Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 9, No.4, pp.321-330, 2010 jmmce.org Printed in the USA. All rights reserved 321 Structural and Optical Characterization Studie s on 2, 4- dinitrophenylhydrazine Single Crystal T. Uma Devi *a, N. Lawrenceb, R. Ramesh Babuc, K. Ramamurthic, G. Bhagavannarayanad aDepartment of Physics, Government Arts College for Women(Autonomous), Pudukkottaii – 622001, India. bDepartment of Physics, St. Joseph’s College (Autonomous), Tiruchirappalli –620002, India. cCrystal Growth and Thin Film Laboratory, School of Physics, Bharathidasan University, Tiruchirappalli –620024, India. d Materials Characterization Division, National Physical Laboratory, Dr. K. S. Krishnan Marg, New Delhi-110 012, India *Corresponding Author: kavin_shri@yahoo.co.in ABSTRACT Single crystal of an organic nonlinear optical (NLO) material, 2,4-Dnitrophenylhydrazine (DNPH), was grown by slow cooling method. Powder X-ray diffraction (PXRD), Fourier transform infrared (FT-IR) FT-Raman and NMR studies have confirmed respectively the crystal structure and functional groups of the grown crystal. Crystalline perfection of single crystals was evaluated by high resolution X-ray diffractometry (HRXRD) using a multicrystal X–ray diffractometer and found that the grown crystals are nearly perfect. UV-Visible-NIR spectral analysis was used to determine the optical constants and band gap of DNPH. Fluorescence spectrum of DNPH was recorded. Keywords: X-ray diffraction, Growth from solutions, Single crystal growth, Nonlinear optic materials 1. INTRODUCTION The properties of hydrazides and hydrazones are of interest due to their biological activities and their use as metal extracting agents [1]. The hydrazone derivatives are used as fungicides, and in the treatment of diseases such as tuberculosis, leprosy and mental disorders. The complexes of 