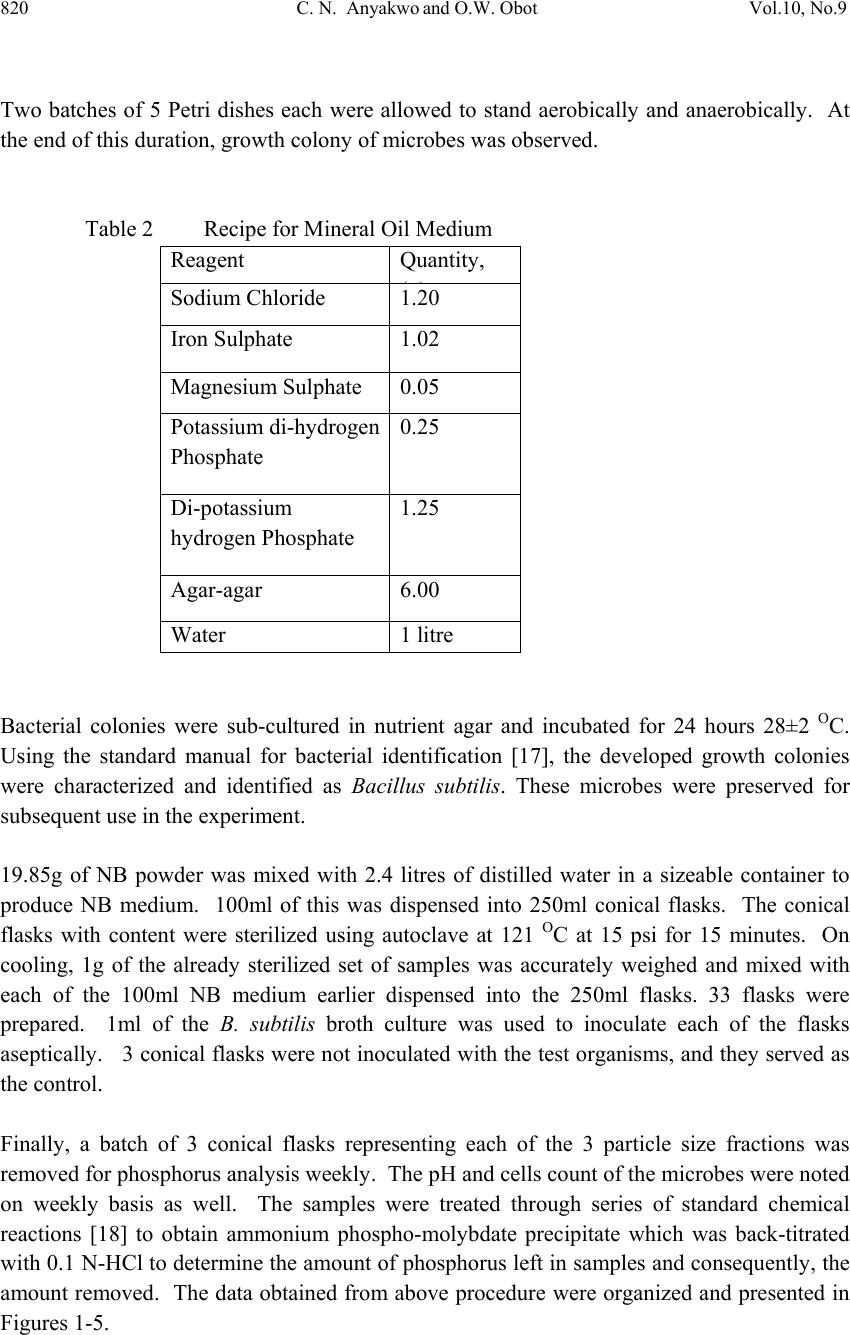 820 C. N. Anyakwo and O.W. Obot Vol.10, No.9 Two batches of 5 Petri dishes each were allowed to stand aerobically and anaerobically. At the end of this duration, growth colony of microbes was observed. Table 2 Recipe for Mineral Oil Medium Reagent Quantity, () Sodium Chloride 1.20 Iron Sulphate 1.02 Magnesium Sulphate 0.05 Potassium di-hydrogen Phosphate 0.25 Di-potassium hydrogen Phosphate 1.25 Agar-agar 6.00 Water 1 litre Bacterial colonies were sub-cultured in nutrient agar and incubated for 24 hours 28±2 OC. Using the standard manual for bacterial identification [17], the developed growth colonies were characterized and identified as Bacillus subtilis. These microbes were preserved for subsequent use in the experiment. 19.85g of NB powder was mixed with 2.4 litres of distilled water in a sizeable container to produce NB medium. 100ml of this was dispensed into 250ml conical flasks. The conical flasks with content were sterilized using autoclave at 121 OC at 15 psi for 15 minutes. On cooling, 1g of the already sterilized set of samples was accurately weighed and mixed with each of the 100ml NB medium earlier dispensed into the 250ml flasks. 33 flasks were prepared. 1ml of the B. subtilis broth culture was used to inoculate each of the flasks aseptically. 3 conical flasks w ere not inoculated with the test organisms, and they served as the control. Finally, a batch of 3 conical flasks representing each of the 3 particle size fractions was removed for phosphorus analysis weekly. The pH and cells count of the microbes w er e noted on weekly basis as well. The samples were treated through series of standard chemical reactions [18] to obtain ammonium phospho-molybdate precipitate which was back-titrated with 0.1 N-HCl to determine the amount of phosphorus left in samples and consequently, the amount removed. The data obtained from above procedure were organized and presented in Figures 1-5. 