Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 9, No.6, pp.559-568, 2010 jmmce.org Printed in the USA. All rights reserved 559 Preparation and Structural Characterization of Vanadium Doped Ni – B Binary Hard Alloys J. A. Ajao Materials and Electronics Division, Centre for Energy Research and Development, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria E-mail: jajao@cerd.gov.ng or johnajao2000@yahoo.com ABSTRACT The microstructure of a series of binary Ni-B alloys containing various amounts of vanadium additions were investigated by Differential Thermal Analysis (DTA), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM) and Energy Dispersive X-ray Analysis (EDXA). Due to the vanadium addition in the alloys quenched from the liquidus, the formation of the Ni 3 B phase was enhanced even when the nominal composition was hypoeutectic. The addition of vanadium led to the formation of τ (Ni 20.4 V 2.6 B 6 ) phase during the present investigation. In addition, the crystallographic orientation relationship of this ternary phase with the nickel matrix was reported. The solid-state eutectoid transformation of the Ni 3 B phase during cooling was also reported and discussed. Keywords: Alloy, Solidification, Microstructure, Solid-state transformation. 1. INTRODUCTION One of the basic components of the Nickel-based hardfacing alloys are the Ni – B hard phases. These hardfacing alloys are specially developed to solve the problems of wear and corrosion in petrochemical, glass, automobile, nuclear and other related industries. They are employed as coatings material deposited by various coating techniques [1-3]. The hard alloys usually consist of nickel as a base metal while chromium, tungsten, molybdenum and vanadium are used as metallic additives and boron, silicon and carbon as non-metallic additives [4]. Addition of chromium promotes the oxidation and corrosion resistance property at elevated temperatures and enhances the hardness of the coating by formation of hard phases. Boron depresses the melting temperature and plays active role in the 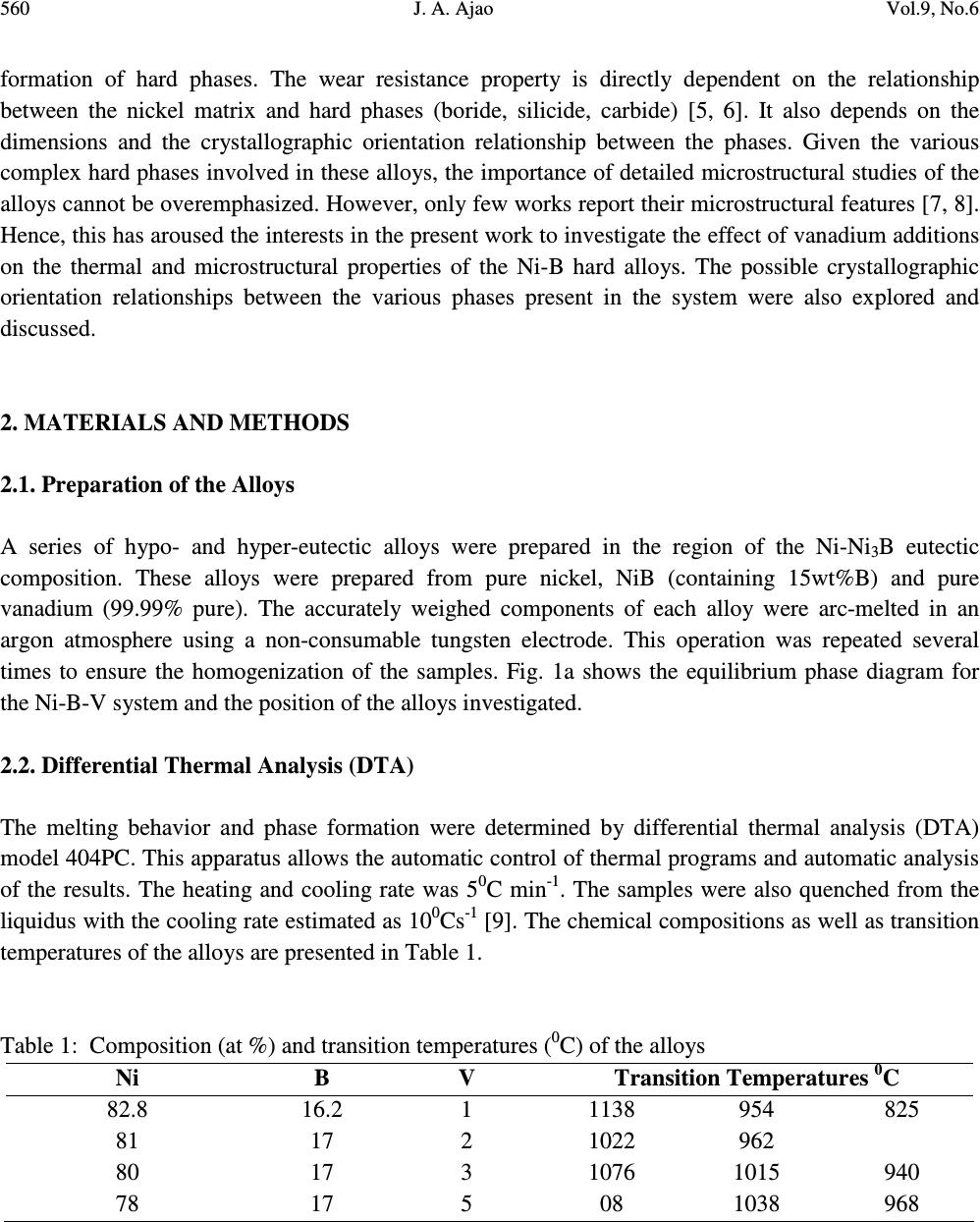 560 J. A. Ajao Vol.9, No.6 