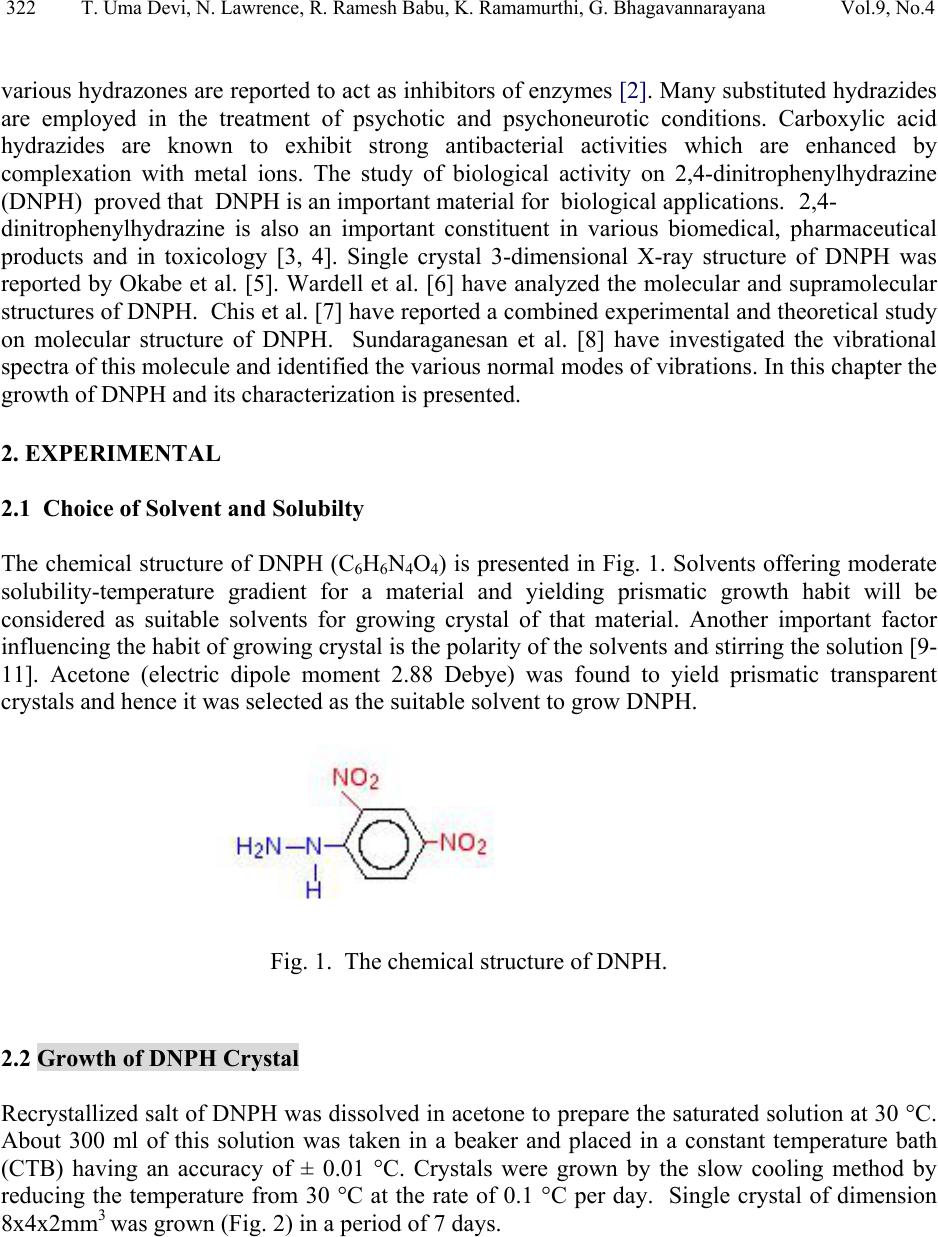 322 T. Uma Devi, N. Lawrence, R. Ramesh Babu, K. Ramamurthi, G. Bhagavannarayana Vol.9, No.4 various hydrazones are reported to act as inhibitors of enzymes [2]. Many substituted hydrazides are employed in the treatment of psychotic and psychoneurotic conditions. Carboxylic acid hydrazides are known to exhibit strong antibacterial activities which are enhanced by complexation with metal ions. The study of biological activity on 2,4-dinitrophenylhydrazine (DNPH) proved that DNPH is an important material for biological applications. 2,4- dinitrophenylhydrazine is also an important constituent in various biomedical, pharmaceutical products and in toxicology [3, 4]. Single crystal 3-dimensional X-ray structure of DNPH was reported by Okabe et al. [5]. Wardell et al. [6] have analyzed the molecular and supramolecular structures of DNPH. Chis et al. [7] have reported a combined experimental and theoretical study on molecular structure of DNPH. Sundaraganesan et al. [8] have investigated the vibrational spectra of this molecule and identified the various normal modes of vibrations. In this chapter the growth of DNPH and its characterization is presented. 2. EXPERIMENTAL 2.1 Choice of Solvent and Solubilty The chemical structure of DNPH (C6H6N4O4) is presented in Fig. 1. Solvents offering moderate solubility-temperature gradient for a material and yielding prismatic growth habit will be considered as suitable solvents for growing crystal of that material. Another important factor influencing the habit of growing crystal is the polarity of the solvents and stirring the solution [9- 11]. Acetone (electric dipole moment 2.88 Debye) was found to yield prismatic transparent crystals and hence it was selected as the suitable solvent to grow DN PH. Fig. 1. The chemical structure of DNPH. 2.2 Growth of DNPH Crystal