Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 9, No.6, pp.519-525, 2010 jmmce.org Printed in the USA. All rights reserved 519 Sonochemical Coating of Ag-TiO 2 Nanoparticles on Textile Fabrics for Stain Repellency and Self-Cleaning- The Indian Scenario: A Review *1 Subhranshu Sekhar Samal, 2 P. Jeyaraman & 3 Vinita Vishwakarma 1, 2, 3 Centre for Nanoscience & Nanotechnology (A joint Initiative of IGCAR, Kalpakkam & Sathyabama University), Chennai, India * Corresponding Author: Subhranshu Sekhar Samal, Scientist-C, CNSNT Sathyabama University Campus, Chennai-600119 E-mail: ssubhransu@gmail.com ABSTRACT Surface modification is an important element of textile manufacturing. The final properties of a textile material are critical in determining how they perform for their given end use. More recently, botany and nanotechnology have united to explore not only the beauty and cleanliness of the leaf, but also its lack of contamination and bacteria, despite its dwelling in dirty ponds. On the basis of lotus leaf concept scientist developed a new concept “Self cleaning textile” the textile surface which can be cleaned itself without using any laundering action. In this paper, the role of silver- Titania nanoparticles synthesized and deposited on different types of fabrics using ultrasound irradiation under an atmosphere of argon gas and decreasing both the cavitational threshold and intensity of ultrasound power has been reviewed. The excellent antibacterial activity of the Ag–Titania fabric composite against Escherichia coli (gram-negative) and Staphylococcus aureus (gram-positive) cultures also reviewed with reference to Indian scenario. Keywords: surface modification/ textile/self cleaning/ultrasound/silver 1. BACKGROUND Nanoparticles possess distinct properties from those of bulk phase or individual molecules. Surface modification is an important element of textile manufacturing. The final properties of a textile material are critical in determining how they perform for their given end use. Nano-  520 Subhranshu Sekhar Samal, P. Jeyaraman & Vinita Vishwakarma Vol.9, No.6 coating the surfaces of textiles and clothing is one approach to the production of highly active surfaces to have UV-blocking, antimicrobial and self-cleaning properties. While antimicrobial properties are exerted by nano-silver, self-cleaning of wine and coffee stains using a solar stimulated light is imparted by nano-TiO 2 coating. Silver nanoparticles can be used as an antimicrobial agent in applications such as