formation of hard phases. The wear resistance property is directly dependent on the relationship between the nickel matrix and hard phases (boride, silicide, carbide) [5, 6]. It also depends on the dimensions and the crystallographic orientation relationship between the phases. Given the various complex hard phases involved in these alloys, the importance of detailed microstructural studies of the alloys cannot be overemphasized. However, only few works report their microstructural features [7, 8]. Hence, this has aroused the interests in the present work to investigate the effect of vanadium additions on the thermal and microstructural properties of the Ni-B hard alloys. The possible crystallographic orientation relationships between the various phases present in the system were also explored and discussed. 2. MATERIALS AND METHODS 2.1. Preparation of the Alloys A series of hypo- and hyper-eutectic alloys were prepared in the region of the Ni-Ni 3 B eutectic composition. These alloys were prepared from pure nickel, NiB (containing 15wt%B) and pure vanadium (99.99% pure). The accurately weighed components of each alloy were arc-melted in an argon atmosphere using a non-consumable tungsten electrode. This operation was repeated several times to ensure the homogenization of the samples. Fig. 1a shows the equilibrium phase diagram for the Ni-B-V system and the position of the alloys investigated. 2.2. Differential Thermal Analysis (DTA) The melting behavior and phase formation were determined by differential thermal analysis (DTA) model 404PC. This apparatus allows the automatic control of thermal programs and automatic analysis of the results. The heating and cooling rate was 5 0 C min -1 . The samples were also quenched from the liquidus with the cooling rate estimated as 10 0 Cs -1 [9]. The chemical compositions as well as transition temperatures of the alloys are presented in Table 1. Table 1: Composition (at %) and transition temperatures ( 0 C) of the alloys Ni B V Transition Temperatures 0 C 82.8 16.2 1 1138 954 825 81 17 2 1022 962 80 17 3 1076 1015 940 78 17 5 08 1038 968  Vol.9, No.6 Preparation and Structural