Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 9, No.5, pp.471-481, 2010 jmmce.org Printed in the USA. All rights reserved 471 Growth and Characterization of Urea Adduct with m-Nitrobenzoic Acid, m-Nitroaniline, and p-Xylene Mixtures P. Jagdish1*, N.P. Rajesh2, S. Natarajan3 1Department of Physics, Sona College of Technology, Salem – 636 005, India 2Centre for Crystal Growth, SSN College of Engineering, Kalavakkam – 636 110, India. 3School of Physics, Madurai Kamaraj University, Madurai – 625 021, India. *Corresponding author: jaggphy1975@yahoo.co.in, jaggphy2002@yahoo.co.in ABSTRACT The urea adduct with the guests of m-nitrobenzoic acid, m-nitroaniline and p-xylene mixtures by adductive crystallization was successfully grown by slow evaporation method. The single crystals obtained were non-hygroscopic and good in size. The crystal structure of the grown crystal has been determined, and it belongs to the monoclinic system with centrosymmertic space group P21/c. Fourier transform infrared spectroscopic studies were performed for the identification of guest components in the compound. The optical absorption spectrum has been recorded in the range 200-1100 nm. The mechanical behavior of the specimen was studied. Key words: X-ray diffraction, Growth from solutions, Organic adduct. 1. INTRODUCTION The development of new optical organic materials is of great interest due to their higher orders of optical transparencies than the conventional inorganic materials. Due to promising optical properties, the organic materials can be utilized in optical device fabrication which can replace the electronic switching circuits [1]. So it is required to grow highly perfect single crystals of organic optical materials for optical devices fabrication with unique properties such as good transparency, large birefringence and frequency mixing