Recrystallized salt of DNPH was dissolved in acetone to prepare the saturated solution at 30 °C. About 300 ml of this solution was taken in a beaker and placed in a constant temperature bath (CTB) having an accuracy of ± 0.01 °C. Crystals were grown by the slow cooling method by reducing the temperature from 30 °C at the rate of 0.1 °C per day. Single crystal of dimension 8x4x2mm3 was grown (Fig. 2) in a period of 7 days.  Vol.9, No.4 Structural and Optical Characterization 323 323 Fig. 2. As grown DNPH. 3. STRUCTURAL ANALYSIS 3.1 Single Crystal X-Ray Diffraction Single crystal X-ray diffraction study shows that DNPH crystallizes in a monoclinic system. There is good agreement between the measured and the corresponding reported values [5] of cell parameter and are presented in Table 1. Also, the powder XRD pattern recorded are indexed and shown in Fig. 3. Fig. 3. X-ray powder diffractogram of DNPH. Table 1 Comparison of unit cell parameters S.No. a ( Å) b (Å) c (Å) β (º) References 1. 4.7812 11.5923 14.0521 98.411 Present Work 2. 4.7917 11.5905 14.0496 98.372 [5] 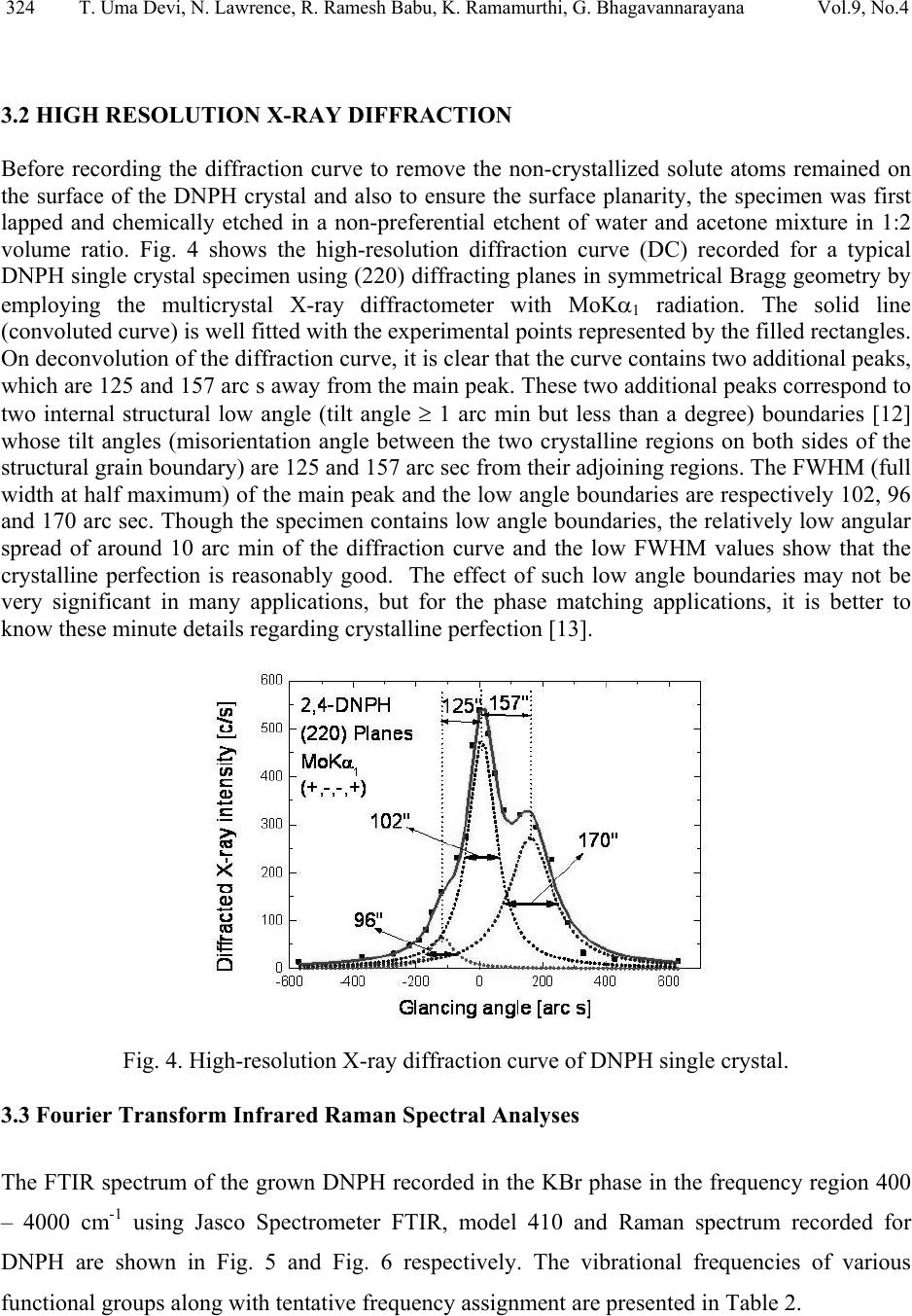 324 T. Uma Devi, N. Lawrence, R. Ramesh Babu, K. Ramamurthi, G. Bhagavannarayana Vol.9, No.4 3.2 HIGH RESOLUTION X-RAY DIFFRACTION Before recording the diffraction curve to remove the non-crystallized solute atoms remained on the surface of the DNPH crystal and also to ensure the surface planarity, the specimen was first lapped and chemically etched in a non-preferential etchent of water and acetone mixture in 1:2 volume ratio. Fig. 4 shows the high-resolution diffraction curve (DC) recorded for a typical DNPH single crystal specimen using (220) diffracting planes in symmetrical Bragg geometry by employing the multicrystal X-ray diffractometer with MoKα1 radiation. The solid line (convoluted curve) is well fi t t e d w it h the experimental