wound dressings and as surface coatings e.g., catheters. Silver colloids have been shown to provide a good antibacterial effect on polymers and textile products. The coating compositions that can modify the surface of textiles are usually composed of nanoparticles, a surfactant, ingredients and a carrier medium. The use of Sonochemical deposition processes could allow many new materials such as metals, and metal oxides to be used as coatings for textile materials. 2. INTRODUCTION The concept of nanotechnology is not new; it was started over forty years ago. Nanotechnology is defined as the utilization of structures with at least one dimension of nanometer size for the construction of materials, devices or systems with novel or significantly improved properties due to their nano-size. Nanotechnology not only produces small structures, but also an anticipated manufacturing technology which can give thorough, inexpensive control of the structure of matter. Nanotechnology can best be described as activities at the level of atoms and molecules that have applications in the real world. Nano-particles commonly used in commercial products are in the range of 1 to 100 nm. Nanotechnology is increasingly attracting worldwide attention because it is widely perceived as offering huge potential in a wide range of end uses. The unique and new properties of nanomaterials have attracted not only scientists and researchers but also industries, due to their huge economical potential. Nanotechnology has real commercial potential for the textile industry. This is mainly due to the fact that conventional methods used to impart different properties to fabrics often do not lead to permanent effects, and will lose their functions after laundering or wearing. Nanotechnology can provide high durability for fabrics, because nano-particles have a large surface area-to-volume ratio and high surface energy, thus presenting better affinity for fabrics and leading to an increase in durability of the function. In addition, a coating of nano-particles on fabrics will not affect their breath ability or hand feel. Therefore, the interest in using nanotechnologies in the textile industry is increasing. The first work on nanotechnology in textiles was undertaken by Nano-Tex, a subsidiary of the US-based Burlington Industries [1].  