in the large range of spectrum including the ultraviolet spectrum [2-6]. In this work, a new organic adduct has been formed with urea, m- nitrobenzoic acid, m-nitroaniline and p-xylene mixtures and was grown as a single crystal. In the present investigation, we report the synthesis, crystal growth, single-crystal X-ray diffraction  472 P. Jagdish, N.P. Rajesh, S. Natarajan Vol.9, No.5 (XRD) studies, Fourier transform infrared (FT-IR) spectroscopy, optical transmission (UV-vis- NIR), Vicker’s micro hardness test of the new organic adduct. 2. EXPERIMENTAL 2.1 Synthesis The organic adduct was synthesized by the combination of urea (Merck, 99.5% pure), m- nitrobenzoic acid (Merck, 99.5% pure), m-nitroaniline (Merck, 99.5% pure) and p-xylene (AR grade) along with methanol (AR grade) as solvent. Homogeneous solutions were prepared by dissolving 17.48 g, 13.812 g and 16.7 g of urea, m-nitrobenzoic acid and m-nitroaniline, respectively, in methanol of 100 ml each. The three solutions thus formed were thoroughly mixed by constant stirring along with p-xylene (100ml) in the ratio 1:1:1:1 and heated to a temperature of 40°C. The solution was then filtered out and kept undisturbed at a temperature of 40°C in a closed beaker with provision for controlled evaporation. After a period of one week, crystalline material of urea adducts with organic mixture was separated out and dried using a vacuum oven. The purity of the synthesized salt was further improved by successive recrystallization of the compound in methanol. 2.2 Crystal Growth Single crystals of urea adduct with m-nitrobenzoic acid, m-nitroaniline and p-xylene mixture were grown by solvent evaporation technique at room temperature. Appropriate selection of solvent for the growth of the material is very important in crystal growth process. Methanol was found to be the suitable solvent