points represented by the fil led recta ngle s. On deconvolution of the diffraction curve, it is clear that the curve contains two additional peaks, which are 125 and 157 arc s away from the main peak. These two additional peaks correspond to two internal structural low angle (tilt angle ≥ 1 arc min but less than a degree) boundaries [12] whose tilt angles (misorientation angle between the two crystalline regions on both sides of the structural grain boundary) are 125 and 157 arc sec from their adjoining regions. The FWHM (full width at half maximum) of the main peak and the low angle boundaries are respectively 102, 96 and 170 arc sec. Though the specimen contains low angle boundaries, the relatively low angular spread of around 10 arc min of the diffraction curve and the low FWHM values show that the crystalline perfection is reasonably good. The effect of such low angle boundaries may not be very significant in many applications, but for the phase matching applications, it is better to know these minute deta i l s regarding crystalline perfection [13]. Fig. 4. High-resolutio n X-ray diffraction curve of DNPH single crystal. 3.3 Fourier Transform Infrared Raman Spectral Analyses The FTIR spectrum of the grown DNPH recorded in the KBr phase in the frequency region 400 – 4000 cm-1 using Jasco Spectrometer FTIR, model 410 and Raman spectrum recorded for DNPH are shown in Fig. 5 and Fig. 6 respectively. The vibrational frequencies of various functional groups along with tentative frequency assignment are presented in Table 2. 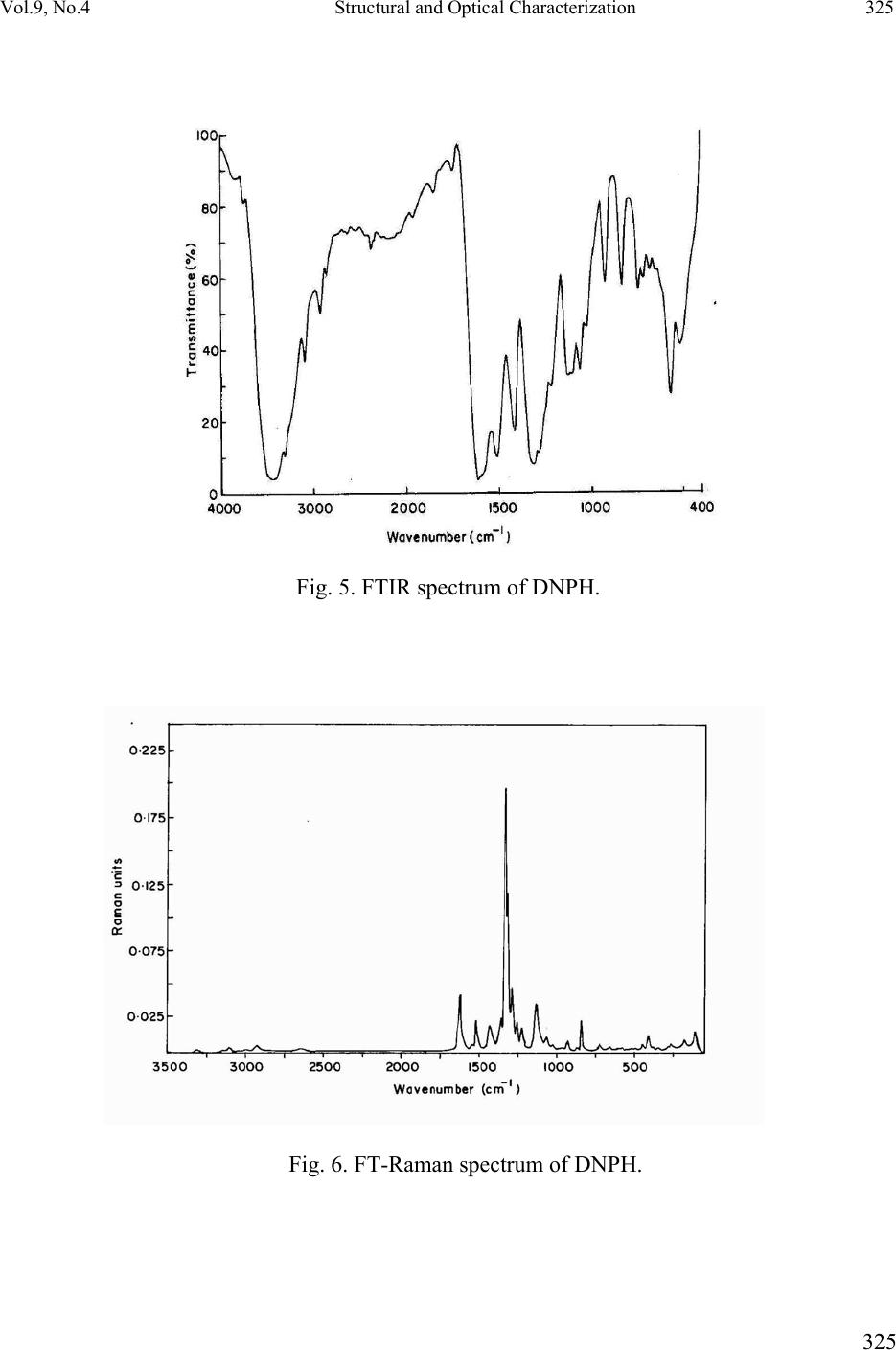 Vol.9, No.4 Structural and Optical Characterization 325 325 Fig. 5. FTIR spectrum of DNPH. Fig. 6. FT-Raman spectrum of DNPH.  326 T. Uma Devi, N. Lawrence, R. Ramesh Babu, K. Ramamurthi, G. Bhagavannarayana Vol.9, No.4 Table 2 Comparison of vibrational frequencies (cm-1) of DNPH