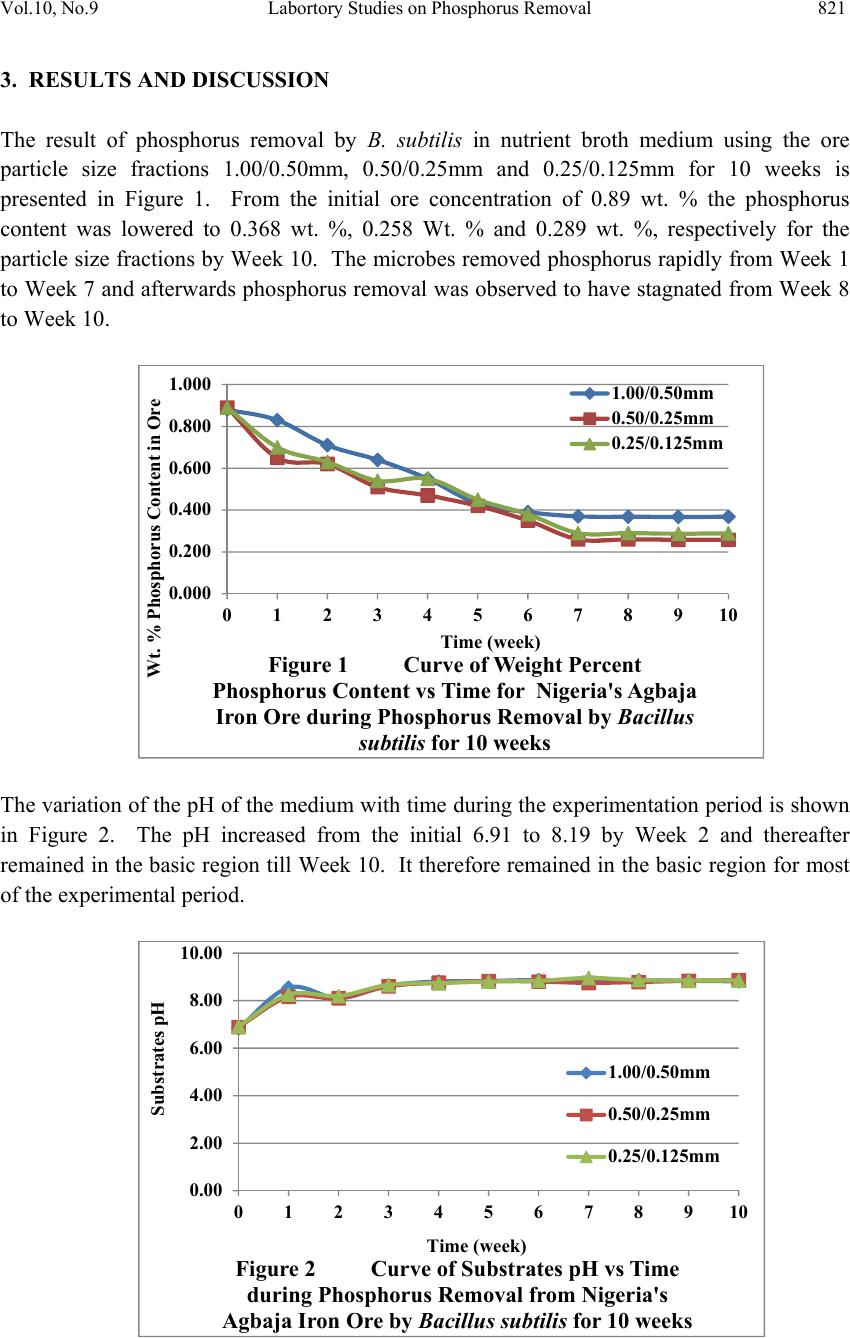 Vol.10, No.9 Labortory Studies on Phosphorus Removal 821 3. RESULTS AND DISCUSSION The result of phosphorus removal by B. subtilis in nutrient broth medium using the ore particle size fractions 1.00/0.50mm, 0.50/0.25mm and 0.25/0.125mm for 10 weeks is presented in Figure 1. From the initial ore concentration of 0.89 wt. % the phosphorus content was lowered to 0.368 wt. %, 0.258 Wt. % and 0.289 wt. %, respectively for the particle size fractions by Week 10. The microbes removed phosphorus rapidly from Week 1 to Week 7 and afterwards phosphorus removal was observed to have stagnated from Week 8 to Week 10. The variation of the pH of the medium with time during the experimentation period is shown in Figure 2. The pH increased from the initial 6.91 to 8.19 by Week 2 and thereafter remained in the basic re gion till Week 10. It therefore remained in the basic region for most of the experimental period. 0.000 0.200 0.400 0.600 0.800 1.000 012345678910 Wt. % Phosphorus Content in Ore Time (week) Figure 1Curve of Weight Percent Phosphorus Content vs Time for Nigeria's Agbaja Iron Ore during Phosphorus Removal by Bacillus subtilisfor 10 weeks 1.00/0.50mm 0.50/0.25mm 0.25/0.125mm 0.00 2.00 4.00 6.00 8.00 10.00 012345678910 Substrates pH Time (week) Figure 2Curve of Substrates pH vs Time during Phosphorus Removal fr om N igeria's Agbaja Iron Ore by Bacillus subtilisfor 10 weeks 1.00/0.50mm 0.50/0.25mm 0.25/0.125mm 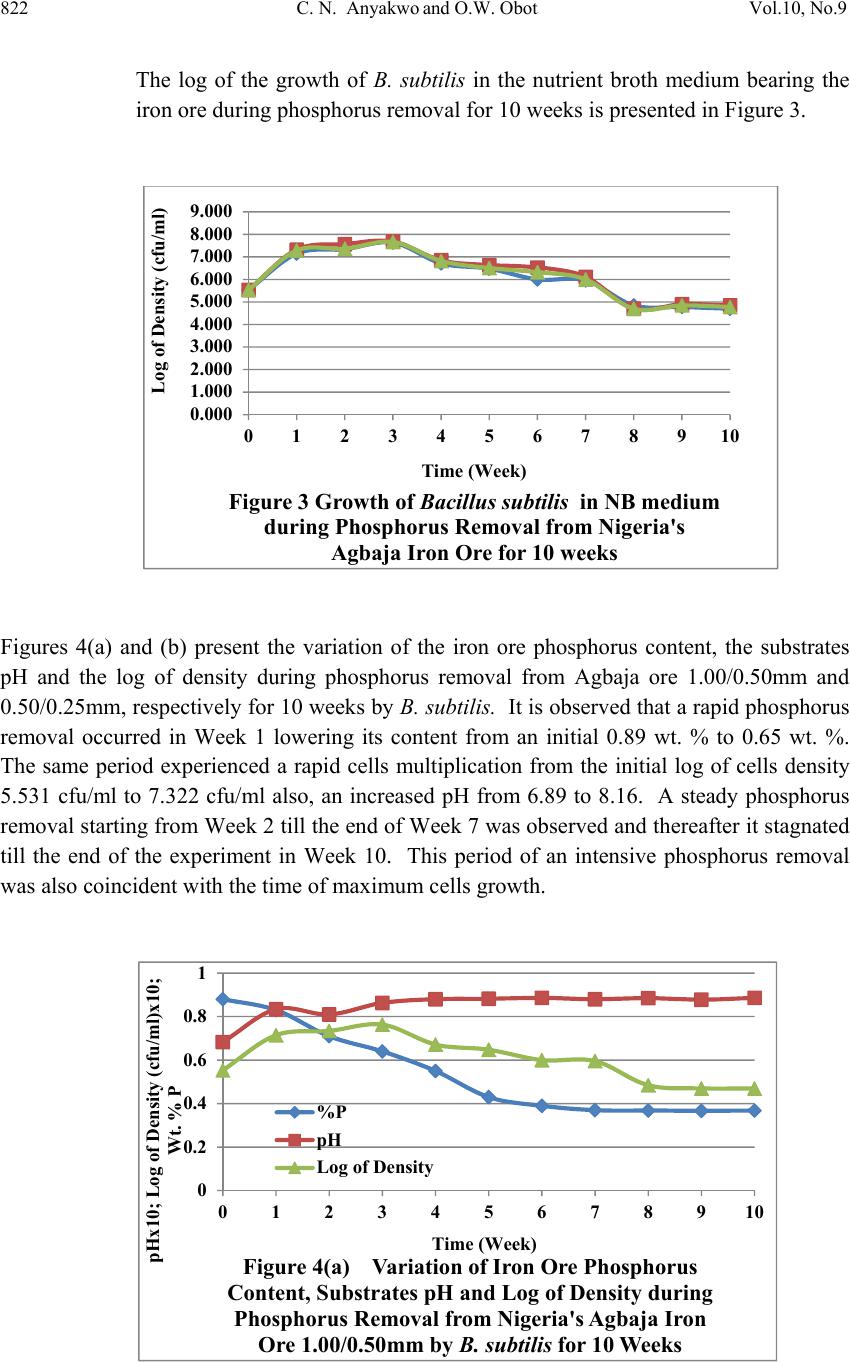 822 C. N. Anyakwo and O.W. Obot Vol.10, No.9 The log of the growth of B. subtilis