for preparing the growth solutions. Recrystallized compound was dissolved in methanol to prepare a saturated solution. The solution was filtered and kept undisturbed in a dust free environment at room temperature. The rate of evaporation of the solvent was controlled critically for a period of thirty days. Finally the single crystals of urea adduct with m-nitrobenzoic acid, m-nitroaniline and p-xylene mixture were harvested. Figure 1 shows the photograph of the harvested single crystal exhibiting birefringence property.  Vol.9, No.5 Growth and Characterization of Urea Adduct 473 Figure 1. Photograph of urea adduct with m-nitrobenzoic acid-m-nitroaniline-p-xylene mixture single crystal (C8H9N3O5). 3. RESULTS AND DISCUSSIONS 3.1 X-ray Diffraction Analysis To obtain the unit cell parameters and to confirm the crystallinity of grown crystals, both single crystal and powder crystal X-ray diffraction studies were carried out. Single crystal X-ray diffraction pattern was recorded using single crystal diffractometer CAD 4/MACH 3 with MoKα radiation in the wavelength 0.71073 Ǻ. The cell parameters were calculated by data reduction and the structure was solved. The unit cell parameters were measured at 293 K. The unit cell dimensions were determined as a = 8.093 (5) Å, b = 12.750 (5) Å, c = 9.502 (5) Å, α =90.00°, β = 93.39 (5)°, γ = 90.00° and the cell volume of 978.8 (9) Å3 with a molecular weight of 227.18 g. The crystal belongs to monoclinic crystal system with space group of P21/c. The crystal data, experimental conditions and structural refinement parameters of urea adduct with m-nitrobenzoic acid-m-nitroaniline-p-xylene mixture single crystal are presented in Table 1. Figure 2 shows the planar molecular structure of the adduct that has been determined by single crystal XRD. The molecular packing diagram given in Figure 3 shows four molecules per unit cell. The calculated cell parameters were also searched through Cambridge Structural Database which revealed that the crystal grown is a new organic adduct. 