Characterization 561 2.3. Characterization of the Alloys Microstructural characterizations of the alloys were carried out using optical and scanning electron microscopes. The samples for SEM observations were slightly etched with an etchant consisting of 5g FeC1 3 + 10ml Conc. HCI dissolved in 50ml H 2 0. The crystallographic nature of the phases present in these samples was examined by X-ray diffraction (XRD). Transmission electron microscopy (TEM) and electron diffraction were performed on thin foils of the samples in a JEM-200CX microscope. Thin foils were obtained by electropolish in an electrolyte containing 57% H 2 SO 4 at room temperature under 10V. 3. RESULTS AND DISCUSSION 3.1. Morphology of the Alloys 3.1.1. Samples Cooled at Rapid Rate A certain number of alloys with varied compositions of vanadium l at % < V < 5 at % were prepared. For alloy containing l at % V and quenched from the liquids, the microstructure observed in SEM shows the primary phase of Ni 3 B (Fig. 1b) surrounded by the lamellar binary eutectic Ni (α) - Ni 3 B. It should be noted that for alloy of similar composition but without vanadium addition, the primary phase observed was Ni (α). Hence, vanadium addition has probably caused the microstructure of the alloy to shift from the hypoeutectic to hypereutectic region during rapid cooling. Detailed observations in TEM of the Ni 3 B primary phase showed the presence of some precipitates in coherence with the matrix. Fig. 2a shows the transmission electron micrograph on these precipitates while Figs. 2b and c present the diffraction pattern and the key diagram on the precipitates respectively. From the diffraction pattern obtained on the precipitates and the energy dispersive X-ray analysis (EDXA) performed on them, a ternary τ (Ni 20.4 V 2.6 B 6 ) phase could be proposed. The lattice parameter of this ternary phase varies between 10.47Å and 10.49Å [10]. Similar ternary phase has been reported in Ni-B-Si [11] and Ni-B-Ti [12, 13] systems. For alloys containing 3 to 5 at %V and quenched from the liquidus, SEM observations only revealed the binary eutectic structure between Ni (α) and Ni 3 B. Unlike