in the nutrient broth medium bearing the iron ore during phosphorus removal for 10 weeks is presented in Figure 3. Figures 4(a) and (b) present the variation of the iron ore phosphorus content, the substrates pH and the log of density during phosphorus removal from Agbaja ore 1.00/0.50mm and 0.50/0.25mm, respectively for 10 weeks by B. subtilis. It is observed that a rapid phosphorus removal occurred in Week 1 lowering its content from an initial 0.89 wt. % to 0.65 wt. %. The same period experienced a rapid cells multiplication from the initial log of cells density 5.531 cfu/ml to 7.322 cfu/ml also, an increased pH from 6.89 to 8.16. A steady phosphorus removal starting from Week 2 till the end of Week 7 was observed and thereafter it stagnated till the end of the experiment in Week 10. This period of an intensive phosphorus removal was also coincident with the time of maximum cells growth. 0.000 1.000 2.000 3.000 4.000 5.000 6.000 7.000 8.000 9.000 012345678910 Log of Density (cfu/ml) Time (Week) Figure 3 Growth of Bacillus subtil isin NB medium during Phosphorus Removal from Nigeria's Agbaja Iron Ore for 10 weeks 0 0.2 0.4 0.6 0.8 1 012345678910 pHx10 ; Log of Den sity (cfu/ml)x10; Wt. % P Time (Week) Figure 4(a) Variation of Iron Ore Phosphorus Content, Substrates pH and Log of Density during Phosphorus Removal from Nigeria's Agbaja Iron Ore 1.00/0.50mm by B. subtilisfor 10 Weeks %P pH Log of Density 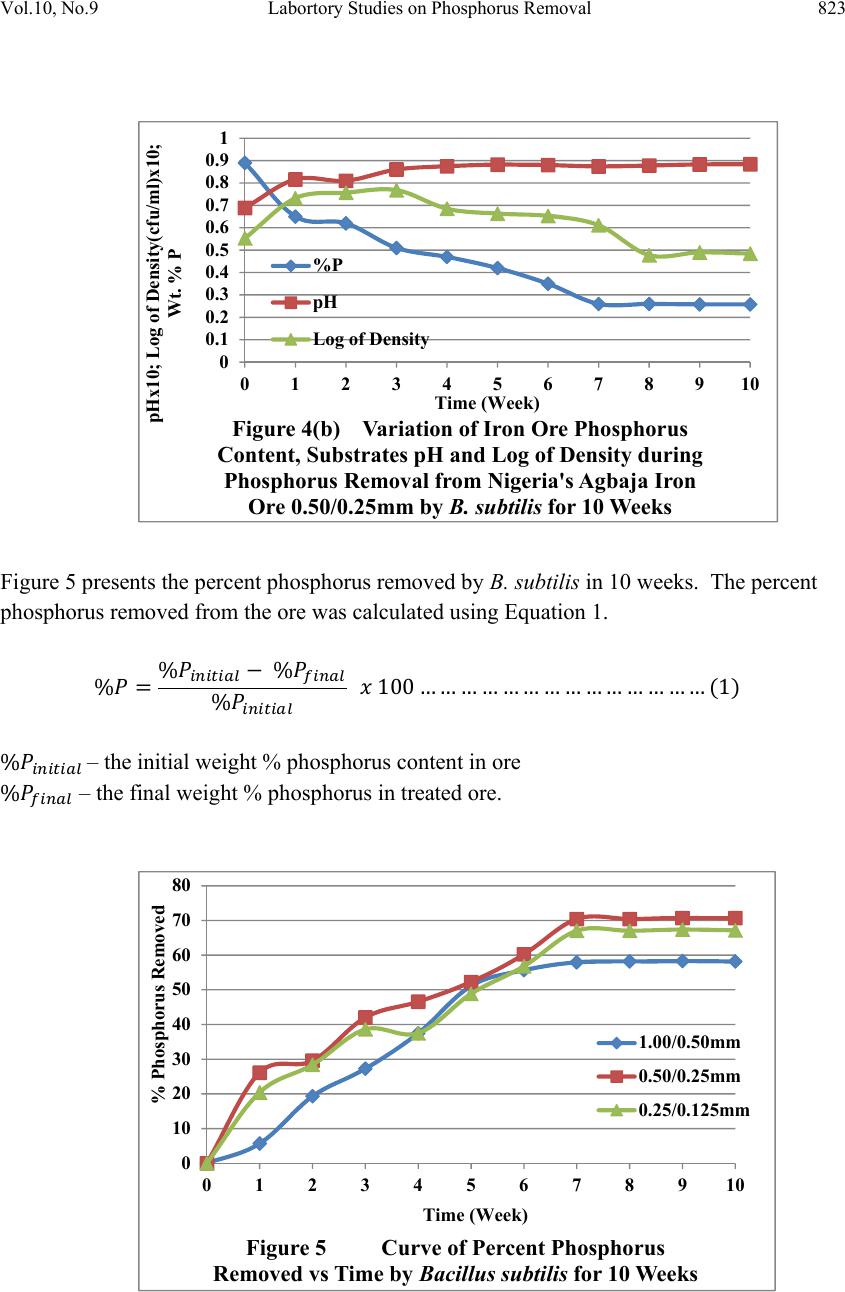 Vol.10, No.9 Labortory Studies on Phosphorus Removal 823 Figure 5 presents the percent phosphorus removed by B. subtilis in 10 weeks. The percent phosphorus removed from the ore was calculated using Equation 1. % % % % 100……………………………………1 % – the initial weight % phosphorus content in ore % – the final weight % phosphorus in treated ore. 