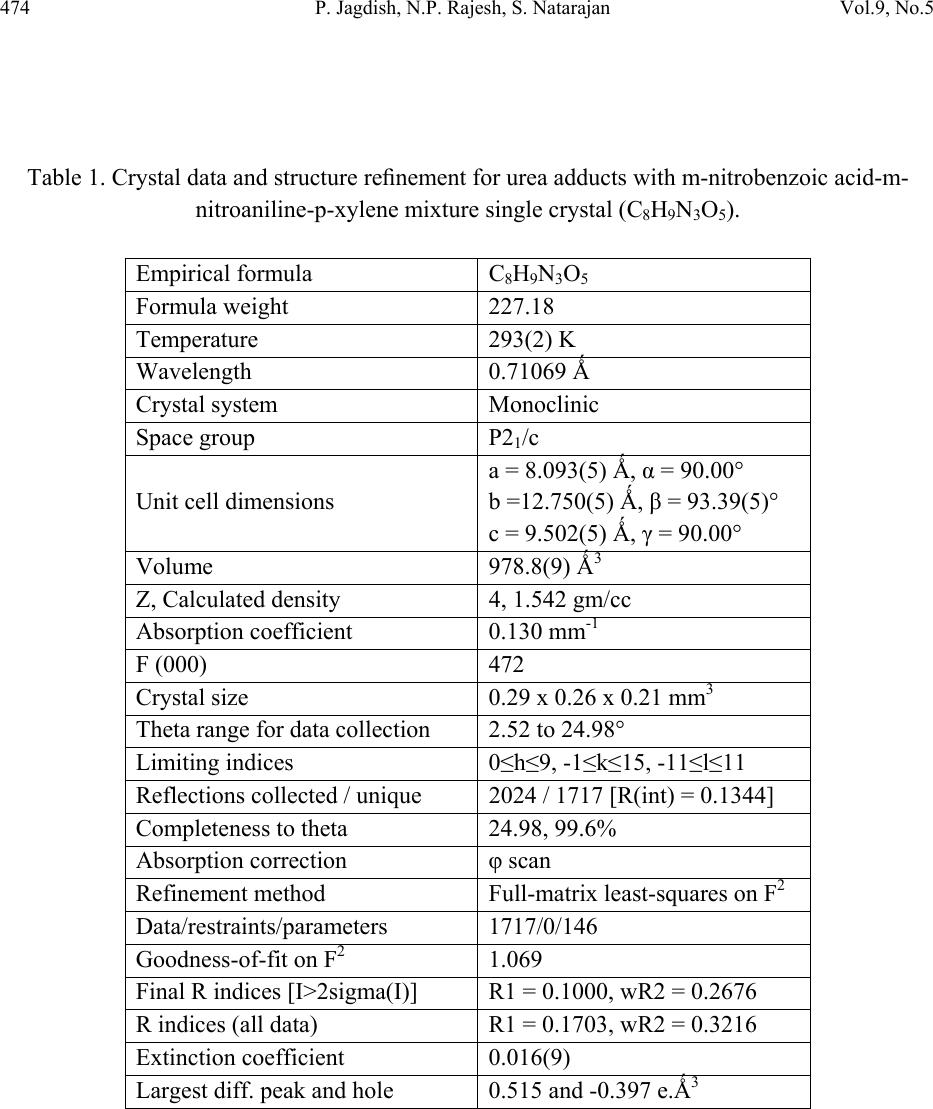 474 P. Jagdish, N.P. Rajesh, S. Natarajan Vol.9, No.5 Table 1. Crystal data and structure refinement for urea adducts with m-nitrobenzoic acid-m- nitroaniline-p-xylene mixture single crystal (C8H9N3O5). Empirical formula C8H9N3O5 Formula weight 227.18 Temperature 293(2) K Wavelength 0.71069 Ǻ Crystal system Monoclinic Space group P21/c Unit cell dimensions a = 8.093(5) Ǻ, α = 90.00° b =12.750(5) Ǻ, β = 93.39(5)° c = 9.502(5) Ǻ, γ = 90.00° Volume 978.8(9) Ǻ3 Z, Calculated density 4, 1.542 gm/cc Absorption coefficient 0.130 mm-1 F (000) 472 Crystal size 0.29 x 0.26 x 0.21 mm3 Theta range for data collection 2.52 to 24.98° Limiting indices 0≤h≤9, -1≤k≤15, -11≤l≤11 Reflections collected / unique 2024 / 1717 [R(int) = 0.1344] Completeness to theta 24.98, 99.6% Absorption correction φ scan Refinement method Full-matrix least-squares on F2 Data/restraints/parameters 1717/0/146 Goodness-of-fit on F2 1.069 Final R indices [I>2sigma(I)] R1 = 0.1000, wR2 = 0.2676 R indices (all data) R1 = 0.1703, wR2 = 0.3216 Extinction coefficient 0.016(9) Largest diff. peak and hole 0.515 and -0.397 e.Ǻ3 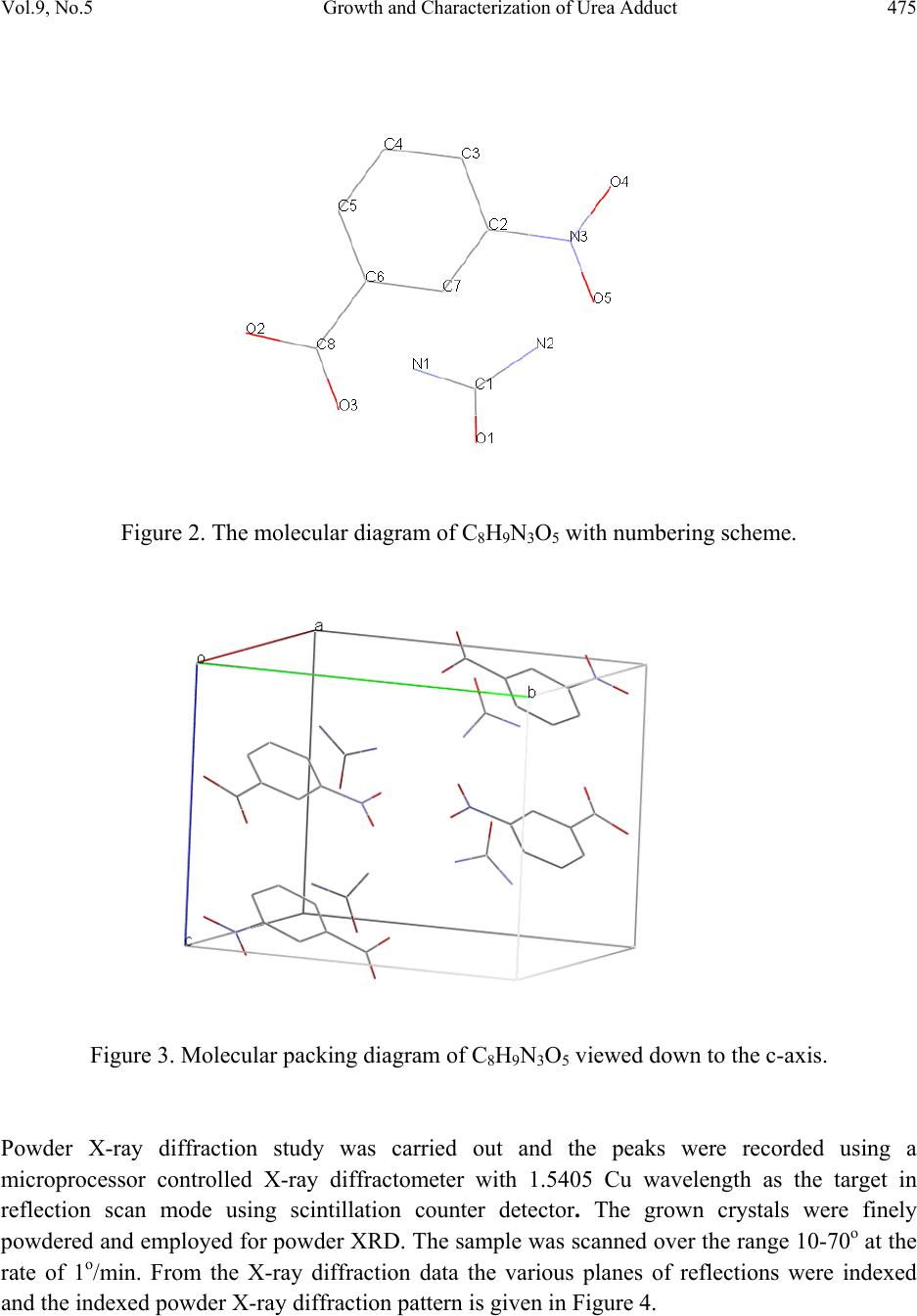 Vol.9, No.5 Growth and Characterization of Urea Adduct 475 Figure 2. The molecular diagram of C8H9N3O5 with numbering scheme. Figure 3. Molecular packing diagram of C8H9N3O5 viewed down to the c-axis. Powder X-ray diffraction study was carried out and the peaks were recorded using a microprocessor controlled X-ray diffractometer with 1.5405 Cu wavelength as the target in reflection scan mode using scintillation counter detector. The grown crystals were finely powdered and employed for powder XRD. The sample was scanned over the range 10-70o at the rate of 1o/min. From the X-ray diffraction data the various planes of reflections were indexed and the indexed powder X-ray diffraction pattern is given in Figure 4. 