Vol.9, No.6 Sonochemical Coating of Ag-TiO 2 Nanoparticles on Textile Fabrics 521 Later, more and more textile companies began to invest in the development of nanotechnologies. The properties imparted to textiles using nanotechnology include water repellence, soil resistance, wrinkle resistance, anti-bacteria, anti-static and UV-protection, flame retardation, improvement of dye ability and so on. Coating is a common technique used to apply nano- particles onto textiles. The coating compositions that can modify the surface of textiles are usually composed of nano-particles, a surfactant, ingredients and a carrier medium [2]. Several methods can apply coating onto fabrics, including spraying, transfer printing, washing, rinsing and padding. Of these methods, padding is the most commonly used [3-5]. The nano-particles are attached to the fabrics with the use of a padder adjusted to suitable pressure and speed, followed by drying and curing. 3. PURPOSE OF RESEARCH Nature has already developed an elegant approach that combines chemistry and physics to create super repellant surfaces as well as self cleaning surfaces. “Lotus leaves” is the best example of self cleaning surfaces. The concept of self cleaning textiles is based on the lotus plant whose leaves are well-known for their ability to ‘self-clean’ by repelling water and dirt. Now day’s people are very busy in their work that they do not have time for clean their daily wear cloths. People who are working in kitchens have headache to wash their garments. Also military peoples have to survive in such drastic condition that they cannot wash their cloths. Nanotechnology provides a new concept of self cleaning textiles which gives self-cleaning as well as fresh cloths every day, this not only technically benefited but techno economically also benefited. There are basically two types of self-cleaning surfaces involving nanotechnology. In the first place extremely water repellent, microscopically rough surfaces: dirt particles can hardly get a hold on them and are, therefore, removed by rain or by a simple rinse in water .The second example is given by photo-catalytic layers: due to a layer of nano crystalline titanium oxide, fouling organic material is destroyed by solar irradiation. Self -cleaning surface having a water contact angle greater than 150 degree and a very low roll off angle. Water through these surfaces easily rolls off and completely cleans the surface in the process. Self cleaning fabrics not only resist coffee and red wine stains but are also repellant to water, dirt, odor and are antibacterial as well. The manufacturing of self-cleaning textiles uses the following nanotechnology: ♦ photo catalyst ♦ microwaves ♦ carbon