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 012345678910 pHx10 ; L og o f D en sity(cf u /ml) x1 0 ; Wt. % P Time (Week) Figure 4(b) Variation of Iron Ore Phosphorus Content, Substrates pH and Log of Density during Phosphorus Removal from Nigeria's Agbaja Iron Ore 0.50/0.25mm by B. subtilisfor 10 Weeks %P pH Log of Density 0 10 20 30 40 50 60 70 80 012345678910 % Phosphorus Removed Time (Week) Figure 5Curve of Percent Phosphorus Removed vs Time by Bacillus subtilisfor 10 Weeks 1.00/0.50mm 0.50/0.25mm 0.25/0.125mm  824 C. N. Anyakwo and O.W. Obot Vol.10, No.9 4. CONCLUSION Bacillus subtilis has the capability to successfully remove phosphorus from Nigeria’s Agbaja iron ore. The result of this research shows that an average of 65.73%P removal across the 3 particle size fractions was achieved. The highest removal of 71.01%P was achieved for 0.50/0.25mm. ACKNOWLEDGEMENT Particularly deserving great thanks are the staff and Heads of Departments of Microbiology and Chemical Engineering of University of Uyo, Akwa Ibom State. Few names will not fail to be mentioned and I wish to thank my Supervisor, Engr. (Dr.) C. N. Anyakwo who, actually not only conceived the work and provided the experimental strategies stage by stage, but also, brought his knowledge and expertise to bear on the entire work. Engr. Peter Asangausung, the Senior Technologist in-charge of the Chemical Engineering Laboratory where the bulk of this work was done, for his resourcefulness, keen interest and constant prayers; Dr. A. O. Ano of the Nigerian Root Crops Research Institute, Umuahia, Abia state, whose elastic patience limit was over stretched many times in an unprecedented manner by my over-keeping borrowed equipment worth millions of naira