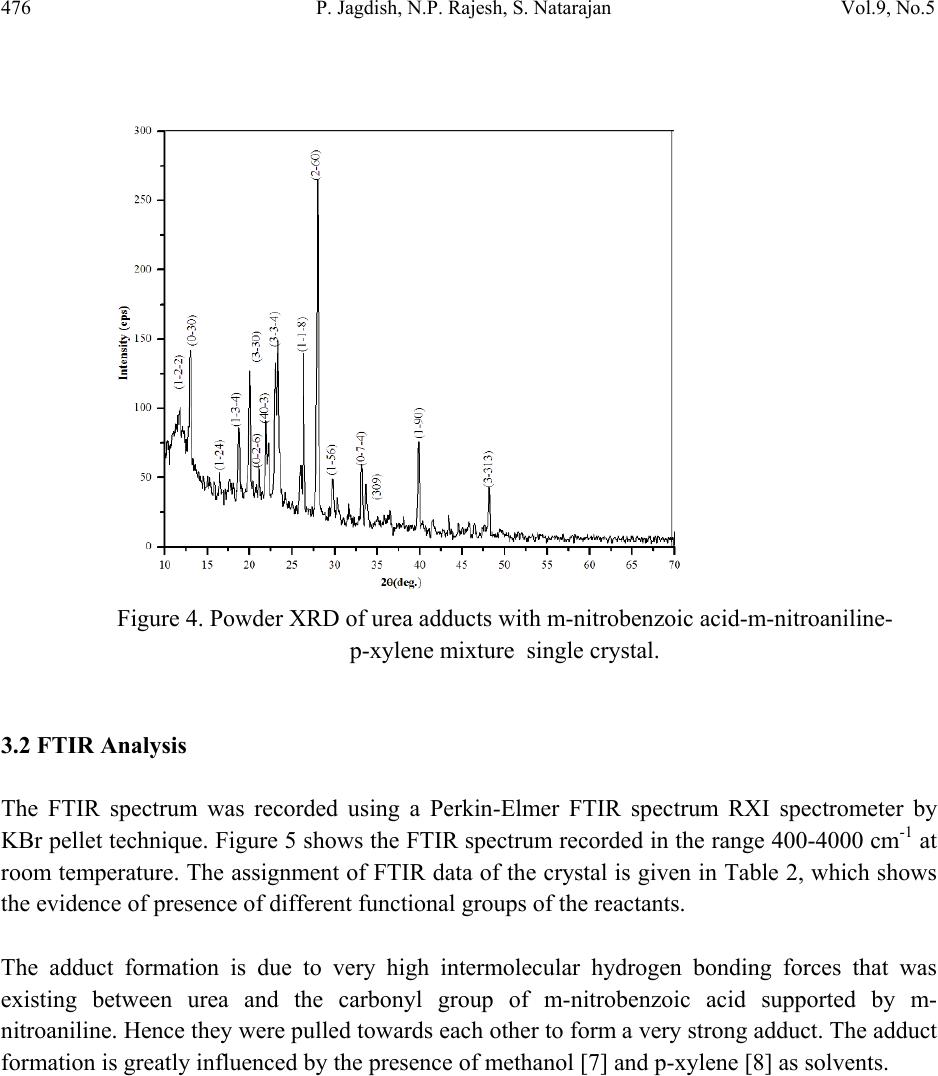 476 P. Jagdish, N.P. Rajesh, S. Natarajan Vol.9, No.5 Figure 4. Powder XRD of urea adducts with m-nitrobenzoic acid-m-nitroaniline- p-xylene mixture single crystal. 3.2 FTIR Analysis The FTIR spectrum was recorded using a Perkin-Elmer FTIR spectrum RXI spectrometer by KBr pellet technique. Figure 5 shows the FTIR spectrum recorded in the range 400-4000 cm-1 at room temperature. The assignment of FTIR data of the crystal is given in Table 2, which shows the evidence of presence of different functional groups of the reactants. The adduct formation is due to very high intermolecular hydrogen bonding forces that was existing between urea and the carbonyl group of m-nitrobenzoic acid supported by m- nitroaniline. Hence they were pulled towards each other to form a very strong adduct. The adduct formation is greatly influenced by the presence of methanol [7] and p-xylene [8] as solvents.  Vol.9, No.5 Growth and Characterization of Urea Adduct 477 Table 2. Assignment of FTIR data of urea adducts with m-nitrobenzoic acid - m-nitroaniline –p- xylene mixture single crystal. Wavenumber (cm-1) Tentative Assignment 3771.17 O-H stretching, C-H stretching – weak to medium 3486.89 O–H stretch(attached to benzene ring), broad very intense, strong 2930.15 C─H Methylene – weak to medium 2859.75 C─H Alkyl methyl – weak to medium 2790.17 C─H Aldehyde – medium 2600.08 C ≡ N (attached to benzene ring) , C ≡ C weak to medium 2425.06 N─H(ammonium ions) multiple broad peaks 1930.67 C═C, C═O broad peak 1630.57 Ester Carbonyl stretch; typically sharp and intense, strong band consistent with C=C, NH