boron, vanadium is more soluble in nickel. Hence the domain of existence of the ternary phase τ (Fig. 1a) is much more reduced. Furthermore, Fig. 3 shows the TEM observations on the binary eutectic Ni (α) – Ni 3 B obtained in alloys containing 3 – 5 at %V quenched from the liquidus. The binary eutectic is lamellar with the lamellae of Ni 3 B (1500Å) about twice the size of the Ni (α) lamellae (800Å) as shown in the transmission electron micrograph of Fig. 3a. 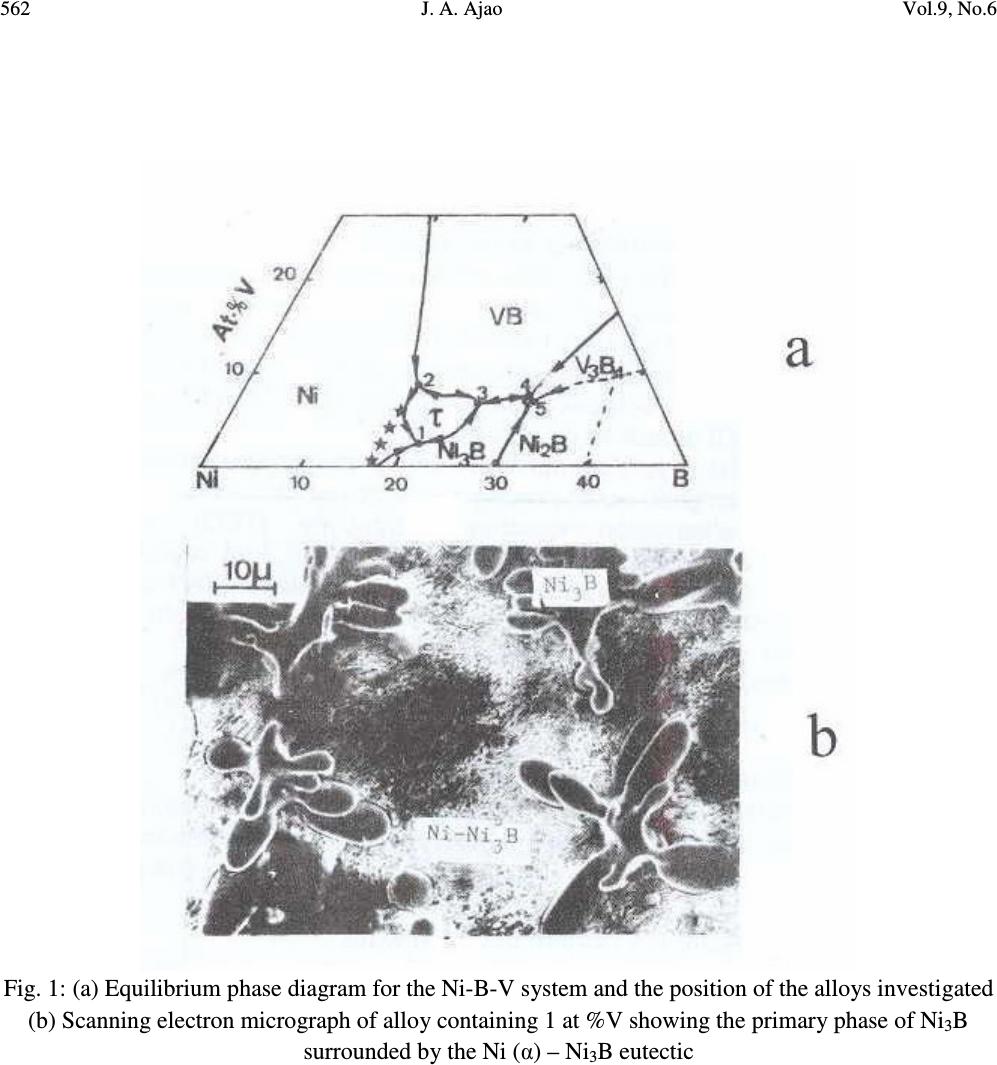 562 J. A. Ajao Vol.9, No.6 Fig. 1: (a) Equilibrium phase diagram for the Ni-B-V system and the position of the alloys investigated (b) Scanning electron micrograph of alloy containing 1 at %V showing the primary phase of Ni 3 B surrounded by the Ni (α) – Ni 3 B eutectic  Vol.9, No.6 Preparation and Structural Characterization 563 Fig. 2: (a) TEM on the τ (Ni 20.4 V 2.6 B 6 ) ternary phase (b and c) Diffraction pattern and the key diagram on the τ precipitates 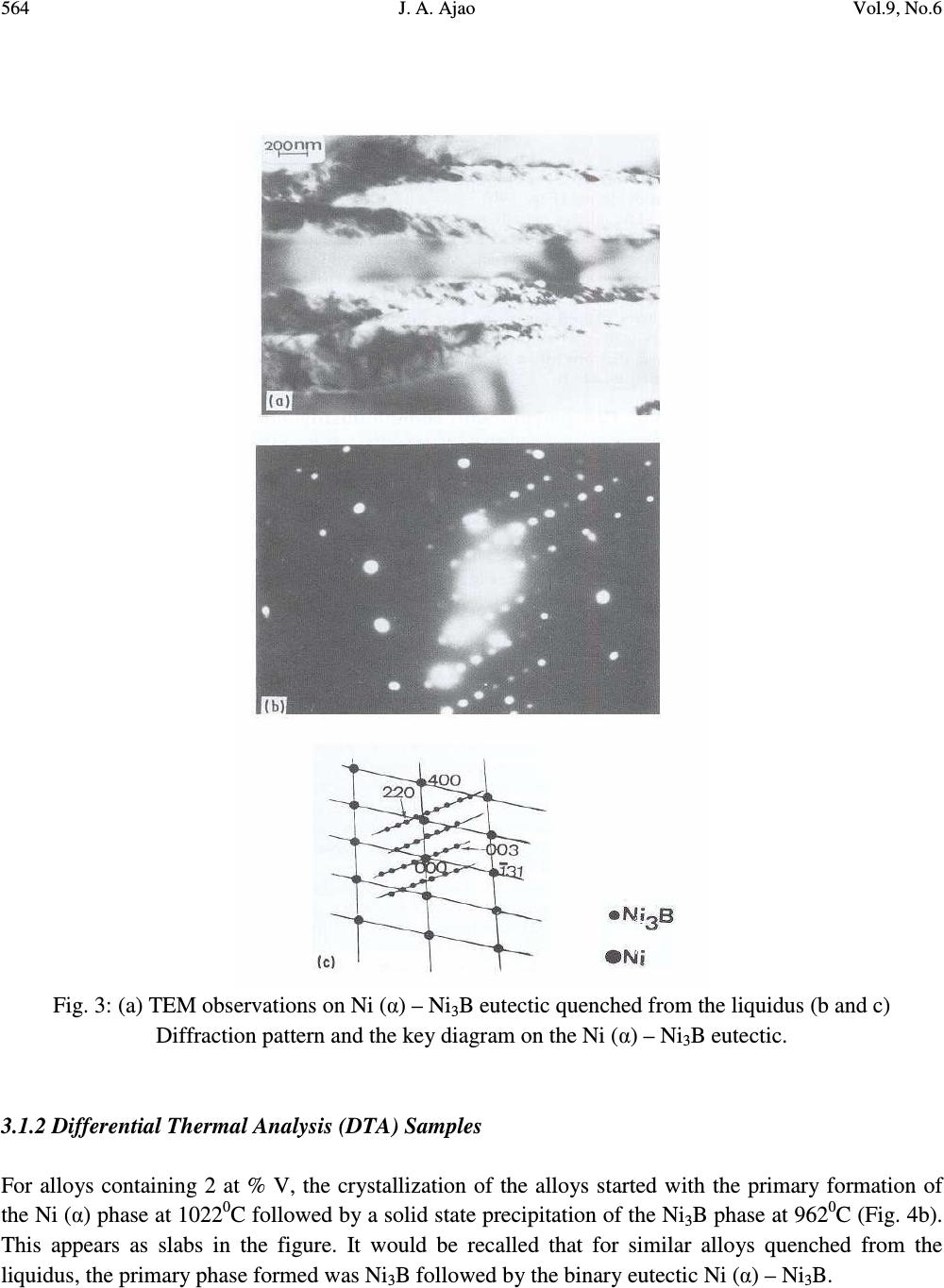 564 J. A. Ajao Vol.9, No.6 Fig. 3: (a) TEM observations on Ni (α) – Ni 3 B eutectic quenched from the liquidus (b and c) Diffraction pattern and the key diagram on the Ni (α) – Ni 3 B eutectic. 3.1.2 Differential Thermal Analysis (DTA) Samples For alloys containing 2 at % V, the crystallization of the alloys started with the primary formation of the Ni (α) phase at 1022 0 C followed by a solid state precipitation of the Ni 3 B phase at 962 0 C (Fig. 4b). This appears as slabs in the figure. It would be recalled that for similar alloys quenched from the liquidus, the primary phase formed was Ni 3 B followed by the binary eutectic Ni (α) – Ni 3 B. 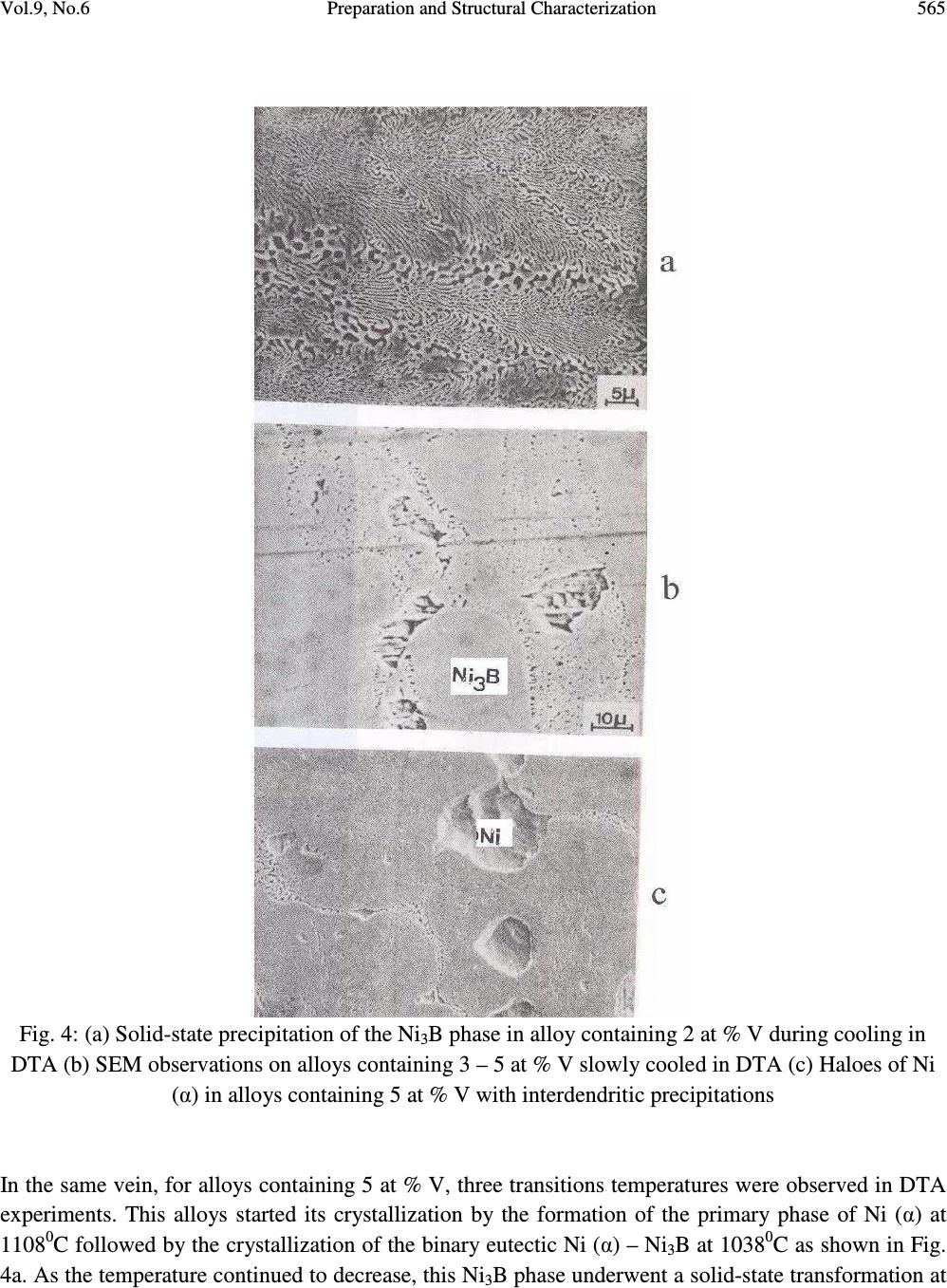 Vol.9, No.6 Preparation and Structural Characterization 565 Fig. 4: (a) Solid-state precipitation of the Ni 3 B phase in alloy containing 2 at % V during cooling in DTA (b) SEM observations on alloys containing 3 – 5 at % V slowly cooled in DTA (c) Haloes of Ni (α) in alloys containing 5 at % V with interdendritic precipitations In the same vein, for alloys containing 5 at % V, three transitions temperatures were observed in DTA experiments. This