in order to complete this research. REFERENCES 1. The Iron Ore Market. Downloaded on 5 December,2010 from www.unctad.org/infocomm/iron/covmar08.htm 2. Uwadiale, G. G. O. O. (1989). Upgrading Nigerian Iron Ores. Journal of Minerals and Metallurgical Processing. AIME, pp.117-123. 3. Uwadiale, G.G.O.O. and Hall, A.J. (1985). Mineralogy of Ironstone from Agbaja Deposit Nigeria in Relation to Beneficiation. Trans. Inst. Minerals & Metallurgy. (Section B. Appl. Earth Science) 94(161-165). 4. Alafara, A. Baba., Adekola, F. A., Folashade, A. O. (2005). Quantitative Leaching of a Nigerian Ore in Hydrochloric Acid. J. Appl. Sci. Environ. Mgt.Vol 9(3) pp.15-20. 5. Astier, J. E., Donzeau, M., and Uwadiale, G. G. O. O. (1989). The Lokoja Oolitic Ironstone Deposit: Possible Use in the Ajaokuta Iron and Steel Plant. Journal of Mining and Geology Vol.25 Nos. (1&2). 6. Adeleye, D. R. (1974). A Fauna from the Ironstone of the Middle Valley. Journal of Mining and Geology 8(pp.45-48). 7. Adeleye, D. R. (1973). Origin of Ironstones, an Example from the Middle Niger Valley. Journal of Sed. Petrology, 43(3). (pp.709-727). 8. Kudrin, V., Steel Making, MIR Publishers, Moscow, 1985, pp 82-83. 9. Uwadiale, G. G. O. O. and Nwoke, M. A. U.; Beneficiation of Agbaja Iron Ore by Reduction Roasting- Magnetic Separation: Semi Pilot Plant Scale-up and Establishment of Residence Point of Phosphorus, National Steel Council, Metallurgical Research and Tests Division, Jos, Nigeria, June 1983.  Vol.10, No.9 Labortory Studies on Phosphorus Removal 825 10. Uwadiale, G.G.O.O. Beneficiation Studies of Agbaja Iron Ore, being text of a lectur e delivered at a staff seminar, Department of chemistry, University of Benin, Nigeria, on May 1983. 