bend-amide II – medium 1521.53 Double bond stretch, N─O (aromatic) sharp-medium to strong 1472.54 C═C stretch, C─H(alkyl) methylene- medium to strong 1351.06 N─O(aromatic), C–H deformation 1269.41 C─O (ethers) aromatic, carboxylic acids 1143.51 C–O stretch; typically broad and intense 1068.77 C─O (primary alcohol) strong - broad, C─N (aliphatic amine) often overlapped 890.43 C─H (aromatic) meta-bisubstituted benzene 779.10 C─H (aromatic) meta-bisubstituted benzene 711.13 C─H (aromatic) mono substituted benzene – strong 3.3 Optical Transmission Studies The UV-vis-NIR study of the single crystal was done using Lambda 35 UV spectrophotometer. Figure 6 shows the optical absorption spectrum of urea adduct single crystals grown from methanol. The crystal shows good optical transmittance in the entire region; therefore this new organic adduct material is best suited for electro-optic modulation [9]. It shows a cutoff at 264.37 nm. This reveals that, in the grown crystal the absorption is almost absent in the visible region due to its high transparency. 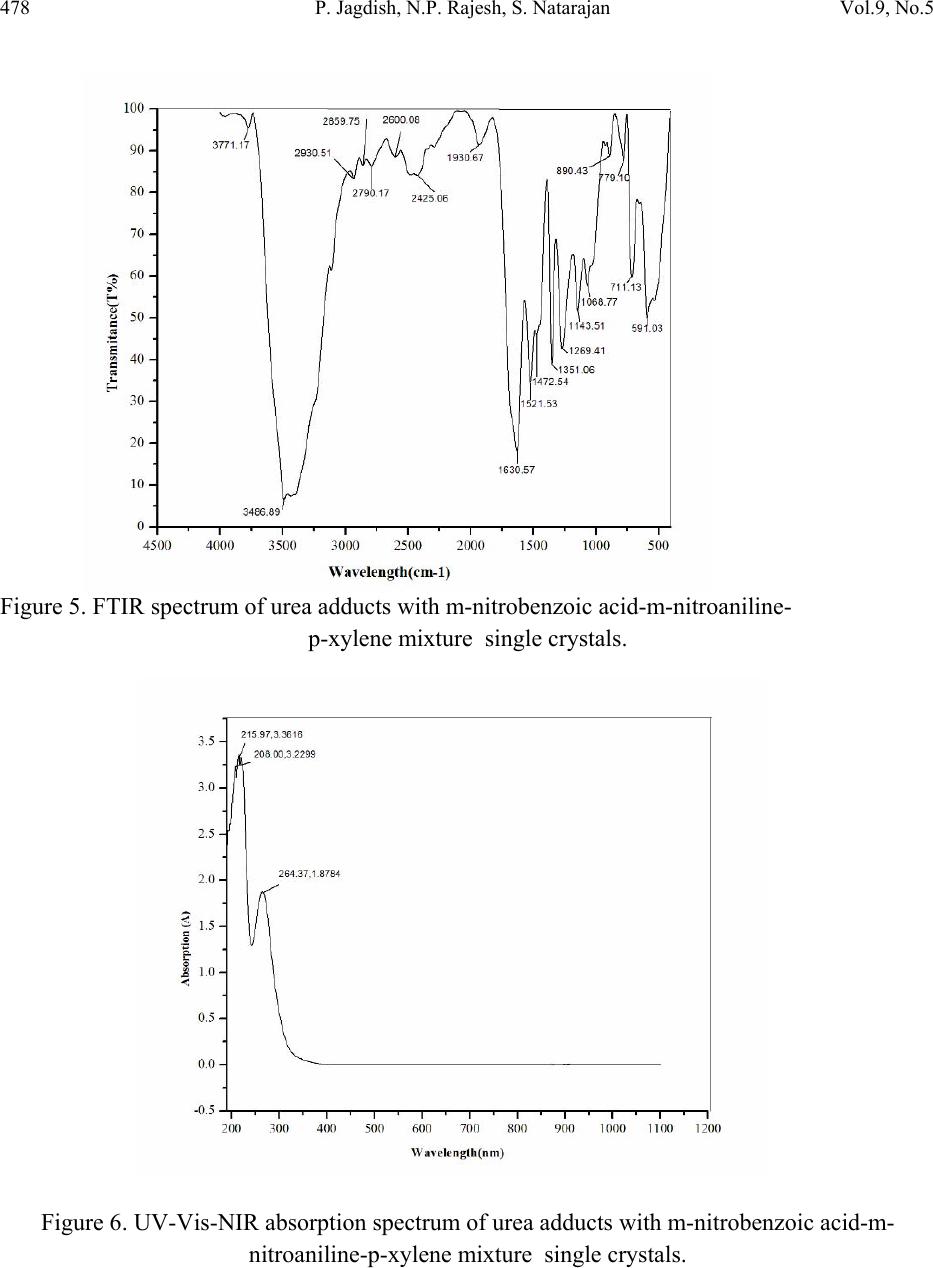 478 P. Jagdish, N.P. Rajesh, S. Natarajan Vol.9, No.5 Figure 5. FTIR spectrum of urea adducts with m-nitrobenzoic acid-m-nitroaniline- p-xylene mixture single crystals. Figure 6. UV-Vis-NIR absorption spectrum of urea adducts with m-nitrobenzoic acid-m- nitroaniline-p-xylene mixture single crystals. 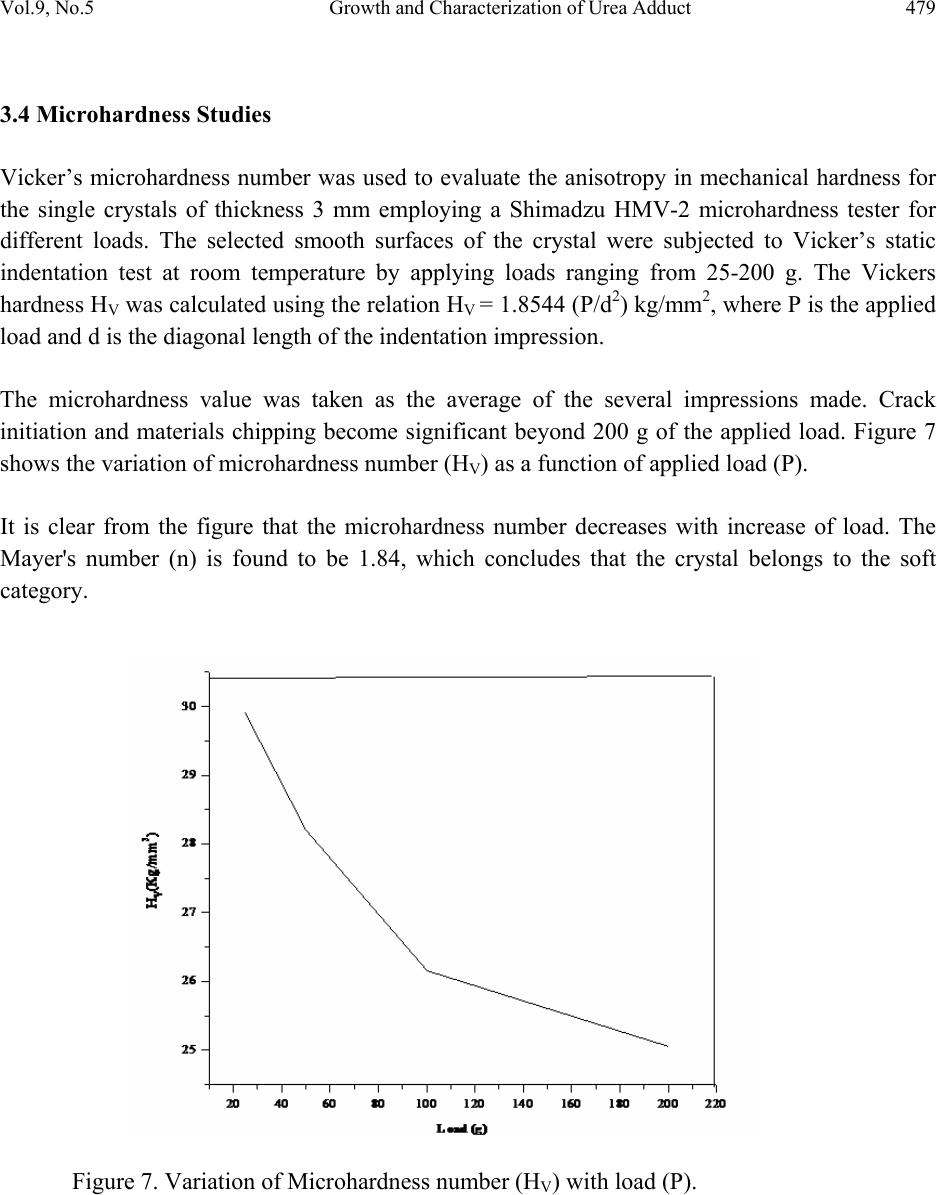 Vol.9, No.5 Growth and Characterization of Urea Adduct 479 3.4 Microhardness Studies Vicker’s microhardness number was used to evaluate the anisotropy in mechanical hardness for the single crystals of thickness 3 mm employing a Shimadzu HMV-2 microhardness tester for different loads. The selected smooth surfaces of the crystal were subjected to Vicker’s static indentation test at room temperature by applying loads