alloys started its crystallization by the formation of the primary phase of Ni (α) at 1108 0 C followed by the crystallization of the binary eutectic Ni (α) – Ni 3 B at 1038 0 C as shown in Fig. 4a. As the temperature continued to decrease, this Ni 3 B phase underwent a solid-state transformation at  566 J. A. Ajao Vol.9, No.6 968 0 C as evidenced in the interdendritic grooves in the micrograph in Fig. 4c. Similar observations have been reported during slow cooling of Ni-B alloys doped with titanium [13]. In fact, at 968 0 C, the non-stoichiometric Ni 3 B was saturated with the atoms of nickel and vanadium at the same time. The rejection of these atoms of nickel and vanadium to the interface gave rise to the solid-state transformation (eutectoid-like structure) as follows: Ni 3 B (Ni, V) → Ni 3 B + Ni (V) [∆H= 20kJ kg -1 ] The relation between the thermal effect due to the change of state and the surface area of the peak in DTA experiment is given by m∆H = kS where m = mass of the melt ∆H = enthalpy of transformation S = surface area of the peak in DTA k = heat transfer coefficient For the alloy undergoing solid-state transformation (with m = 0.002kg), the enthalpy of transformation has been estimated as 20 x 10 3 J.kg -1 [9]. From the DTA trace, the surface area of the peak during transition at 954 0 C was calculated as 55 x 10 -6 . Hence, from the above relation and data, the heat transfer coefficient (k) between the crucible and the melt has been estimated as 8 x10 5 W m 2 K -1 . This is in good agreement with the value obtained by Casanova et al. [15]. It is also important to report that the hardness measurements carried out on these samples showed a higher value for alloys with vanadium additions due probably to the formation of some pockets of VB hard phases which might be present in the alloys. 