nanotubes ♦ metal oxide colloidal ♦ silver nanoparticles 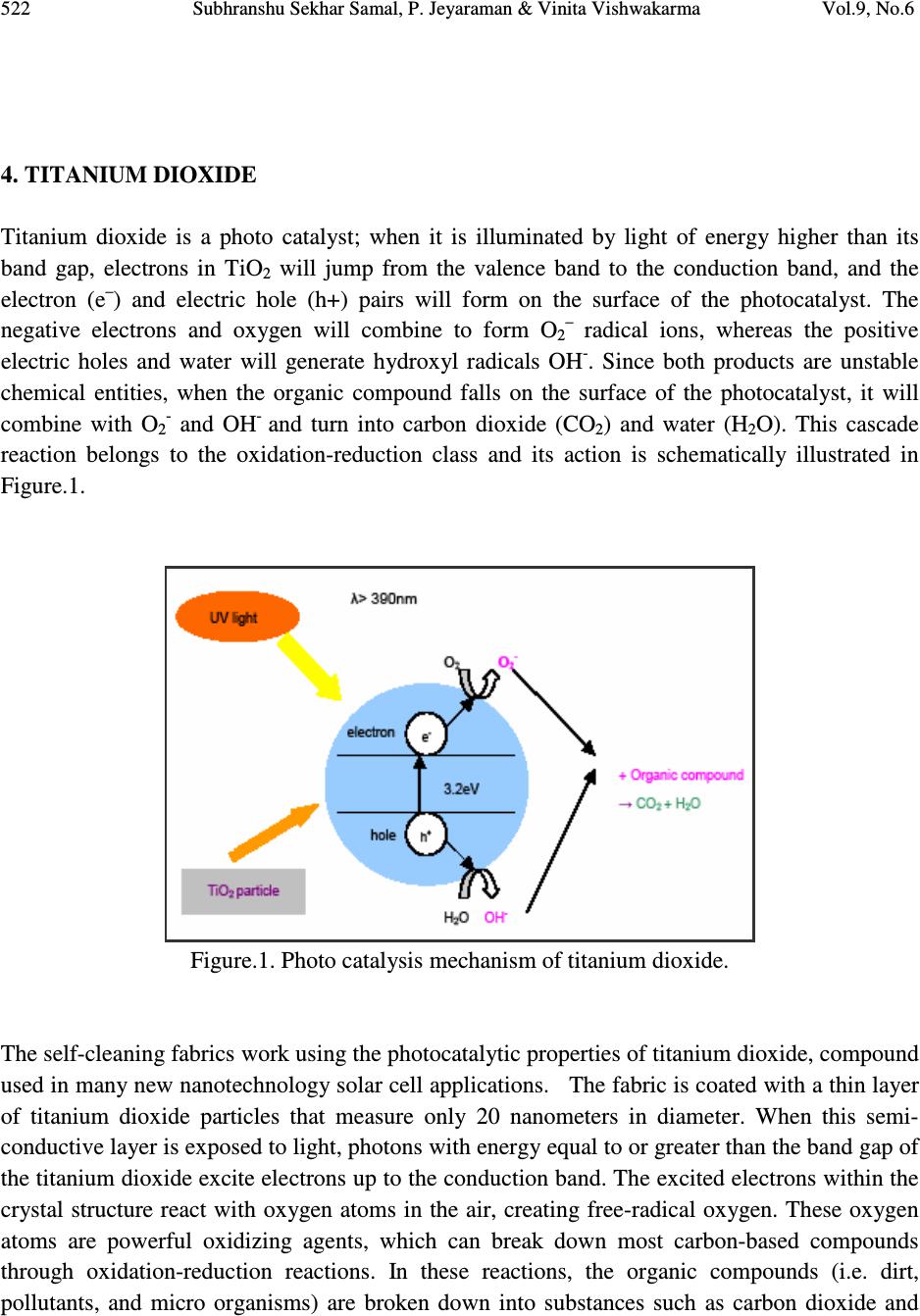 522 Subhranshu Sekhar Samal, P. Jeyaraman & Vinita Vishwakarma Vol.9, No.6 4. TITANIUM DIOXIDE Titanium dioxide is a photo catalyst; when it is illuminated by light of energy higher than its band gap, electrons in TiO 2 will jump from the valence band to the conduction band, and the electron (e – ) and electric hole (h+) pairs will form on the surface of the photocatalyst. The negative electrons and oxygen will combine to form O 2– radical ions, whereas the positive electric holes and water will generate hydroxyl radicals OH - . Since both products are unstable chemical entities, when the organic compound falls on the surface of the photocatalyst, it will combine with O 2- and OH - and turn into carbon dioxide (CO 2 ) and water (H 2 O). This cascade reaction belongs to the oxidation-reduction class and its action is schematically illustrated in Figure.1. Figure.1. Photo catalysis mechanism of titanium dioxide. The self-cleaning fabrics work using the photocatalytic properties of titanium dioxide, compound used in many new nanotechnology solar cell applications. The fabric is coated with a thin layer of titanium dioxide particles that measure only 20 nanometers in diameter. When this semi- conductive layer is exposed to light, photons with energy equal to or greater than the band gap of the titanium dioxide excite electrons up to the conduction band. The excited electrons within the crystal structure react with oxygen atoms in the air, creating free-radical oxygen. These oxygen atoms are powerful oxidizing agents, which can break down most carbon-based compounds through oxidation-reduction reactions. In these reactions, the organic compounds (i.e. dirt, pollutants, and micro organisms) are broken down into substances such as carbon dioxide and 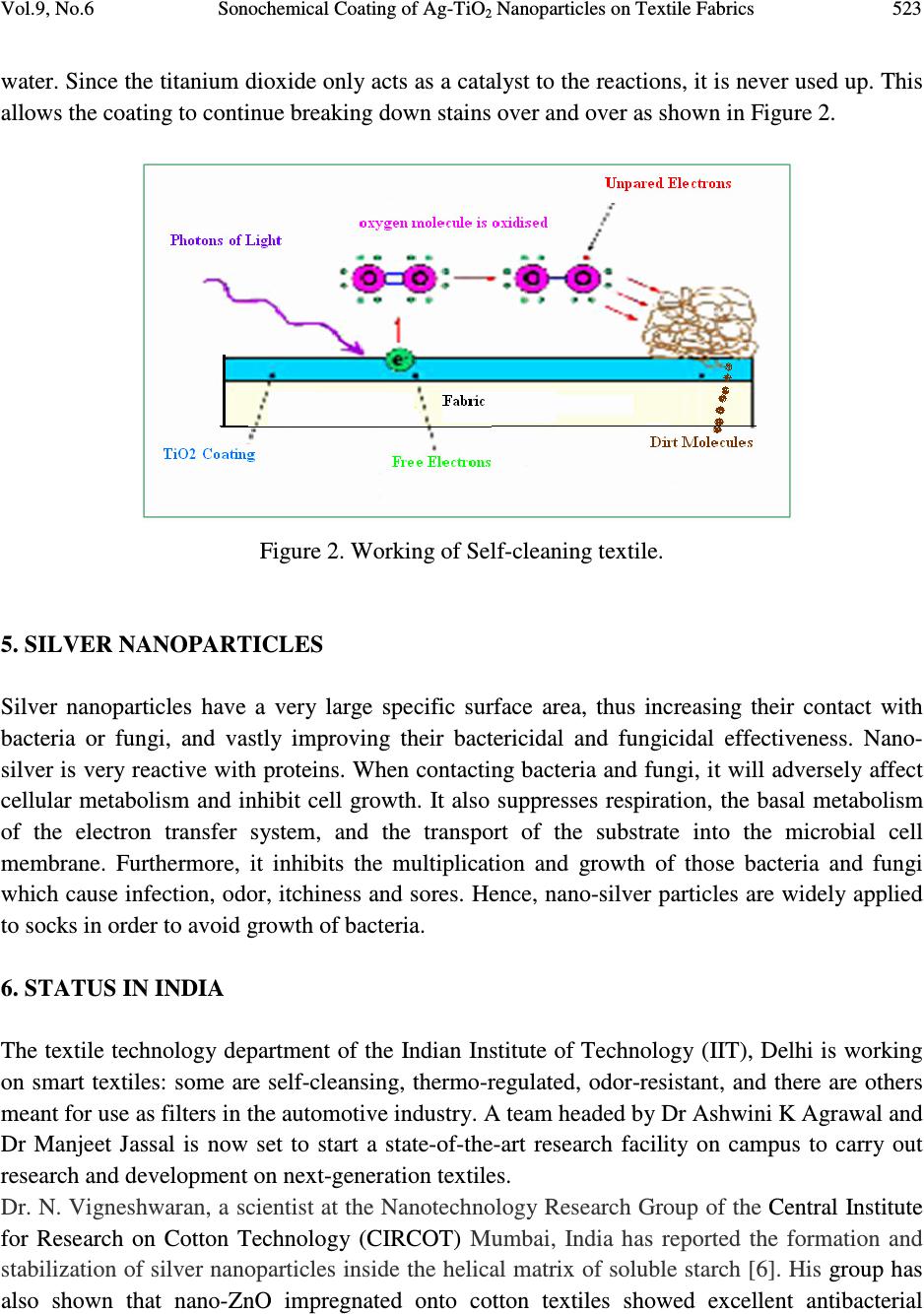 Vol.9, No.6 Sonochemical Coating of Ag-TiO 2 Nanoparticles on Textile Fabrics 523 water. Since the titanium dioxide only acts as a catalyst to the reactions, it is never used up. This allows the coating to continue breaking down stains over and over as shown in Figure 2. Figure 2. Working of Self-cleaning textile. 