ranging from 25-200 g. The Vickers hardness HV was calculated using the relation HV = 1.8544 (P/d2) kg/mm2, where P is the applied load and d is the diagonal length of the indentation impression. The microhardness value was taken as the average of the several impressions made. Crack initiation and materials chipping become significant beyond 200 g of the applied load. Figure 7 shows the variation of microhardness number (HV) as a function of applied load (P). It is clear from the figure that the microhardness number decreases with increase of load. The Mayer's number (n) is found to be 1.84, which concludes that the crystal belongs to the soft category. Figure 7. Variation of Microhardness number (HV) with load (P).  480 P. Jagdish, N.P. Rajesh, S. Natarajan Vol.9, No.5 4. CONCLUSIONS The urea adduct with m-nitrobenzoic acid, m-nitroaniline and p-xylene mixture was synthesized and grown as single crystal by slow evaporation solution growth technique at room temperature. The grown crystals are nonhygroscopic and have well developed habit. The single crystal X-ray diffraction study confirmed its crystal structure belonging to monoclinic crystal system with centrosymmetric space group P21/c. The molecular packing shows four molecules per unit cell. The FTIR spectrum shows the incorporation of different functional of the guest reactants. The adduct formation of urea with its guests in a very strong manner is highly influenced by p-xylene and methanol. The strong forces provided by the secondary bonding between the amide and carboxyl group has favored the electron transfer between them. The excellent transparency in the entire UV-vis-NIR region facilitates this material to various optical applications. The microhardness studies concludes the category of the organic adduct crystal as soft category. ACKNOWLEDGEMENT This work, supported