3.2 Crystallographic Orientation Relationship between Ni (α) and Ni 3 B The superposed diffraction pattern of the two phases is shown in Fig. 3b while the key diagram is presented in Fig. 3c. From the superposed electron diffraction pattern of the two phases, the following relative crystallographic orientation relationship between the two phases could be deduced: (200) Ni (α) // (221) Ni3B [013] Ni(α) // [110] Ni3B This orientation relationship is quite different from those obtained from pure Ni (α) - Ni 3 B eutectic quenched from the liquidus [9] showing the pronounced influence of vanadium addition. However, this mutual crystallographic orientation between the two phases is comparable to the orientation relationship observed between them in meltspun Ni-B alloys [14]. From the above crystallographic orientation relationship, the lattice mismatch could be estimated as 33% for the [013] Ni(α) // [110] Ni3B direction. This value is far greater than 10% which could have made the planes special. These  Vol.9, No.6 Preparation and Structural Characterization 567 observations show the apparent effect of cooling conditions on the orientation and the growth of the interface. 4. CONCLUSION A series of Ni-B alloys containing various amount of vanadium additions were studied using various characterization techniques. Precipitates of a ternary phase identified as the τ- phase and coherent with the matrix were observed in the alloys during quench from the liquidus. The mutual crystallographic orientation relationship of this ternary phase with the matrix was also reported. In addition, solid-state transformation of the Ni 3 B phase during slow cooling was reported and an explanation attempted. ACKNOWLEDGEMENTS The author is thankful to Dr. D. A. Pelemo for helpful discussions on electron microscopy. The Centre for Energy Research and Development, Obafemi Awolowo University, Ile-Ife, Nigeria is appreciated for granting the author leave of absence during the preparation of this work. REFERENCES [1] Fernandez, E., Cadenas, M., Gonzalez, R., Navas, C., Fernandez, R. and J. de Damborenea, 2005, “Wear behaviour of laser clad NiCrBSi coating”, Wear 259: 870-875. [2] Gonzalez, R., Garcıa, M. A., Penuelas, I., Cadenas, Rocıo, Fernandez, Ma. Del., Hernandez Battez, A., and Felgueroso, D., 2007, “Microstructural study of NiCrBSi coatings obtained by different processes”, Wear 263: 619–624. [3] Knotek, O., Lohage, P. and Reimann, H., 1983, “Nickel-based