with tentative frequency assignment IR (cm-1) Raman (cm-1) Assignments [14] Present Work Ref[8] Present work Ref[8] 3312 3325 3318 N-H symmetric stretching 3099 3087 3101 3102 C- H stretching 1847 1614 1511 1860 1606 1518 1620 1521 1606 1530 C -C stretching 1420 1426 1431 1426 C- C stretching - - 1364 1333 1373 1335 N= O symmetric stretching C- N stretching 1314 1319 1316 1319 N- O symmetric stretching - - 1287 1257 1224 1280 1223 NH2 twisting N-H in-plane bending 1126 1130 1135 1135 C- H in-plane bending 1061 1061 1070 1066 C- C- C trigonal bending 922 923 931 923 C- C ring breathing 833 839 840 839 NO2 scissoring 744 744 729 716 NO2 wagging 718 716 - - C- C- C in-plane bending 682 692 662 - C- C- C in-plane bending 569 516 530 508 453 412 453 C- C- C out-of-plane bending - - 271 225 C-NO2 out-of-plane bending - - 110 107 NO2 torsion  Vol.9, No.4 Structural and Optical Characterization 327 327 3.4. NMR Spectroscopy The proton NMR spectrum of DNPH was recorded using JEOL GSX 400 model at 23 °C. Powder form of the DNPH crystal was dissolved in acetone. The chemical shift values of the protons are plotted on the X-axis and the intensity is plotted on the Y axis (Fig. 7). There are signals at 7.90 ppm (doublet), 8.35 ppm (doublet) and 8.90 ppm (singlet), indicating the presence of aromatic hydrogens at the positions 3H, 5H and 6H, respectively. A sharp singlet at 10.80 ppm is due to the presence of NH group proton. Also the NH2 groups produce signal at 2.82 ppm. Appearance of additional peaks in the spectrum indicates that the compound is in the pure form. Fig. 7. NMR spectrum of DNPH. 3.5 Optical Transmittance The optical transmittance spectrum of DNPH was recorded using Shimadzu model 1601 in the range of 300 – 1000 nm and is shown in Fig. 8. Optically clear single crystal of thickness about 2 mm was used for this study. There is no appreciable absorption of light in the entire visible range. The transmittance between 500 nm and 1000 nm is approximately 23 %. The short wavelength cutoff occurs at 400 nm. 