5. SILVER NANOPARTICLES Silver nanoparticles have a very large specific surface area, thus increasing their contact with bacteria or fungi, and vastly improving their bactericidal and fungicidal effectiveness. Nano- silver is very reactive with proteins. When contacting bacteria and fungi, it will adversely affect cellular metabolism and inhibit cell growth. It also suppresses respiration, the basal metabolism of the electron transfer system, and the transport of the substrate into the microbial cell membrane. Furthermore, it inhibits the multiplication and growth of those bacteria and fungi which cause infection, odor, itchiness and sores. Hence, nano-silver particles are widely applied to socks in order to avoid growth of bacteria. 6. STATUS IN INDIA The textile technology department of the Indian Institute of Technology (IIT), Delhi is working on smart textiles: some are self-cleansing, thermo-regulated, odor-resistant, and there are others meant for use as filters in the automotive industry. A team headed by Dr Ashwini K Agrawal and Dr Manjeet Jassal is now set to start a state-of-the-art research facility on campus to carry out research and development on next-generation textiles. Dr. N. Vigneshwaran, a scientist at the Nanotechnology Research Group of the Central Institute for Research on Cotton Technology (CIRCOT) Mumbai, India has reported the formation and stabilization of silver nanoparticles inside the helical matrix of soluble starch [6]. His group has also shown that nano-ZnO impregnated onto cotton textiles showed excellent antibacterial 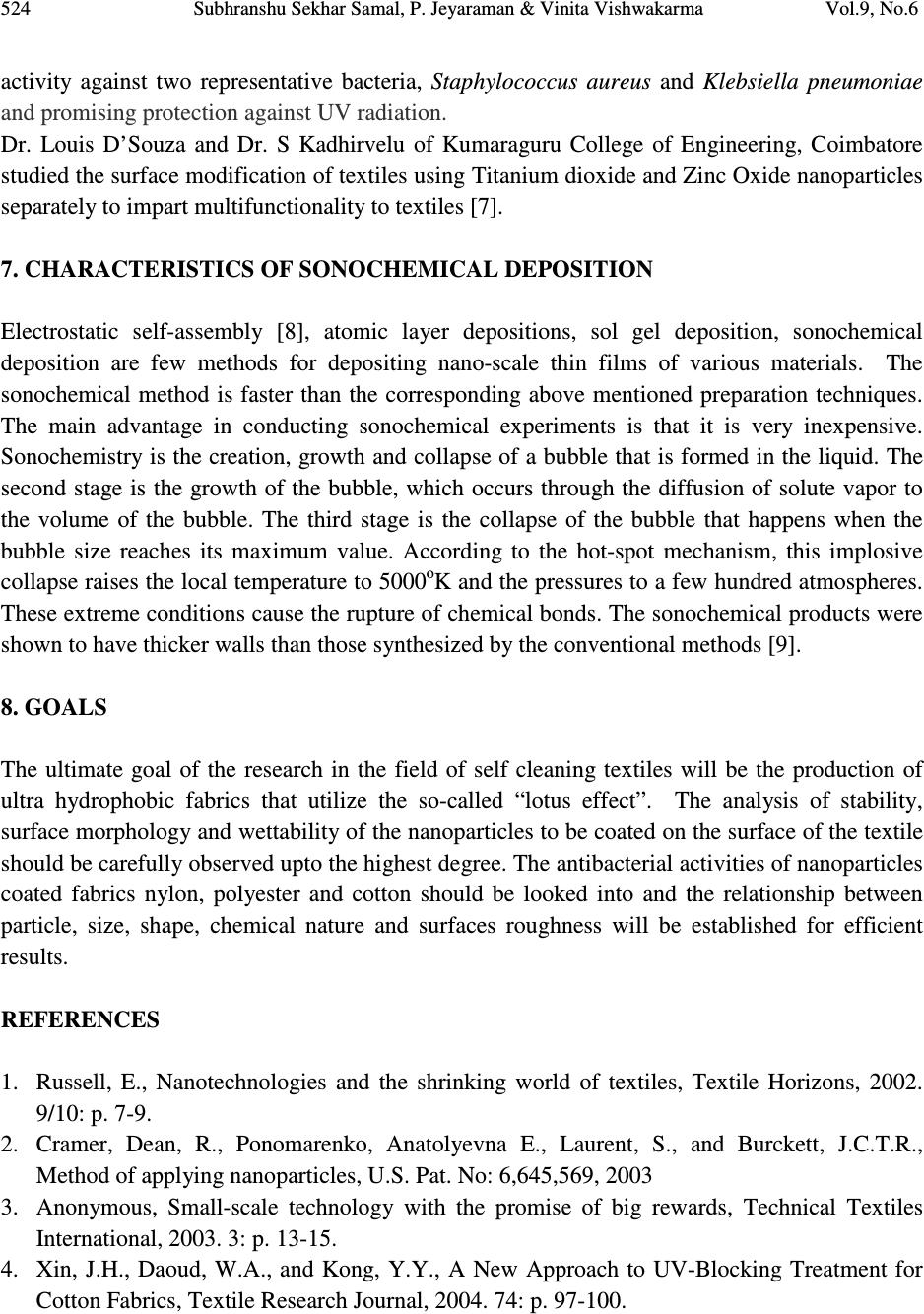 524 Subhranshu Sekhar Samal, P. Jeyaraman & Vinita Vishwakarma Vol.9, No.6 activity against two representative bacteria, Staphylococcus aureus and Klebsiella pneumoniae and promising protection against UV radiation. Dr. Louis D’Souza and Dr. S Kadhirvelu of Kumaraguru College of Engineering, Coimbatore studied the surface modification of textiles using Titanium dioxide and Zinc Oxide nanoparticles separately to impart multifunctionality to textiles [7]. 7. CHARACTERISTICS OF SONOCHEMICAL DEPOSITION Electrostatic self-assembly [8], atomic layer depositions, sol gel deposition, sonochemical deposition are few methods for depositing nano-scale thin films of various materials. The sonochemical method is faster than the corresponding above mentioned preparation techniques. The main advantage in conducting sonochemical experiments is that it is very inexpensive. Sonochemistry is the creation, growth and collapse of a bubble that is formed in the liquid. The second stage is the growth of the bubble, which occurs through the diffusion of solute vapor to the volume of the bubble. The third stage is the collapse of the bubble that happens when the bubble size reaches its maximum value. According to the hot-spot mechanism, this implosive collapse raises the local temperature to 5000 o K and the pressures to a few hundred atmospheres. These extreme conditions cause the rupture of chemical bonds. The sonochemical products were shown to have thicker walls than those synthesized by the conventional methods [9]. 