Wear-resistant Coatings by Vacuum Melting”, Thin Solid Films 108:449-458. [4] Knotek, O., Reimann, H. and Lohage, P., 1981, “Reactions between Ni-Cr-B-Si matrixes and carbide additives in coating during fusion treatment”, Thin Solid Films 83:361 – 367. [5] Lebaili, S., Durand-Charre, M. and Hamar-Thibault, S., 1988, “The Metallurgical Structure of as – solidified Ni-Cr-B-Si-C Hardfacing Alloys”, J. Mat. Sci. 23:3603–3611. [6] Chin-you Hsu, Jien-Wei Yeh, Swe-Kai Chen and Tao-Tsung Shun., 2004, “Wear Resistance and High-Temperature Compression Strength of FCC CuCoNiCrAl 0.5 Fe Alloy with Boron Addition”, Metallurgical and Materials Transactions A Vol 35A: 1465 - 1470. [7] Lebaili, S, Ajao, J. and Hamar-Thibault, S., 1992, “Preparation and Characterization of meltspun nickel-based alloys containing heavy metals”, J. Alloys Compd. 188: 87-93. [8] Li, Q., Zhang, D., Lei, T., Chen, C. and Chen, W., 2001, “Comparison of laser-clad and furnace- melted Ni-based alloy microstructures”, Surf. Coat. Technol. 137:122–135.  568 J. A. Ajao Vol.9, No.6 [9] Ajao, J. and Hamar-Thibault, S., 1988, “Influence of Additions on the Solidification behavior of Ni-B alloys – crystallography of Ni-Bi3B eutectic”, J. Mater. Sci. 23: 1112 – 1125. [10] Stadelmaier, H. H. and Balance, G. B., 1967, “The Ni-V-B Ternary System”, Metall. 21, 691-695. [11] Knotek, O. and Lugscheider, E., 1974, “On the Structure of Ni-Cr-B-Si Hardfacing Alloys and their Bonding Reactions”, J. Vacuum Sci. Tech. 11:798 – 801. [12] Finch, C. B., Becher, P. F., Ferber, M. K. Tennery, V.J. and Yust, C. S., 1982, “Growth and Properties of Ni 20.3 Ti 2.7 B 6 (τ- phase) Crystals”, J. Cryst. Growth 58: 647 – 649. [13] Ajao, J. A., 2009, “Phase transitions in some nickel-rich nickel–boron–titanium hard alloys”, J. Alloys Compds. DOI:10.1016/j.jallcom.2009.12.091. [14] Ajao, J. and Hamar-Thibault, S., 1989, “Structure Observations by High Resolution Electron Microscopy of Ni-B Meltspun Alloys”, J. Mater. Sci. 24:3647–3659. [15] Casanova, P., Joud, J.C. Senillou, C. and Yavari, A. R., 1984, “Elaboration des Rubans larges de Verres Metalliques”, Mem. Sci. Rev. Met. 81:553–559. |