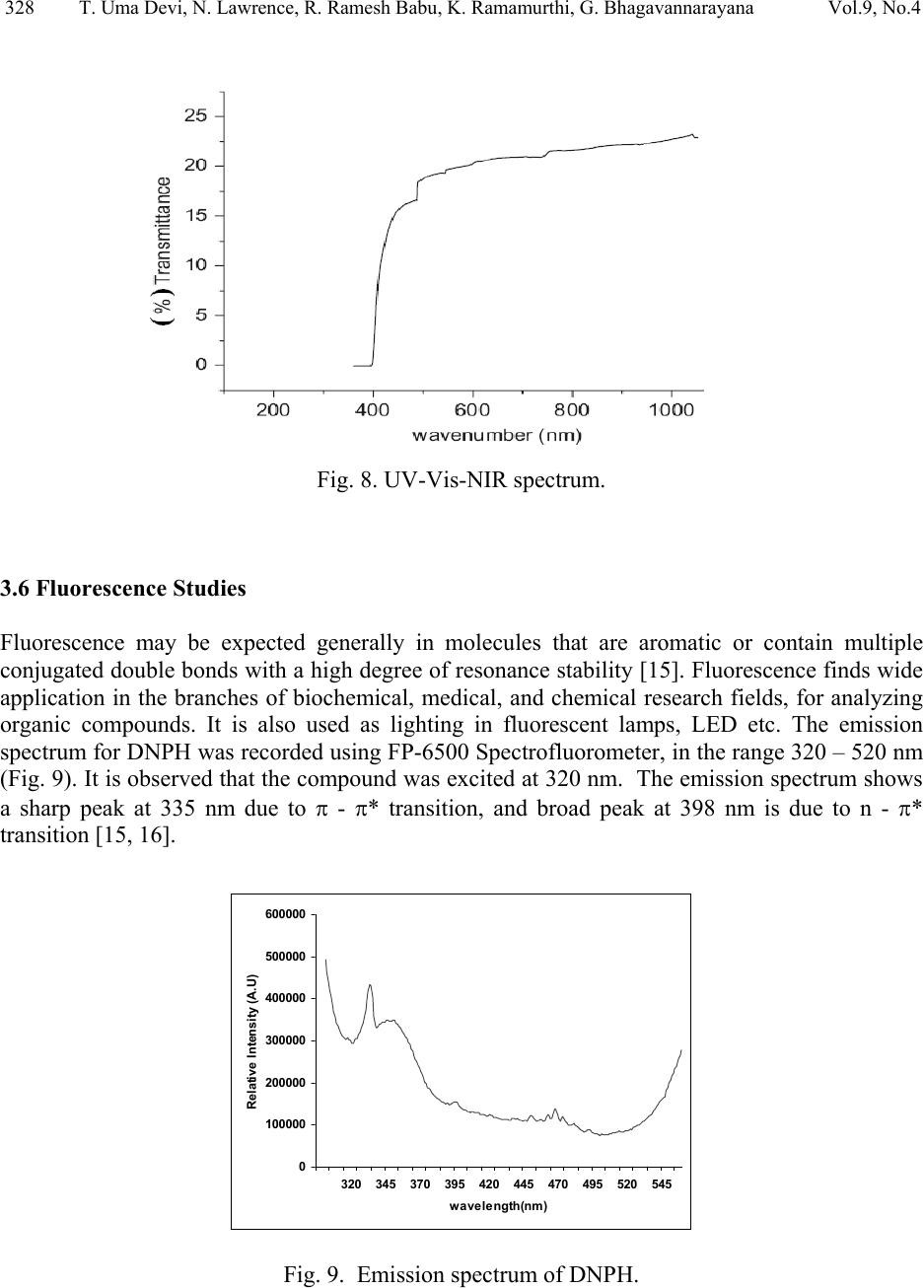 328 T. Uma Devi, N. Lawrence, R. Ramesh Babu, K. Ramamurthi, G. Bhagavannarayana Vol.9, No.4 Fig. 8. UV-Vis-NIR spectrum. 3.6 Fluorescence Studies Fluorescence may be expected generally in molecules that are aromatic or contain multiple conjugated double bonds with a high degree of resonance stability [15]. Fluorescence finds wide application in the branches of biochemical, medical, and chemical research fields, for analyzing organic compounds. It is also used as lighting in fluorescent lamps, LED etc. The emission spectrum for DNPH was recorded using FP-6500 Spectrofluorometer, in the range 320 – 520 nm (Fig. 9). It is observed that the compound was excited at 320 nm. The emission spectrum shows a sharp peak at 335 nm due to π - π* transition, and broad peak at 398 nm is due to n - π* transition [15, 16]. 0 100000 200000 300000 400000 500000 600000 320 345 370 395 420 445 470 495 520 545 wavelength(nm) Relative Intensity (A.U) Fig. 9. Emission spectrum of DNPH.  Vol.9, No.4 Structural and Optical Characterization 329 329 4. CONCLUSION DNPH single crystals of dimension 8x4x2 mm3 were grown by slow cooling method. The XRD studies confirm the structural identity of the grown crystals. The HRXRD study indicates that the grown crystal has very low angle boundary, which is due to the solvent incorporation into the crystal. FT-IR and FT-Raman spectra revealed the presence of various functional groups. NMR study confirms the placement of protons in DNPH molecule. The UV-Visible spectrum of DNPH showed that the crystal is transparent in the range 500 – 1000 nm. Fluorescence spectrum shows that DNPH fluoresces. A Z-scan technique analysis related to nonlinear optics may yield many interesting aspects of the title compound. REFERENCES: 1. H. Vidrio, G. Fernandez, M. Medina, E. Alvarez, F. Orallo, Vascul Pharmacol. 