by the Department of Science and Technology, New Delhi, India under the grant of project ref- SR/FTP/PS-20/2005, is hereby gratefully acknowledged. One of the authors (P. Jagdish) thanks Sona College of Technology, Salem, India for their help and encouragement to carry out this research work. REFERENCES [1] David P. Shoemaker, Jerry Donohuem, Verner Schomaker, Robert B. Corey, 1950, “The Crystal Structure of Ls-Threonine.” J. Am. Chem. Soc. Vol. 72(6), pp. 2328-2349. [2] D.S. Chemla and J. Zyss, 1987, Nonlinear optical properties of organic molecules and crystals, Academic Press. [3] R.T. Denton, F.S. Chen, A.A. Ballman, 1967, “Lithium Tantalate Light Modulators” J. Appl. Phys. Vol. 38, pp. 1611-1617. [4] M. Ebrahimzadeh, M.H. Dunn, F. Akerboom, 1989, “Highly efficient visible urea optical parametric oscillator pumped by XeCl excimer laser” Optical Lett. Vol. 14, pp. 560-562. [5] D.J. Halfpenny, J.N. Sherwood, 1990, “Synchrotron-radiation section topography of large uncut crystals of organic nonlinear optical materials.” Philos. Msg. Lett. Vol. 62, pp. 1-7. [6] Joseph Zyss, 1982, “New organic molecular materials for nonlinear optics,” J. Non. Crystalline Solids, Vol. 47, pp. 211-225. [7] A.A. Gundyrev, L.P. Kazakova, M.V. Karaibog, 1977, “Influence of alcohols on urea- adduct formation with solid paraffins.” Chem. And Tech. of Fuels and Oils, Vol. 13, pp. 101- 104.  Vol.9, No.5 Growth and Characterization of Urea Adduct 481 [8] Alfred Fischer, John Neilson Ramsay, 1974, “Formation of Adducts in the Nitration of p- Xylene. Exchange and Rearomatization of p-Xylene Adducts.” Can. J. Chem. Vol. 52, pp. 3960-3970. [9] A. S. Haja Hameed, G. Ravi, A. Nixon Azariah, T. Mahalingam, P. Ramasamy, 2003, “Growth and optical characterization of organic nonlinear optical crystal: indole-3- Aldehyde.” Phys. Chem. Sol. Vol. 64, pp. 147-153. |