11. Amadi, N. J., Odunaike, A. A and Marthur, G. P; Preliminary Bench Scale Beneficiation Studies with Three Lumps of Iron Ore Sample from Agbaja, Central Metallurgical Research and Development Institute, Jos, Nigeria, 1982. 12. Jain, N., Sharma, D. (2004). Biohydrometallurgy for Nonsulfidic Minerals – a Review. Geomicrobiology Journal, 21:3, p.135 – 144. 13. Delvasto, P. et al, 2005. Exploring the possibilities of biological beneficiation of iron- ores: The phosphorus problem. In: Proceedings of the 15th Steelmaking Conference, 5th Ironmaking Conference & 1st Environm ent and Recycling Symposium IAS (CD-ROM). Argentinean Steelmaking Institute (IAS). San Nicolás, Buenos Aires, Argentina, pp. 71-82. 14. Dephosphorisation of an Iron Ore by a Filamentous Fungus, 2007. Pedro Delvasto, Antonio Ballester, Jesus Angel Munos, Maria Luisa Blasquez, Felisa Gonzalez and Camino Garcia-Balboa. Proceedings…VII Meeting of the southern Hemisphere on Mineral Technolo gy, Brazil. 15. Pradhan, N. et al., 2006, ‘‘Beneficiation of Iron Ore Slime using Aspergillus niger and Bacillus circulans’’, Bioresource Technology 97: 15, P. 1876-1879 16. Anyakwo, C. N., Obot, O. W., 2008, ‘‘Phosphorus Removal from Nigeria’s Agbaja Iron Ore by Aspergillus niger’’, International Research Journal in Engineering, Science & Technology, Vol. 5 No. 1, pp 54-58. 17. Holt, John G., Krieg, Noel R., Sneath, Peter H. A., Stanley, James T., and Williams, Stanley T., 1994, “Bergy’s Manual of Determinative Bacteriology,’’ 9th Edition, Lippincott Williams and Wilkins, Maryland, USA. pp. 559,562. 18. Jain S. K., (1982). An Introduction to Metallurgical Analysis: Chemical and Instrumental, India, New Delhi, Vikas Publishing House. 19. Bacterial Growth, Wikipedia – downloaded 5 January, 2011 from www.http://en.m.wikipedia.org/wiki/Bacterial_Growth |