40 (2003) 13. 2. S. Patil, A. Kanase, P.H. Kulkarni, Indian J. Exp. Biol. 38 (2000) 253. 3. I. Kosalec, M. Bakmaz, S. Pepeljnjak, S. Vladimir-Knezevic, Acta Pharm. 54 (2004) 65. 4. S. Gupta, R.C. Husser, R.S. Geske, S.E. Welty, C.Smith, Toxicol. Sci. 54 (2000) 203. 5 N. Okabe, T. Nakamura, H. Pukuda, Acta Crystallogr. C49 (1993) 1678. 6. J.L. Wardell, J.N. Low, C. Glidewell, Acta Cryst. C62 (2006) o318. 7. V. Chis, S. Filip, V.Miclaus, A. Pirnau, C. Tanaselia, V.Almasan, M. Vasilescu, J.mol.struct. 744 (2005) 363. 8. N. Sundaraganesan, S. Ayyappan, H. Umamaheswari, B. D. Joshua, Spectrochim. Acta A 66 (2007) 17. 9. R. Ramesh Babu, S. Kumaresan, N. Vijayan, M. Gunasekaran, R.Gopalakrishnan, P. Kannan, P. Ramasamy J.Cryst. Growth 256 (2003) 387. 10. S. A. deVries, P. Goedtkindt, W. J. Huisman, M. J. Zwanenburg, R.Feidenhans’l, S. L. Bennett, D. M. Smilgies, A. Stierle, J. J. De Yoreo, W. J. P. van Enckevort, P. Bennema, E. Vlieg, J. Cryst. Growth 205 (1999) 202. 11. K. Udaya Lakshmi, K. Ramamurthi, Cryst. Res. Technol. 40, 1167 (2005). 12. K. Lal, G. Bhagavannarayana, J. Appl. Cryst. 22, 209 (1989). 13. G. Bhagavannarayana, R.V. Ananthamurthy, G.C. Budakoti, B. Kumar, K.S.Bartwal, J. Appl.Cryst. 38, 768 (2005). 14. G. Socrates, Infrared Characteristic Group frequencies; Wiley-Interscience: Chichester, U.K., 1980.  330 T. Uma Devi, N. Lawrence, R. Ramesh Babu, K. Ramamurthi, G. Bhagavannarayana Vol.9, No.4 15. Hobart H. Willard, Lynne L. Merritt jr., John A. Dean, Frank A.Settle jr., Instrumental Methods of Analysis,Sixth Edition, Wadsworth Publishing Company. USA, 1986 pp.609. 16. N. J. Turro, Molecular Photochemistry, W. A. Benjamin, Inc. Publishers, Massachusetts, 1965. |