8. GOALS The ultimate goal of the research in the field of self cleaning textiles will be the production of ultra hydrophobic fabrics that utilize the so-called “lotus effect”. The analysis of stability, surface morphology and wettability of the nanoparticles to be coated on the surface of the textile should be carefully observed upto the highest degree. The antibacterial activities of nanoparticles coated fabrics nylon, polyester and cotton should be looked into and the relationship between particle, size, shape, chemical nature and surfaces roughness will be established for efficient results. REFERENCES 1. Russell, E., Nanotechnologies and the shrinking world of textiles, Textile Horizons, 2002. 9/10: p. 7-9. 2. Cramer, Dean, R., Ponomarenko, Anatolyevna E., Laurent, S., and Burckett, J.C.T.R., Method of applying nanoparticles, U.S. Pat. No: 6,645,569, 2003 3. Anonymous, Small-scale technology with the promise of big rewards, Technical Textiles International, 2003. 3: p. 13-15. 4. Xin, J.H., Daoud, W.A., and Kong, Y.Y., A New Approach to UV-Blocking Treatment for Cotton Fabrics, Textile Research Journal, 2004. 74: p. 97-100.  Vol.9, No.6 Sonochemical Coating of Ag-TiO 2 Nanoparticles on Textile Fabrics 525 5. Yeo, S.Y., Lee, H.J., and Jeong, S.H., Preparation of nanocomposite fibers for permanent antibacterial effect, Journal of Materials Science, 2003. 38: p. 2143-2147. 6. NadanathangamVigneshwaran, Sampath Kumar, A A Kathe,P V Varadarajan and Virendra Prasad, Functional finishing of cotton fabrics usingzinc oxide–soluble starch nanocomposites, Nanotechnology 17(2006) 5087–5095 7. S. Kathirvelu, Dr. Louis D’Souza and Bhaarathi Dhurai., A comparative study of multifunctional finishing of cotton and P/C blended fabrics treated with titanium dioxide/zinc oxide nanoparticles, Indian Journal of Science and Technology, Vol.1 No 7 (Dec. 2008) 8. Lei Qian, Juan P Hinestroza, Application of Nanotechnology for high performance textiles, Journal of textile and apparel, technology and management, Vol. No.4, Issue No.1 (Summer 2004) 9. Vitalijus Janickis, Ingrida Ancutienė, Modification of polyester textile by conductive copper sulfide layers, CHEMIJA. Vol. 20. No. 2. P. 136–140 (2009) |

